Saprolegniosis in aquaculture and how to control it?
Abstract
Saprolegniosis, also called water mould, induces a cotton or wool-like white growth on fish skin. It can kill fish at all stages of life, from eggs to adults. It is caused by oomycetes from the genus Saprolegnia and causes fish mortality and huge financial losses to fish farms and hatcheries. Saprolegnia species are endemic and ubiquitous in all freshwater habitats around the world. The exposing factors for saprolegniosis are still largely unknown, but stressors such as temperature shocks, poor water quality, handling and high fish density have been associated with outbreaks. For decades, malachite green was the most effective treatment against Saprolegnia infection, but it has been banned due to its carcinogenic and toxic effects. This has forced farmers to use alternative disinfection methods against Saprolegnia infection, such as hydrogen peroxide, formalin, Bronopol, NaCl, acetic acid and ozone, although many may have safety concerns or are impractical to use. This has led to the investigation of plant-based compounds with antifungal and antibacterial properties against saprolegniosis. These include extracts of certain herbs, onion, garlic, extracts of the plant Chrysanthemum, essential oils of Eryngium campestre, Mentha piperita, Cuminum cyminum and Thymus linearis, which include a variety of phenolic compounds and fatty acids with antifungal properties. This review combines the current knowledge regarding the predisposing factors to Saprolegnia infections and current methods to prevent and treat them, including those under further research. Thus far, many compounds have been tested and studied, but an effective, suitable and safe compound to treat Saprolegnia infection remains to be found.
1 INTRODUCTION
Saprolegniosis is a disease which can kill fish in all stages of life, from eggs to adult fish (Duan et al., 2018; Hirsch et al., 2008; Ruthig, 2009). The disease is caused by the oomycetes from genus Saprolegnia (Bruno et al., 2011; Coker, 1923; Duan et al., 2018), although other Saprolegniaceae such as species from genus Achlya can cause identical symptoms (Chukanhom & Hatai, 2004; Pavić et al., 2022). It is one of the major obstacles in fish production worldwide. It poses a serious threat to many species, including rainbow trout, salmon, tilapia and carp, as well as amphibians and crustaceans (Lone & Manohar, 2018; Peréz et al., 2003; Shah et al., 2021).
Saprolegniosis, also called water mould, induces a cotton or wool-like white growth on the fish skin and gills (Yanong, 2003). Lesions are often initially small and focal, but they rapidly enlarge (Zahran & Risha, 2013), causing epidermal damage and cellular necrosis (Noga, 2010). Saprolegniosis leads to an impaired ability of osmoregulation, loss of body fluids and finally respiratory failure (van West, 2006). The disease is lethal in most cases (Fernandez-Beneitez et al., 2008; Magwaza et al., 2017; van West, 2006).
Saprolegnia spp. is ubiquitous in freshwater ecosystems and is the main genus of water moulds responsible for significant fungal infections of freshwater fish and eggs (Pelczar et al., 2008). It causes significant declines in crayfish, fish and amphibian populations (Bruno et al., 2011; Liu et al., 2015; Pavić et al., 2021; van den Berg et al., 2013) and has been recognized as a threat to biodiversity (Fisher et al., 2012; Gozlan et al., 2014). Continuous infections have led to a decline in fish populations and species diversity (Romansic et al., 2009). Saprolegniosis has been observed in various fish species, amphibians (Blaustein et al., 1994), crustaceans (Dieguez-Uribeondo et al., 1994) and aquatic insects (Sarowar et al., 2013). It also threatens some highly endangered species (Fernández-Benéitez et al., 2008; Kiesecker et al., 2001).
Saprolegnia spp. is considered responsible for the decline of natural and cultured populations of salmonids, cyprinids and acipensers (Johari et al., 2014; van West, 2006), as well as amphibians, crayfish, crustaceans (Hirsch et al., 2008; Kiesecker et al., 2010) and other aquatic species (van den Berg et al., 2013; van West, 2006). In the environment, saprolegniosis has been observed (Hussein et al., 2001; Qureshi et al., 2001) not only in brackish (El-Hissy & Khallil, 1989) and freshwater environments (El-Hissy et al., 2000) but also in lagoons (Ali, 2005; El-Hissy et al., 2004). It also occurs frequently in fish hatcheries (Shokouh Saljoghi et al., 2020), on fish farms, and even in hobby fish tanks, causing infections (Hussein et al., 2002; Johari et al., 2014; Okumuş, 2002).
Saprolegnia spp. has been found worldwide: in most European countries, China, Japan, Southeast Asian countries, India, North and South America and parts of Australia (Hatai & Hoshai, 1994; Ravindra et al., 2022; Rowland et al., 2000; Magray et al., 2019). However, there is more genetic variation among Saprolegnia strains within each country than between countries (Elameen et al., 2021). According to some estimates, every freshwater fish is exposed to at least one oomycete during its lifetime (Neish, 1997), and about 10% of hatched salmon die due to Saprolegnia parasitica (Adel et al., 2020; Robertson et al., 2009). In aquaculture, Saprolegnia infects especially freshwater-cultured salmonids such as Atlantic salmon Salmo salar and rainbow trout Oncorhynchus mykiss but also species like eel, perch and catfish (Bruno et al., 2011; Das et al., 2012). Saprolegniosis causes mortality and presents a great threat to animal well-being (Magray et al., 2019; Phillips & Subasinghe, 2008).
Fish are one of the most important food sources in many countries worldwide (Hussain et al., 2011; Rao, 2023; Rubbani et al., 2011). Globally, fish resources are declining, driven by economic and human population growth (Limburg et al., 2011). This has led to increased fish farming, which has become a commercially important industry and sector in food production worldwide (Shah et al., 2021). Over the years, production has intensified but has unfortunately led to more disease outbreaks (El Gamal et al., 2023; Shaheen et al., 2015), which are problematic for aquaculture and its development (Shah et al., 2021; Shokouh Saljoghi et al., 2020).
Fish diseases are a major cause of financial losses in aquaculture. Oomycete (including water mould) infections have the second biggest impact after bacterial diseases (Johari et al., 2014). Saprolegnia and Achlya are the two typical species infecting fish and causing saprolegniosis (Mastan, 2015). Both cause fish mortality and huge financial losses to fish farms and hatcheries (Duan et al., 2018; Mostafa et al., 2020; Songe et al., 2016; Thoen et al., 2011) worldwide (Pavić et al., 2022). According to some estimates, saprolegniosis causes losses of billion dollars in Atlantic salmon, rainbow and brown trout and other species (Bruno et al., 2011; Van West, 2006). For example, in channel catfish (Ictalurus punctatus), saprolegniosis causes losses of approximately $40 million in the United States (Bruno et al., 2011; Liu et al., 2015). In India, disease incidences of S. parasitica have become more common in the widely raised striped catfish (Pangasianodon hypophthalmus) in recent decades and caused major financial losses on fish farms and damage in natural ecosystems (Ravindra et al., 2022; van West, 2006).
Thus far, a safe and effective treatment for saprolegniosis has yet to be established. Previously used and relatively effective treatments have been banned due to their carcinogenic or toxic properties (Corcoran et al., 2010; Mostafa et al., 2020), and it is planned to ban other treatment chemicals in the future (Bruno et al., 2011). However, promising results have been received by a selective breeding programme for Atlantic salmon S. salar (Misk et al., 2022), which indicated lower mortality for Saprolegnia infection in fish from resistant families.
As early as 2000, Rowland et al. (2000) published an extensive study of infectious diseases in silver perch (Bidyanus bidyanus), including saprolegniosis, which contained an extensive section on therapeutic chemicals. However, this review concentrated on the treatment of warm water species in Australia. Recently, Barde et al. (2020) have reviewed saprolegniosis, its taxonomy and life cycle but only briefly mentioned other treatments besides the banned malachite green. Buchmann (2022) reviewed methods to control all parasitic diseases in aquaculture, whereas Tedesco et al. (2022) reviewed natural compounds with therapeutic effects against Saprolegnia spp. Furthermore, He et al. (2023) extensively reviewed strategies to treat malachite green residues in the environment. However, a recent and comprehensive study of the known factors affecting saprolegniosis in aquaculture has been lacking.
