Human Skin Equivalents Demonstrate Need for Neuro-Immuno-Cutaneous System
Abstract
A variety of human skin equivalents (HSEs) has been designed for clinical use or for exploratory skin research. In vitro HSE models have been used to target relationships between the skin and nervous or immune systems but have not yet considered the neuro-immuno-cutaneous (NIC) system. In this study, HSEs are described, with and without neural and immune components, to discern these types of effects. These systems are composed of only primary human cells and contain an epidermis, dermis, hypodermis (with immune cells), and human induced neural stem cells for the neuronal component. RNA sequencing is utilized to confirm differences between sample groups and to identify unique or important genes with respect to sample type. Only samples with both neural and immune components result in the upregulation of genes in all the key biological pathways explored. The analysis of protein secretion confirms that this group has measurable functions related to all key cell types. Overall, this novel skin tissue system confirms that designing HSEs that include the NIC system results in a tissue model that reflects key functions. These systems could be used to identify selected targets of interest in skin research related to healthy or diseased states.
1 Introduction
Human skin equivalents (HSEs) have undergone significant improvement in recent years, with innovations to the design, complexity, stability, and applications of these tissue models.1-7 The human skin is a complex organ composed of numerous cell types and responsible for discrete and connected pathway interactions, such as the neurological systems and immune system.8-15
There is interest in the neurological and immune components in skin, but most studies only focus on one system at a time, not both.1, 4, 16 The in vitro HSE system described herein is a multilayered, full thickness skin model, containing only primary human cells, including human induced neural stem cells (hiNSCs), and tissue-inherent immune cells from lipoaspirate utilized in the hypodermis.6 In order to compare the effect of the tissue components (i.e., hiNSCs, hypodermis, and the dermis/epidermis), a parametric study was performed of the system in vitro. Through RNA Sequencing (RNASeq) and protein analysis it was determined that each sample group (dermis/epidermis alone, dermis/epidermis + hiNSCs, etc.) was distinct in terms of functions, gene expression profiles, and enriched biological pathways. Each sample group expressed genes related to several phenomena key to skin biology, including skin development, neuronal system development, inflammation, and immune system process.
Through RNASeq, numerous genes were up- or downregulated in a distinct manner with respect to the specific HSE study group (i.e., each HSE group had distinct gene expression) that would not have been possible to identify as quickly or thoroughly by other techniques such as real-time reverse transcriptase polymerase chain reaction (qRT-PCR) or microarrays due to lower sensitivity and throughput.17, 18 Further, RNASeq allowed the identification of genes