The initial cause of saprolegniosis outbreak is still unknown, but stressors, such as poor water quality, handling and high fish density, have been associated with disease outbreaks (Whistler, 1997; Zahran & Risha, 2013). Based on this, this study reviews the currently known causes and affecting factors of Saprolegnia infections in aquaculture, focusing on water quality and stressors which may occur in aquaculture. Additionally, previously used, current and recently studied methods have been reviewed for treating saprolegniosis.
2 SAPROLEGNIA SPP
2.1 Classification
Fungal pathogens which infect freshwater fish belong to the oomycetes class (Daugherty et al., 1998). Oomycetes are taxonomically distributed into three subclasses: Rhipidiomycetidae, Peronosporomycetidae and Saprolegniomycetidae. Animal pathogenic oomycetes belong to Saprolegniomycetidae (Jiang et al., 2013; van West, 2006) which consist of Leptomitales and Saprolegniales (Hart & Reynolds, 2002; Magray et al., 2019). The Saprolegniales include eight genera: Saprolegnia, Achlya, Aphanomyces, Calyptratheca, Thraustotheca, Leptolegnia, Pythiopsis and Leptomitus. The three first are the most common in causing saprolegniosis in aquaculture (Bly et al., 1994; Hatai & Hoshiai, 1993; Rao, 2023).
Saprolegniaceae were first introduced by Ledermüller in 1760 (Magray et al., 2019). The classification for the Saprolegnia spp. group is as follows: kingdom: Chromista (Stramenopiles), phylum: Oomycota, class: Oomycetes, order: Saprolegniales, family: Saprolegniaceae and genus: Saprolegnia (Beakes & Ford, 1983; Magray et al., 2019).
Saprolegnia spp. is both saprobic and parasitic, which means that it obtains nutrition from decaying organic matter and from living hosts (Seymour, 1970). Previously, oomycetes, fungal-like eukaryotes, were classified as fungi due to their filamentous growth, because they live on decaying matter (Duan et al., 2018). However, they are more closely related to algae due to their bi-flagellated zoospores, gametangial copulation, cellulose cell wall structure and biochemistry (Duan et al., 2018; Paul & Steciow, 2004). Currently, oomycetes are included in brown algae and diatoms (Beakes et al., 2012; Derevnina et al., 2016; Duan et al., 2018).
2.2 Reproduction of S. parasitica
Oomycetes are a widespread group of parasites (Carris et al., 2012; Rezinciuc et al., 2018). Oomycetes are considered successful because they can overcome host resistance and even jump to new hosts (Derevnina et al., 2016). They also have a flexible mating system and reproduce sexually, asexually, or through interspecific hybridization (Hardham, 2001). They rely on asexual secondary zoospores (Kim & Judelson, 2003; Walker & van West, 2007) which form cysts and attach to their hosts prior to the infection (Raftoyannis & Dick, 2006). The descriptions of developmental stages of oomycetes, including primary and secondary zoospores, primary and secondary cysts, and germinated cysts, are often shown in a schematic diagram of the oomycetes’ life cycle (e.g., in Mueller, 1994; van West, 2006). Oomycetes can invade a wide range of hosts, including fungi, algae, plants, invertebrates and vertebrates (Robold & Hardham, 2005; Zhang et al., 2013). They have adaptable mechanisms for host colonization. They contain diverse molecules of translocated host-targeting proteins (Wawra et al., 2012), the degrading enzymes glycosyl hydrolases and proteases (Jiang et al., 2013), or adhesive biopolymers (Epstein & Nicholson, 2006). In close proximity to a potential host, oomycetes release effector proteins and toxins into the host tissues to manipulate their immune responses (Bly et al., 1992, 1993).
Among the oomycetes, S. parasitica is one of the most harmful fish pathogens (Magray et al., 2021; Matthews et al., 2021). The presence of oomycetes in an aquatic environment is one of the most important reasons for saprolegniosis (Bly et al., 1992, 1993). S. parasitica can infect a wide range of hosts, including important species raised for food (Jaies et al., 2020; Magray et al., 2021).
Saprolegnia spp. development begins with a slight enlargement of the terminal portion of a hypha and cytoplasm and nuclei accumulate in the enlarged tip, called the sporangium, which produces masses of zoospores (Rao, 2023). Saprolegnia spp. proliferates rapidly at low temperatures and produces zoospores (Matthews, 2019). The tip of the sporangia breaks down and releases the primary zoospore, which has two flagella at the anterior end. They move independently for some time and then encyst. The cyst germinates after a period into hyphae or bursts to release secondary zoospores, which are reniform with two flagella laterally placed on the concave side (Rao, 2023). After swimming for some time, they encyst and finally germinate into hyphae.
Sexual reproduction in Saprolegnia spp. takes place by the sex organs developing at tip of the hyphae (Rao, 2023). The female organ oogonium is a globose body, and the male organ antheridium is slender and sinuous. Sexual reproduction occurs by gametangia contact, in which the fusion of the haploid oosphere occurs with nuclei, producing a zygote (Beakes & Gay, 1977). The zygote then transforms into a sporangium, in which the protoplast divides into several zoospores. It takes nearly 3 months from fertilization to germination into hyphae (Rao, 2023).
Saprolegnia spp. has various components on its cell wall. The major ones are cellulose, β-(1 → 3)- and β-(1 → 6)-glucans and chitin in small amounts (Parra-Laca et al., 2015; Rzeszutek et al., 2019). Chitin contributes to mechanical strength to intracellular turgor pressure, playing an important role in mycelial tip growth (Guerriero et al., 2010). The cell wall molecules can evoke immune response in a host (Parra-Laca et al., 2015). Long zoospores with hook-like end-structures aid its attachment to the host (van West, 2006). In S. parasitica, the zoospores may be generated repeatedly for up to six generations by a process known as repeated zoospore emergence (Robertson et al., 2009; van West, 2006).
2.3 Infection
Saprolegnia spp. infects different organisms, including insects, fish, amphibians and turtles (MacGregor, 1921). The infection can also be transmitted between species or via contact with infected soil (Kiesecker et al., 2001). Adhesive compounds allow attachment to the cell substratum and prevent the removal of pathogen (Bechinger et al., 1999; Rezinciuc et al., 2018). Biological attachment is based on adhesive polymers which vary in structure and capabilities. S. parasitica contains hooked hairs or spines on the secondary cysts to attach to fish (Rezinciuc et al., 2018; Wood et al., 1988). The hooked hairs are more than 2 μm long, organized in bundles and are rapidly formed during secondary cyst formation (Beakes, 1983; Beakes et al., 1994). The bundles of hooked hairs are involved in pathogen attachment, and the strength of cyst attachment of Saprolegnia spp. appears to be correlated with the length of bundles (Pickering et al., 1979; Rezinciuc et al., 2018). Additionally, the more efficient attachment of S. parasitica cysts may be due to the different stickiness properties and composition of the cyst extracellular matrix compared to other Saprolegnia species (Rezinciuc et al., 2018).
Once Saprolegnia spp. has infected its host, it transfers effector proteins into the tissues of the host and manipulates its immune responses (Wawra et al., 2012) and can induce perceptible changes in the gills and kidney (Belmonte et al., 2014), taking the form of profusely branched, non-septate mycelia. Infected organisms become covered with white hyphal filaments (Blaustein et al., 1994), which can spread via contact with growing hyphae or through colonization by free-swimming zoospores (Wood & Willoughby, 1986). The early legions are grey or white and often appear as circular colonies. They form white cotton-wool-like tufts or fungal mats in deep skin lesions on fish, which become grey as cysts are produced (Eissa et al., 2013; Mahfouz et al., 2019). White or grey mycelium patches on the skin may also penetrate the muscles (Mahfouz et al., 2019). Mycelia can colonize eggs and lead to their suffocation and death (Cao et al., 2012; Mousavi et al., 2009). In severe cases, the lesions can cover 80% of the body. Saprolegnia spp. typically penetrates the superficial musculature, but in some cases, it can be even deeper. More rarely, the Saprolegnia spp. can be located on the oesophageal or gill region of fish. Furthermore, epithelial damage on skin, gills and gut, or other pathogens can offer a route of entry (Roberts, 2001).
Saprolegnia infection can occur in almost any part of the fish’ body, but some areas are more easily infected (Pickering & Willoughby, 1982; Richards & Pickering, 1978). The pathogen primarily affects epidermal tissues, fins or head and eventually spreads over the entire surface of the fish's body (Figure 1). It may also spread to internal organs liver, kidney and alimentary canal (van West, 2006). Male fish are more vulnerable along the dorsal surface, females on the caudal and ventral fins. There are usually only weak inflammatory responses in the tissues of the fish. The site of infection and the patterns may vary between farm-raised and wild fish (Beakes, 1983; Pickering & Willoughby, 1982), and fish reared in a hatchery are typically infected around the fins. Saprolegnia infections are often restricted to the epidermis, dermis and, in some cases, the superficial musculature (Pickering & Richards, 1980).

The degenerative changes in tissues may represent damage caused by proteolytic enzymes in the fungal hyphae (Figure 2, Pickering & Willoughby, 1982; Roberge et al., 2007). The most harmful effect of the infection is related to the osmoregulatory system of the fish. Teleost fish are constantly subject to an osmotic flux of water and loss of ions across the gills. These are normally balanced by the uptake of salts and production of urine but require the skin to form an impermeable layer (Evans, 2008; Pickering & Willoughby, 1982). After a Saprolegnia infection, the osmotic pressure and salt content of the blood are inversely related to the severity of the infection (Richards & Pickering, 1979). The Saprolegnia spp. seems to destroy the essential waterproofing properties, resulting in a lethal dilution of body fluids (Pickering & Willoughby, 1988).
The changes beneath the skin surface include dermal necrosis and oedema and in later stages, extensive haemorrhage and deeper myofibrillar necrosis (Hatai & Hoshai, 1994). The tissue damage is probably caused by a proteolytic chymotrypsin-like enzyme in the Saprolegnia spp. (Mueller, 1994). The ultimate cause of death is the severe hemodilution caused by haemorrhage and the destruction of the water-proofing properties of the fish's integument (Pickering & Willoughby, 1988).
Das et al. (2012) studied the pathogen causing mortality in Indian major carp (Catla catla) fingerlings. They observed that from the onset of infection, fish died within 12–15 days. The first signs were visible red or grey patches of filamentous mycelium. The development of patches was probably due to the rubbing of the body against a hard surface due to the attachment of filamentous mycelium, which causes itching. During an acute infection, which took 7–8 days from the beginning of infection, a cotton-like structure appeared that radiated out in circular, crescent-shaped, or whorled pattern (Das et al., 2012). The fish died within 3–4 days.
Saprolegnia spp. invades epidermal tissues, generally beginning on the head or fins, and spreads over the entire surface of the body (Neish, 1997; Willoughby & Roberts, 1992; Zaki et al., 2008). Das et al. (2012) observed that the red patches first appeared in the middle part of the surface of the body and then gradually spread to other parts. Infections on fish often occur in the epidermis and dermis but sometimes in the superficial musculature. Fungal infection eventually destroys the osmoregulatory system and causes a lethal dilution of body fluids (Pickering & Willoughby, 1988). Saprolegniosis also causes skin lesions, erratic swimming behaviour and sunken eyes (Majhi et al., 2005). As a fungal infection can be seen by the naked eye, a fungal reproductive process has been initiated, and natural recovery is highly unlikely (Das et al., 2012).
3 PREDISPOSING FACTORS TO SAPROLEGNIOSIS
3.1 Stressful conditions
Thus far, the initial cause of saprolegniosis outbreak is unknown. Many stressors, such as an unsuitable water temperature, poor water quality, handling and high fish density, have been associated with outbreaks (Whistler, 1997; Zahran & Risha, 2013). However, longitudinal studies of abiotic and biotic factors remain rare (Duan et al., 2018). Numerous studies have been conducted, but the prevalence of S. parasitica in the environment or ecological parameters influencing its occurrence remain very limited (Elameen et al., 2021; Masigol et al., 2020). It is unclear why some farms have frequent outbreaks, whereas others remain disease-free.
In aquaculture, numerous factors are involved in the formation of saprolegniosis, but Saprolegnia-associated mortality appears to increase in the presence of abiotic stressors (Kiesecker et al., 2001). For example, overcrowding, an insufficient water flow rate, low oxygen content, poor water quality, injuries from handling, malnutrition, crowding, stress, temperature shocks, spawning, external parasitism, or infrequent removal of dead fish or eggs can lead to disease occurrences (Pavić et al., 2021; Piper et al., 1982; Bruno et al., 2011; Tedesco et al., 2022). High stocking densities and injuries formed in intensive breeding techniques have increased the occurrence of mycotic diseases, including saprolegniosis (Olah & Farkas, 1978).
Stressful rearing conditions (temperature shocks, infections by bacteria and fungi, pollutants, overcrowding, injuries from biting and due to rough handling and transportation, low levels of dissolved oxygen, temperature fluctuations, osmotic shocks and poor water quality) can weaken the immune system of the reared species and increase susceptibility to S. parasitica (Bly et al., 1993; Hatai & Hoshiai, 1994; Howe & Stehly, 1998; Pavić et al., 2021; Roberge et al., 2007). Insufficient removal of dead fish and eggs can result in a higher spore load and act as a predisposing factor for saprolegniosis (Bruno et al., 2011; Tedesco et al., 2022).
Saprolegniosis is a common problem for salmon and trout hatcheries and is often associated with broodstock, fertilized ova and parr (Hatai & Hoshiai, 1993), especially when wild or broodstock fish are held and handled for the taking of eggs or milt (Howe & Stehly, 1998). Hatchery managers often need to treat fish regularly with an antimicrobial or anti-oomycete chemical during stressful periods to prevent saprolegniosis.
Stress related to increased water temperatures can increase susceptibility to saprolegniosis (Casas-Mulet et al., 2021; Stewart et al., 2018) but also transformation to different life stages (Pickering, 1994). Pickering (1993) concluded that stress stimulates the hypothalamic-pituitary-interrenal axis of salmonids and leads to increased cortisol levels. It is an immunosuppressant and probably causes the suppression of the defence system, increasing the stress-induced likelihood of saprolegniosis (Pickering, 1994).
Incorrect handling procedures can lead to damage to the fish skin and increased stress, which are both associated with a higher susceptibility to saprolegniosis (Beckmann et al., 2020; Bruno et al., 2011). Pavić et al. (2022) observed and reported how the health status of the fish significantly influenced S. parasitica on the skin of injured rainbow O. mykiss and brown trout Salmo trutta, which had significantly higher S. parasitica loads than the apparently healthy fish.
Both abiotic and biotic stressors may act synergistically with pathogens to increase the adverse effects on hosts (Romansic et al., 2006). If both pathogens and stressors are present, the effects on hosts may be more aggravated than when either is present alone (Khan, 1990). Saprolegniosis can also be a secondary to viral or bacterial infection (O'Brien, 1974), or fish can be impacted by several pathogens (Daszak et al., 2003), and the effects are influenced as co-factors (Christin et al., 2003; Kiesecker, 2002). Additionally, an inadequate diet and nutritional deficiencies also play a role. An insufficient supply of ascorbic acid is especially important for collagen production and for sufficient mucus production and tissue regeneration (Ashley et al., 1975; Pavić et al., 2022).
The quality and quantity of mucus in various parts of the fish's body may play a role in saprolegniosis (Noga, 1993). Mucus concentration tends to be lower on the fins than on the remaining sites on the fish's body, allowing faster zoospore attachment to the fins than the rest of body (Durborow et al., 2003; Richards & Pickering, 1978).
The control of saprolegniosis depends on good husbandry practices in hatcheries and on fish farms. It includes the prevention of pathogens, the maintenance of good water quality, a decrease of environmental stressors, adequate nutrition (Meyer, 1991; Tedesco et al., 2022) and proper handling (Ali et al., 2019; Rao, 2023). The defence systems of fish include enforcement of their immune system and mucus formation on skin, which play a role in the prevention of saprolegniosis (Quiniou et al., 1998; Mueller, 1994).
3.2 Water quality
According to the current understanding, water quality influences outbreaks of saprolegniosis. Previous studies have shown that varying pH, O2 and total ammonium nitrogen have no direct effect on the development of saprolegniosis (Bly et al., 1993), but environmental factors such as water temperature, salinity and microorganisms seem to be connected with outbreaks (Mifsud & Rowland, 2008). Saprolegnia spp. strains grow well at wide temperatures and pH ranges (5–40°C, pH 2–11, Olah & Farkas, 1978). However, zoospore production decreases when the water temperature increases above 20°C, and their germination is inhibited in acidic (pH < 4) conditions (Dieguez-Uribeondo, 1995; Kitancharoen et al., 1996; Pavić et al., 2021). Additionally, pH can affect zoospore growth: abundant hyphae at low pH and spherical schizons at neutral pH (Kocan, 2013; Mahboub & Shaheen, 2021).
High organic load and nitrogen compounds have been connected with Saprolegnia spp. infections (Pavić et al., 2022). Even sublethal concentrations of ammonia and nitrites can increase the susceptibility of trout to saprolegniosis (Carballo & Munoz, 1991). Romansic et al. (2006) reported that the effects of nitrate and Saprolegnia spp. were synergistic, not additive. They stated that nitrate might decrease zoospore production or kill zoospores, inducing physiological response mechanisms in the host which might lead to resistance to Saprolegnia spp.
Saprolegniosis rarely occurs in seawater because Saprolegnia spp. cannot survive in the marine environment at a salinity above 1.75% (as NaCl, Mueller, 1994). As early as 1979, Taylor and Bailey (1979) suggested that seawater was an effective substitute for malachite green in preventing saprolegniosis. Salt (NaCl) is a safe and inexpensive parasiticide and osmoregulatory aid (Mifsud & Rowland, 2008). However, its controlling effect varies with fish species, salt concentration and the strain of oomycetes (Chukanhom & Hatai, 2004; Mifsud & Rowland, 2008). For example, the survival of silver perch treated with 2–3 g L−1 was 100%, but for untreated fish, only 66.7% (Mifsud & Rowland, 2008).
A high organic load can be associated with Saprolegnia infections (Pavić et al., 2022). Organic compounds often include humic substances (HSs) in natural waterbodies, especially in the Nordic countries (Thorsen, 1999). HSs are complex organic molecules that constitute the main part (50%–80%) of dissolved natural organic material in freshwater ecosystems (Meinelt et al., 2007; Wetzel, 2001). For example, concentrations of dissolved organic carbon range from 1 to 100 mg L−1 HS in oligotrophic ecosystems (Steinberg, 2003; Wetzel, 2001). Aquatic HS consist of water-soluble substances with carboxylic, phenolic, quinoid and aliphatic groups, and cations of certain elements (e.g., Na, K, Mg, Ca, Fe, Al and Cu). HSs are large and complex organic molecules, but no specific structural formulas can be given. Statistical parameters can be defined based on their molecular weight, aromaticity and elemental analysis.
HS may act as a natural biocide or herbicide (Karasyova et al., 2007; Prokhotskaya & Steinberg, 2007). Even antibiotic properties of humic and fulvic acids have been reported (Gryndler et al., 2005). The effects of HS on living organisms have been reviewed by Steinberg et al. (2006), but not the effects of HS on fungi or S. parasitica. Meinelt et al. (2007) tested the effects of HS (20 HS and natural organic matter, NOM) on S. parasitica in vitro. The HSs were collected and isolated from real aquatic environments in central and northern Europe and were characterized by high-performance size-exclusion chromatography. The results of Meinelt et al. (2007) showed that HS might enhance and reduce the vegetative growth of the water mould S. parasitica. This is based on NOMs rich in carbohydrates and amino acids which support fungal growth, whereas humic material with high aromaticity and high C:H and C:CH2 ratios inhibits the growth of water mould. Later, Pavić et al. (2022) showed that HSs and other dissolved organic matter could inhibit mycelial growth (Meinelt et al., 2007).
There are differences between hosts in their susceptibility to Saprolegnia infection (Kiesicker & Blaustein, 1995, 1997). For example, water temperature, pH, pollutants and exposure to UV radiation can alter susceptibility to saprolegniosis (Lefcort et al., 1997). For example, El Gamal et al. (2023) focused on investigating outbreaks on fish farms rearing tilapia (apart from salmonids, the focus of most studies) that received untreated water with different contamination loads and its relationship with saprolegniosis outbreaks. Their paper was the first to study the link between saprolegniosis and improper water quality sources used on tilapia farms.
Water parameters such as electrical conductivity (EC), cations Ca2+ and Na+, and anions SO42− and Cl− are considered highly relevant for explaining the S. parasitica load in water (Pavić et al., 2022). NO3− and F− remained barely below the level of significance, whereas COD, TOC, K+, TP, NH4+, pH and Mg2+ had a smaller influence on the S. parasitica load (Pavić et al., 2022).
Fluoride (F−) has shown a negative correlation with S. parasitica in water (Pavić et al., 2022), suggesting an inhibitory effect of fluoride on S. parasitica. In the environment, excessive fluoride concentrations are known to affect microbial communities by inducing negative effects on microbial physiology (Marquis et al., 2003; Mendes et al., 2014). To our knowledge, no studies have been conducted so far on the toxicity of fluoride to oomycetes.
The strongest positive correlation with S. parasitica was found with EC and Ca2+. Pavić et al. (2022) concluded that calcium ions could positively influence the developmental stages and infection process of oomycetes (Burr & Beakes, 1994; Rezinciuc et al., 2018). Ca2+ ions regulate adhesion, encystment and germination processes (Pavić et al., 2022; Rezinciuc et al., 2018). Additionally, Pavić et al. (2022) showed that Ca2+ concentrations above average (global median 4 mg L−1, Weyhenmeyer et al., 2019) in surface waters favoured the growth of S. parasitica.
Furthermore, EC, describing the total ion content (mainly Na+, Cl− and Ca2+), has been found to be positively correlated with the S. parasitica load (Pavić et al., 2022). In natural freshwater ecosystems, sodium and chloride concentrations vary from country to country. In high concentrations (above 1000 mg L−1), sodium chloride is used as a non-toxic method to control Saprolegnia sp. Sodium chloride can reduce the formation, release and proliferation of sporangia and the growth of the pathogen (Ali, 2005; Pavić et al., 2022).
Freshwater aquaculture facilities are often connected with rivers/streams, suggesting the potential transfer of Saprolegnia pathogens from fish farms to downstream freshwater environments or from water bodies to farms (El Gamal et al., 2023). The escaping fish or water drainage from or to fish farms can therefore result in pathogen transfer with the natural environment.
3.3 Temperature
Temperature as an abiotic stressor in fish is frequently cited as a critical contributing factor to Saprolegnia infection (Bly & Clem, 1991; Bly et al., 1992). Saprolegniosis outbreaks often occur when temperatures are near the physiological low end of a particular fish species. Cultured fish often suffer from saprolegniosis during the winter months when the temperature falls below 10°C (Naskar et al., 2005). This may be due to decreased immunity because many oomycetes are more active in the cooler months of the year (Rao, 2023). Different species of Saprolegnia have different optimal growth temperatures, but increased concentrations are often found at low temperatures. However, other studies suggest that specific water temperature was not correlated with saprolegniosis occurrences but with the change in temperature (Korkea-aho, Wiklund, et al., 2022; Korkea-aho, Viljamaa-Dirks, et al., 2022). The change in temperature may lead to stress reactions and immunosuppression in fish and more likely lead to saprolegniosis.
Saprolegniosis occurs more frequently in winter (34%) than in spring (17%) (Mahboub & Shaheen, 2021). Saprolegnia spp. strains grow faster at low temperatures and germinate at 5°C in the early spring. Previous studies have reported seasonal variation of prevalence of Saprolegnia spp.: 47% in December and only 9% in August (Das et al., 2012; Mahboub & Shaheen, 2021) and for tilapia 88.8%, 93.3%, 88.8% and 84.4% in December, January, February and March (Abd El-Rahman et al., 2012). For example, the zoospore levels of Saprolegnia spp. were more than 5 spores mL−1 in the winter and below 1 spore mL−1 during the summer months (Bly et al., 1993).
Saprolegnia spp. has a wide temperature tolerance range, from 3 to 33°C (Aly & El-Ashram, 2000; Willoughby & Roberts, 1992). Low temperatures in the early spring and late fall can cause saprolegniosis, therefore referred to as ‘winter kill’ (Bly et al., 1993). Bly et al. (1992) were the first to report this in channel catfish, I. punctatus. They found that the syndrome was linked to cold fronts when the water temperature dropped (5–9°C in 12 h and from 20 to 10°C in 24 h, Bly et al., 1992, 1993). A rapid decrease and low water temperature has also been linked to high levels of zoospores of S. parasitica (Bly et al., 1992; Zahran & Risha, 2013). The mycelial growth rate of S. parasitica was highest between 15 and 25°C and zoospore viability at around 10°C (Matthews, 2019).
For example, a sudden decrease in water temperature (from 20 to <8°C), coupled with an increase in water pH (>9), has caused saprolegniosis occurrences in cultured fish (Das et al., 2012). Bruno et al. (2011) reported that sudden changes in temperature could make fish vulnerable to saprolegniosis due to the increased physiological stress. Indian major carp species grew better at temperatures from 26 to 33°C (Das et al., 2004), and their feeding, swimming, oxygen consumption and thermal regulation rates were significantly influenced at low temperatures (Das et al., 2012). However, the stress of high temperature and poor oxygen levels may also induce saprolegniosis (Rao, 2023; Roth, 1972).
Bly et al. (1993) also reported that a rapid drop in temperature led to immunosuppression in catfish, and the presence of fungal zoospores led to fungal infections. Similarly, Ravindra et al. (2022) stated that disease incidences were often associated with stressful conditions such as a low water temperature, or when the host was immunocompromised (Duan et al., 2018; Van den Berg et al., 2013). The combination of a rapid drop in temperature and its duration led to a saprolegniosis outbreak (Bly et al., 1993; Zahran & Risha, 2013).
4 TREATMENTS
4.1 Malachite green
Malachite green is a diamino derivative of rosaniline dye (Culp & Beland, 1996). Malachite green has fungicidal properties, which has led to its use in controlling Saprolegnia infections in freshwater systems (Culp & Beland, 1996; Hughes, 1994; Stueland et al., 2005). It was long considered the most effective treatment (Srivastava et al., 2004). Malachite green was originally used in the dye industry in dying silk, wool, jute and leather (Merck, 1989), but it has also been used as a medical disinfectant and anthelmintic (Nelson, 1974). It also serves as an additive in the paper industry, a pigment in ceramics, and has even been used as a food colouring agent in milk products in India (Khanna et al., 1973).
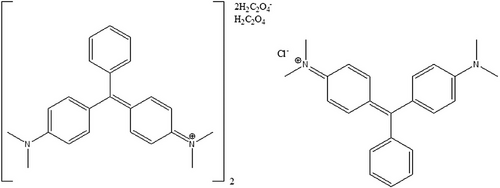
Malachite green has been referred to as basic green 4 based on its colour index, acryl brilliant green, aniline green, Aizen malachite green, astra malachite green, Benzal green, China green, benzaldehyde green, diamond green P extra, solid green 0, light green N and fast green (Culp & Beland, 1996). The CAS name and number for malachite green oxalate is N-[4-[[4-(dimethylamino)-phenyl]phenylmethylene]-2,5-cyclohexadien-1-ylidenel-N-methylmethanaminium oxalate 2437-29-8 (CI 42000).
In ambient conditions, malachite green is a green crystalline powder and occurs as chloride and oxalate salts (Figure 3), as a neutral carbonol form, or in the reduced leuco form (Culp & Beland, 1996). It is soluble in water (66.67 g L−1) as chloride salt (Reynolds, 1982), but it decomposes in air due to oxidation to a diarylketone (Bradley, 1958). In a neutral aqueous solution, malachite green occurs as dye salt and a lipophilic carbinol base (Alderman, 1985). The carbinol base is colourless and almost insoluble in water (500 μg L−1). In acidic conditions, malachite green salt occurs as completely ionized (at pH 4), whereas, in basic conditions (pH 10.1), malachite green exists as the carbinol. In aquaculture, it was used as zinc-free oxalate, whereas a double salt with zinc chloride was used as a dye (Culp & Beland, 1996).
Malachite green was first used in aquaculture in 1936 (Foster & Woodbury, 1936) to control fungi. It has been used routinely since the 1930s due to its efficiency against fungal infections (Schnick, 1988). Among more than 180 tested antifungal compounds, malachite green was the most efficient with low toxicity towards fish (Meyer & Schnick, 1989). Besides the prevention of oomycete fungi, malachite green can control Proliferative kidney disease in rainbow trout (Culp & Beland, 1996).
Malachite green was banned in edible fish in the United States, Europe (Legislative Decree no. 119/92, Forneris et al., 2003), and worldwide in 2002 due to its carcinogenic and toxic effects and teratogenic and mutagenic properties (Duan et al., 2018; Kumar et al., 2020; Shah et al., 2021; Stueland et al., 2005). This has forced farmers to use alternative disinfection methods against saprolegniosis (Sandoval-Sierra et al., 2014). Currently, no sufficiently effective treatment has been found, which has for its part led to the spread of saprolegniosis (Kumar et al., 2020) and struggle for many fish farmers (Derevnina et al., 2016). Although malachite green has been banned, and its risks are known, it is still used as an anti-oomycete chemical in some countries (Kalatehjari et al., 2015; Sattari & Roustayi, 1999). For example, it can still be used for ornamental fish (He et al., 2023). To overcome problems related to Saprolegnia spp., an effective antimicrobial agent is desperately required which is inoffensive to fish and safe for human health and the environment (Madhuri et al., 2012).
Malachite green was first detected in fish by Poe and Wilson (1983). In fish, malachite green is absorbed through the gills and skin, transformed to the leuco form and stored in the lipid tissue (Culp & Beland, 1996). Carbinol forms of malachite green are more lipophilic than its cations. Leucomalachite green is oxidized to malachite green when frozen. Intact malachite green is excreted quite rapidly, but leucomalachite green has a half-life of about 40 days.
Exposure to malachite green causes reproductive abnormalities in rabbits and fish (Meyer & Jorgenson, 1983) and enhances the formation of hepatic tumours in rats (Fernandes et al., 1991), possibly due to its structural similarity with other carcinogenic triphenylmethane dyes (Littlefield et al., 1985). In addition to its carcinogenic and teratogenic properties, malachite green is capable of intercalating and binding of DNA (Corcoran et al., 2010; Rosenkranz & Carr, 1971).
4.2 Other chemical treatments
Treatment of water mould infection is very difficult, and legal drugs are limited (Zahran & Risha, 2013). The currently most applied chemicals against Saprolegnia spp. are formaldehyde, peracetic acid and hydrogen peroxide (Adel et al., 2020). Only a few aquatic anti-oomycete agents are available, and other options besides malachite green have unfortunately been less effective against Saprolegnia spp. (Table 1). Multiple chemicals have been tested to control saprolegniosis and other parasites, as recently reviewed by Buchmann (2022), but their harmful effects on farming personnel or the environment often prevent their wider use (Kumar et al., 2020). Many attempts have been made to prevent saprolegniosis outbreaks by curative and prophylactic anti-oomycete chemicals (Hussein et al., 2002). Although many compounds have been tested and studied, a very suitable and safe compound to treat saprolegniosis remains to be found (Kumar et al., 2020).
| Treatment | Concentration | Effect in fish/eggs/in vitro | Reference |
|---|---|---|---|
| Bronopol (2-bromo-2-nitro-1,3-propanediol) |
10–50 mg L−1 MIC 100–250 mg L−1 MLC |
Growth of Saprolegnia parasitica in vitro | Stueland et al. (2005) |
| Bronopol (2-bromo-2-nitro-1,3-propanediol) |
100 mg L−1 60 min 200 mg L−1 60 min |
Hyphal growth of S. parasitica: colony radius 0–38.5 mm Colony radius 0 mm |
Oono and Hatai (2007) |
| Chloramine T (C) + formalin (F) | 8 mg L−1 (C) + 123 mg L−1 (F) | Spot disease (Ichthyophthiriasis) decreased 63% in salmonids | Rintamäki-Kinnunen et al. (2005) |
| Copper nanoparticles | 10 mg L−1 | Relative S. parasitica colony size 60% of control | Kalatehrjari et al. (2015) |
| Copper sulphate | 20–40 mg L−1 | 45.8%–50.5% survival of sunshine bass larvae | Straus et al. (2016) |
|
Formaldehyde Formalin (37% formaldehyde) |
1–2 mL L−1 0, 50, 100, 150 mg L−1 |
58.6% survival of eggs 67%(0), 35%(50), 29% (100), 40%(150) mortality of rainbow trout |
Forneris et al. (2003) and Gieseker et al. (2006) |
| Formaldehyde | 40–80 mg L−1 | 76%–97% reduced infection on rainbow trout | Jaafar et al. (2013) |
| Hydrogen peroxide | 15–30 mg L−1 | 44%–85% reduced infection on rainbow trout | Jaafar et al. (2013) |
| Hydrogen peroxide | 560 mg L−1 for 30 min | 56% rainbow trout parasite-free | Rach et al. (2000) |
| Malachite greena | 10 mg L−1 | Lowest spore count in vitro | Caruana et al. (2012) |
| NaCl | 4 g L−1 for 2 min, 3× weekly | 50% fish mortality | Das et al. (2012) |
| NaCl + KMnO4 | 4 g L−1 NaCl for 2 min + 5 mg L−1 KMnO4 for 10 min | 8% fish mortality | Das et al. (2012) |
| NaCl + ascorbic acid | 0.17 M NaCl + 5.68 mM ascorbic acid | Reduced protease activity, inhibited sporangial formation | Ali (2005) |
| Ozone | 0.01–0.2 mg L−1 d−1 | 42.6%–49.1% survival of eggs | Forneris et al. (2003) |
| Peracetic acid | 0.1–0.3 mg L−1 | 42%–94% on rainbow trout | Jaafar et al. (2013) |
| Silver zeolite | 600 mg L−1, 0.06% | 100% inhibition in vitro | Johari et al. (2014) |
| Sodium percarbonate | 40–120 mg L−1 | 42%–84% reduced infection on rainbow trout | Jaafar et al. (2013) |
- Abbreviations: MIC, minimum inhibitory concentration; MLC, minimum lethal concentration against strains of S. parasitica.
- aBanned worldwide in 2002 (van West, 2006).
Formalin, an aqueous solution of 37% formaldehyde, is commonly used (Abd El-Gawad et al., 2015) and fairly effective in treating saprolegniosis (Table 1, Das et al., 2012; Mitchell & Collins, 1997). Formalin is currently registered as a disinfectant and/or parasiticide for use in aquaculture, depending on country (Leal et al., 2018). Formalin can be administered by adding formalin directly to fish tank water, including RAS farms (Noga, 2012), usually at concentrations ranging from 20 to 50 mg L−1 (Buchmann, 2022).
Formalin is widely used but is not effective enough to control Saprolegnia infections in fish, fish eggs (Bruno et al., 2011; Okumuş, 2002) and larvae (Gieseker et al., 2006). In some circumstances, formalin has been found to be very effective against Saprolegnia infections (Rach et al., 2005; Schreier et al., 1996). However, there are concerns about its effects on the environment and the handling personnel (Fitzpatrick et al., 1995). It currently has a classification as a 1B carcinogen by the European Commission (EC, 2014), indicating that it may be banned as a treatment option (Abd El-Gawad et al., 2015; Bruno et al., 2011; Liu et al., 2015).
Malachite green and formalin are the most potent anti-oomycete agents although they have acute effects on aquatic ecosystems (Schreier et al., 1996) and are teratogenic (Meyer & Jorgenson, 1983) and immunosuppressive for fish if used repeatedly (Hussein et al., 2002; Prost & Sopinska, 1989). Restrictions in the use of these chemicals have led to a search for an effective new treatment.
Various treatments have been used to control saprolegniosis, such as hydrogen peroxide (Ali et al., 2019; Kitancharoen et al., 1997), formalin (Bly et al., 1996), Bronopol (Pyceze), NaCl, acetic acid, povidone iodine (Fuangsawat et al., 2011), ozone, UV (Rahkonen & Koski, 2002), KMnO4 (Sherif & Abdel-Hakim, 2016), NaOCl (Khomvilai et al., 2005), ClO2 (Prasatporn et al., 2010), chitosan (Min et al., 1994), triclosan (Kumar et al., 2020) and copper fibres (Emara et al., 2020; Miura et al., 2005). They have all shown lower efficiency than malachite green (Bruno et al., 2011; Liu et al., 2015) which was banned in 2002 (due to toxicity, Fernandez et al., 1991). Many include safety concerns, causticity can cause tissue damage (Burka et al., 1997), or their application in large volumes of water is impractical (Branson, 2002; Oono et al., 2008). They can even change the biological properties of water and contaminate the environment (Battaglin & Fairchild, 2002; Özçelik et al., 2020). Additionally, probiotics (Lategan et al., 2004) and biological control agents have been studied, such as Pythium (Hussein & Hatai, 2002) and Aeromonas (Lategan et al., 2004; Sherif & Abdel-Hakim, 2016), but they also have limitations. Vaccination against saprolegniosis has also been proposed, but its application is costly and impractical (Fregeneda-Grandes & Olesen, 2007; Minor et al., 2014).
Bronopol (2-bromo-2-nitropropane-1,3-diol) and its methyl derivative (2-methyl-4-isothiazolin-3-one) oxidize thiol groups of protein and inhibit dehydrogenases. They have been considered promising for the control of Saprolegnia spp. (Oono & Hatai, 2007). However, some of the Saprolegnia strains are tolerant for bronopol (Rezinciuc et al., 2014).
Hydrogen peroxide, sodium percarbonate and peracetic acid are used for bathing infected fish and kill various parasites (Bruzio & Buchmann, 2010; Meinelt et al., 2009). Hydrogen peroxide appears to be a promising chemical for the treatment of Saprolegnia spp. (Fitzpatrick et al., 1995; Marking et al., 1994) with minimal impact on the environment (Mitchell & Collins, 1997). For example, it has been useful in treating infections in catfish (Howe et al., 1999). However, it can induce adverse side effects such as reduced fish growth and damage to the gills (Mustafa, 2019), and the species, life stage and water temperature should be considered when treating Saprolegnia spp. with hydrogen peroxide (Rach et al., 1997). Hydrogen peroxide appears an effective anti-oomycete agent in trout eggs (Lovetro, 1998; Rach et al., 1997); but in many cases, it is accepted only as a disinfectant.
KMnO4 has been tested and found to be an effective anti-oomycete agent in eggs of several species. Zahran and Risha (2013) reported that KMnO4 had a beneficial effect against saprolegniosis and showed a protective role against oxidative damage in saprolegniosis-infected Nile tilapia. Das et al. (2012) suggested that at the beginning of Saprolegnia infection, fish should be treated in two steps: dip treatment with 4 g salt per litre of water for 2 min, followed by 5 ppm KMnO4 for 10 min, resulting in faster recovery.
Peracetic acid has proven activity against S. parasitica but it causes lacrimation and irritation to the upper respiratory tract in humans (Marchand et al., 2012). Boric acid inhibits the germination and colonization of spores and mycelial growth (Ali et al., 2014) but excess amounts affect DNA integrity and reduce fish growth (Öz et al., 2018). Among the current treatments, the least harmful is NaCl, but it is effective only in high concentrations which prevents its use in freshwater systems (Das et al., 2012).
The tolerance of Saprolegnia spp. to high salinity has thus far been inadequately studied (Ali, 2005). Salt dips release osmotic stress in fish, affecting water mould infection (Straus et al., 2009; Treves-Brown, 2000). In previous studies, seawater at 29 g L−1 and salt water (NaCl solution) at 15 g L−1 were lethal to Saprolegnia spp. (Marking et al., 1994; Pickering, 1994) and effective for controlling saprolegniosis (Willoughby, 1994).
Ali (2005) reported that the vegetative growth of S. parasitica decreased as the concentration of NaCl increased and the vegetative hyphae were branched and crippled at 0.07 and 0.10 M. The synergistic action of salinity and ascorbic acid significantly enhanced sporangial production and to some extent, sporangial release at various concentrations compared to salinity alone. Only the two highest concentrations of NaCl supplied with 5.68 mM ascorbic acid inhibited sporangial formation (Ali, 2005). The different concentrations of NaCl and ascorbic acid significantly promoted the cellulolytic activity of S. parasitica at low and moderate concentrations but inhibited it at high concentrations. The growth of S. parasitica declined as concentrations of NaCl and ascorbic acid increased until 0.14 M.
Ascorbic acid affected both sporangia formation and release to a lesser extent than NaCl, which was gradually inhibited by increasing the salt level up to 3.41 mM (Ali, 2005). Low concentrations of ascorbic acid had no effect on the formation of reproductive organs, whereas moderate concentration affected their number, and morphogenesis and high levels completely inhibited their formation (Ali, 2005). These findings are promising for finding an efficient and non-toxic treatment.
Ozone is considered relatively safe and poses no risk to the environment due to its short half-life. The complete elimination of certain bacteria (Aeromonas salmonicida, Vibrio anguillarum, Vibrio salmonicida and Yersinia ruckeri) has been observed (Liltved et al., 1995). The use of ozone requires particular care in adult fish, and it leads to death, even at low concentrations (Forneris et al., 2003). Forneris et al. (2003) studied the effects of O3 at 0.01; 0.03; and 0.2 mg L−1 for 10 min daily and compared them with similar formaldehyde treatments to treat fish eggs. They reported similar efficiency with both compounds, but the 0.3 mg O3 L−1 dose was above the toxicity threshold.
Many studies have been performed on various inorganic substances and heavy metals (Jung et al., 1999; Yeo et al., 1995) which have wide antibacterial properties (Cho et al., 2005). For example, silver, copper, zinc and other metals bound to inorganic carriers are more efficient than inorganic disinfectants. Various silver-containing antibacterial and antifungal materials have recently been developed (Shahverdi et al., 2007; Shokouh Saljoghi et al., 2020; Wang et al., 2007; Xu et al., 2011), and some are in commercial use. Silver has a wide antibacterial spectrum, and it is relatively safe to use (Cho et al., 2005). For example, Shokouh Saljoghi et al. (2020) used nano-silver zeolite (SZ) and bentonite on fish pathogens and in in vitro experiments and studied their effect on Saprolegnia spp. They concluded that nano-SZ and bentonite had antifungal activities and potential candidates for indirect use in aquaculture. Additionally, SZ and silver nanoparticles have been found efficient against Saprolegnia spp. at 600 mg L−1 concentration (Johari et al., 2014) and even to increase the hatchability of eggs and larval survival (Soltani et al., 2010).
Silver, copper, zinc and other metals can be bound to inorganic carriers for slow release and act better as disinfectants compared to conventional organic options (Bailey, 1983; Johari et al., 2014). Inorganic carriers include zeolite, apatite, phosphates, titanium dioxides and glass. Zeolite is a porous material of hydrated sodium aluminosilicate, showing a strong affinity with silver ions, and it can electrostatically bind up to 40% (w/w) (Uchida, 1995). SZ has been the subject of studies for medicine and the hygiene industry (Morishita et al., 1998; Nikawa et al., 1997).
Silver ions affect DNA molecules, causing a loss of the replication abilities of DNA, and interact with thiol groups in protein, inactivating the bacterial proteins. For example, Feng et al. (2000) investigated the inhibition mechanism of silver ions on microorganisms. Johari et al. (2014) studied the antifungal activity of SZ and determined the minimum inhibitory concentrations. They used powdered SZ in glucose yeast extract, and the growth of Saprolegnia spp. on the SZ agar was compared to SZ-free agar. The results showed that SZ inhibited the growth of Saprolegnia spp. The minimum inhibitory concentration for Saprolegnia spp. was at 600 mg L−1. This showed that SZ was a good candidate to replace other toxic antifungal agents. However, the study of Johari et al. (2014) needs to be repeated at lower temperatures (i.e., 10–12°C) to understand the inhibitory effects of SZ and its potential use in systems for cold water fish species.
Mixing approximately 0.06% SZ into the structure of aquaculture equipment seemed to minimize Saprolegnia spp. growth (Johari et al., 2014). It is now necessary to find the best applicable methods to use SZ in aquaculture systems, such as fishponds, hatcheries and aquariums. For example, SZ can be incorporated into the filter media used in the water filtration systems of recirculation systems and hatcheries to control bacterial and fungal diseases transmitted and spread through water (Johari et al., 2014). On the other hand, SZ can be included in the polymeric structures of aquaculture systems such as fibreglass or polyethylene troughs, trays, culture tanks and other rearing instruments as an antimicrobial and antifouling agent (Johari et al., 2014).
Copper nanoparticles are used in many applications in agriculture, livestock, medicine and many other areas (Borkow & Gabbay, 2009; Kalatehjari et al., 2015). Copper and copper-based compounds are known to remove a wide range of yeasts and fungi (Belli et al., 2006; Zatcoff et al., 2008), but their antifungal properties depend on concentration (Cioffi et al., 2004).
Kalatehjari et al. (2015) studied if copper nanoparticles could act against S. parasitica. They measured the minimum lethal concentration of copper nanoparticles on Saprolegnia sp. in yeast extract glucose chloramphenicol agar. They observed that copper nanoparticles from 10 ppm had antifungal effects on Saprolegnia sp. The antifungal effects of copper nanoparticles were positively correlated with both concentration and time of exposure.
Certain antibiotics have been studied to treat Red Skin Disease and Saprolegnia infections (Toranzo et al., 2005; Xi et al., 2019). For example, oxytetracycline and sulfadiazine have been administered to bighead carp during breeding (He et al., 2016; Toranzo et al., 2005; Xi et al., 2019). Antibiotics were already tested in 1978, when Oláh and Farkas (1978) studied the effects of 33 antibiotics against Saprolegnia spp. and Achlya spp. They observed significant inhibiting effects only for nalidixic acid against Saprolegnia spp. More recently, pentacyclic indolosesquiterpenes (Oridamycins A and B) were studied and showed antiparasitic activity against S. parasitica at 3.0 μg mL−1 (Takada et al., 2010). Additionally, two angucycline compounds related to saquayamycin antibiotics were found effective (Saprolmycin A 3.9 and E 7.8 ng mL−1) as anti-saprolegniosis candidates (Nakagawa et al., 2012). However, a suitable antibiotic against saprolegniosis is not commercially available.
4.3 Plant-based treatments
Plant extracts are known to play an essential role in several biological activities. Several plant extracts can improve fish growth and immunity or cure diseases. Certain plants and plant extracts contain significant amounts of antifungal and antibacterial compounds with medicinal properties (Milutinović et al., 2021; Shah et al., 2021). For example, pomegranate extracts, clove and Thymus linearis have been proposed as potential treatments for saprolegniosis (Shah et al., 2021).
Various herbs have been utilized in their crude or extracted forms to stimulate fish immune systems (Table 2). These include onion (Allium cepa) and garlic (Allium sativum), which have been used medicinally since ancient times (Núñez-Torres et al., 2022; Özçelik et al., 2020) and contain various bioactive compounds such as organosulfurs, flavonols, ascorbic acids and carbohydrate prebiotics. These compounds have anti-inflammatory, antimicrobial, antioxidative, antistress, anticancer and immunomodulatory effects (Sagar et al., 2022). Furthermore, the phenolic compounds and fatty acids of plant extracts have shown antimicrobial activities (Maftuch Kurniawati et al., 2016; Puupponen-Pimiä et al., 2001; Stanković et al., 2012).
| Treatment | Concentration | Effects for fish/inhibition | Reference |
|---|---|---|---|
| Essential oils of Eryngium campestre, Mentha piperita, Cuminum cyminum |
0.5 μg mL−1 for Cuminum cyminum 1 μg mL−1 for Mentha piperita and Eryngium campestre |
Zone of inhibition C. cyminum 27.4 mm; M. piperita 22.6 mm; E. campestre 24.8 mm | Adel et al. (2020) |
| Extracts of Cyperus esculentus and Carthamus tinctorius | 0.1 mg L−1 | >80% and 70% inhibition against Saprolegnia parasitica | Emara et al. (2020) |
| Solution of catappa leaf (Terminalia catappa) | 500 mg L−1 | 59% survival rate of juvenile gourami (Osphronemus goramy) infected with Saprolegnia sp. | Grandiosa et al. (2022) |
| Thymoquinone Nigella sativa oil | 4 μg mL−1 in 160 μg mL−1 for 30 min | Zoospores of S. parasitica could not germinate | Hussein et al. (2002) |
| Extract of garlic Allium sativum | 500 mg L−1 20 min d−1 | Absence of fungal structures of cotton specks 39% and depigmentation 33.5% | Núñez-Torres et al. (2022) |
| Pennyroyal + Myrtus communis, Thymus daenensis essence oils | 5 μL mL−1 + 10 μL mL−1 | The best antifungal effect for rainbow trout (Oncorhynchus mykiss) eggs against S. parasitica | Salehi et al. (2015) |
| Ethanolic extract of Thymus linearis |
0.32 mg mL−1 5.12 mg mL−1 |
Hyphal growth inhibition 54.5% ± 0.9% 100% inhibition against S. parasitica |
Shah et al. (2021) |
Various organophosphates have been tested in aquaculture. Concentrations as low as below 1 mg L−1 are known to limit infections (Kabata, 1985). Even pyrethroids have been used as a parasiticide (Kabata, 1985). They are extracts of the plant Chrysanthemum. However, pyrethroids are toxic to fish, and their application requires caution (Bakke et al., 2018).
The essential oils of Eryngium campestre, Mentha piperita and Cuminum cyminum have shown antifungal activity against S. parasitica (Adel et al., 2020). The major constituents in the studied essential oils were bornyl acetate (17.9%) in E. campestre, menthol (48.5%) in M. piperita and α-pinene (29.1%) in C. cyminum. Adel et al. (2020) reported minimum fungicidal concentrations of 0.5 μg mL−1 for C. cyminum and 1 μg mL−1 for M. piperita and E. campestre. The results suggest that the essential oils may be potential plant-based antifungal components against S. parasitica.
In previous years, anti-oomycetes activities of some plant extracts Ceramium rubrum, Magnolia officinalis and Zingiber officinale have been observed (Caruana et al., 2012; Huang et al., 2015). The medicinal properties of the genus Thymus L. have long been known (Chraibi et al., 2016; Javed et al., 2013). For example, T. linearis has antibacterial (Gilani et al., 2010), anti-cancer (Hussain et al., 2012) and anti-viral properties (Hafidh et al., 2009). Shah et al. (2021) assessed a plant extract of T. linearis to evaluate anti-oomycetes activity against S. parasitica. T. linearis extract was able to reduce hyphal growth by 54.45% at 0.32 mg mL−1 and 100% at 5.12 mg mL−1, leading to a complete reduction of zoospore production.
Emara et al. (2020) studied 59 plants or plant parts and screened for possible antifungal properties against S. parasitica. They found eight plant extracts in which Cyperus esculentus induced the highest inhibition rate. C. esculentus and Carthamus tinctorius extracts were the most effective at a concentration of 0.1 mg L−1. Additionally, methanolic extracts of C. esculentus, Chenopodium murale plant, C. tinctorius flowers, Ipomoea batatas leaves, Juniperus phoenicea fruits, Luffa aegyptiaca leaves and fruit rend, and Ricinus communis leaves showed high inhibitory action against Saprolegnia spp. at low concentrations. They should be studied in more detail to assess their potential for aquaculture applications against saprolegniosis.
Catappa leaf plant (Terminalia catappa) has antifungal properties (Caruso et al., 2013). Previously, catappa leaves have been studied, focusing on fish infected with Aeromonas hydrophila bacteria or treating Saprolegnia sp. on fish eggs (Yakubu et al., 2020). Later, Grandiosa et al. (2022) studied if catappa leaf solution was suitable in treating juvenile gourami infected with Saprolegnia sp. They used doses of 500, 1000, 1500 and 2000 mg L−1 to prevent any harmful side-effects or toxicity. They observed that the growth of Saprolegnia sp. was reduced (Grandiosa et al., 2022), and the highest survival rate of juvenile gourami was found in treatment with 500 ppm (59%). This is presumably due to the phytochemicals in catappa leaf, which function as antimicrobials. Catappa leaf contains flavonoids, saponins, triterpenes, diterpenes, phenolic compounds and tannins (Pauly, 2001). However, although the treatment increased the survival of gourami, catappa leaf treatment could not fully remove infections of Saprolegnia.
Thus far, suitable compounds have been sought among plant extracts with fungicidal properties (Rai et al., 2002), and essential oils (Madhuri et al., 2012; Tampieri et al., 2003). Salehi et al. (2015) demonstrated that Eucalyptus globulus, Thymus daenensis and forty other essential oils were effective in suppressing the fungal growth of S. parasitica. The best results were observed with T. daenensis and Myrtus communis essence oils in the presence of pennyroyal essence oil.
Mostafa et al. (2020) studied the antifungal potency of the plant extracts of Nigella sativa, Punica granatum, Thymus vulgaris and Z. officinale against saprolegniosis. Their results showed that two plant extracts provided a significant inhibition of mycelial growth of the pathogenic Saprolegnia diclina isolate. P. granatum extract showed a potential suppressive effect on the growth parameter of S. diclina at a concentration of 0.5 mg mL−1, followed by extract of T. vulgaris, whereas the other plant extracts had no suppressive potency at the same concentration.
N. sativa (black cumin) is an herbaceous plant which grows annually in countries near the Mediterranean Sea. Its seeds have traditionally been used for medicinal purposes. Hussein et al. (2002) studied the antimycotic activity of thymoquinone (2-isopropyl-5-methyl-1, 4-benzoquinone), a major carbonaceous component of N. sativa oil against water mould. N. sativa oil had a fungicidal effect and inhibited the growth of water mould at a concentration of 160 μg mL−1. The zoospores of S. parasitica could not germinate after exposure to 4 μg mL−1 thymoquinone for 30 min (Hussein et al., 2002). However, thymoquinone is toxic to salmonids and cyprinids, suggesting that its toxicity against other fish species should be tested.
5 CONCLUSIONS
The factors influencing Saprolegnia infections in the environment and on fish farms are still largely uncertain. Stressors such as malnutrition, crowding, stress, temperature shocks, or unsuitable water temperature, spawning, poor water quality, injuries from handling and high fish density have been associated with outbreaks. For example, water temperature and changes of temperature, a high organic load, nitrogen compounds, salinity, conductivity, the content of Ca2+ ions and microorganisms seem to be connected with saprolegniosis occurrences. However, it is unclear why some farms have frequent outbreaks, whereas others remain disease-free. In the future, connections among different water quality parameters with saprolegniosis occurrences need to be systematically and scientifically studied. For fish farmers, this could act as warning signs for saprolegniosis and as a signal for starting a treatment.
For long, malachite green was the most effective treatment against saprolegniosis. It was banned in edible fish world-wide in 2002 due to its carcinogenic and toxic effects. This has forced farmers to use alternative disinfection methods against Saprolegnia spp. Many compounds have since been tested and studied, but a suitable and safe compound to treat saprolegniosis remains to be found. Formalin, an aqueous solution of 37% formaldehyde, is widely used, but it is not efficient enough to sufficiently control fungal infections in fish and eggs. Other treatments have been used to control saprolegniosis, such as Bronopol (Pyceze), salt (NaCl), acetic acid, povidone iodine, ozone, hydrogen peroxide, UV, potassium permanganate, sodium hypochlorite and chlorine dioxide, to name only a few. Many include safety concerns, their causticity can cause tissue damage to reared species, or their application in large volumes of water is impractical. It is of high importance to find an effective and non-toxic treatment agent, and research efforts should be made to achieve this goal.
Certain plants and plant extracts contain significant amounts of antifungal and antibacterial compounds. Various plant-based compounds, such as phenolic compounds, fatty acids, organosulphates and pyrethroids, have been tested in aquaculture against Saprolegnia spp. and other fungal diseases. For example, these can be found in onion (A. cepa), garlic (A. sativum), extracts of Chrysanthemum, C. rubrum, M. officianalis and Z. officianalis, and essential oils of E. campestre, M. piperita, C. cyminum and T. linearis. However, more research is required before a suitable compound and method for large-scale rearing conditions are established.
In aquaculture, there is an urgent need for a new and effective treatment agent without harmful side-effects to the reared species, environment or farming personnel. It is possible that a suitable treatment can even be found among the plant-based compounds. Several compounds and combinations have been tested, but further studies are still required to attain this goal. Before an effective treatment is available in commercial use, better understanding regarding the affecting factors is required to prevent occurrences of saprolegniosis.
AUTHOR CONTRIBUTIONS
Petra Camilla Lindholm-Lehto: Conceptualization; data curation; project administration; writing—original draft. Päivi Pylkkö: Funding acquisition; writing—review and editing.
ACKNOWLEDGEMENTS
Financial support provided by Natural Resources Institute Finland (Luke) is gratefully acknowledged.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
FUNDING INFORMATION
Natural Resources Institute Finland (Luke)
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




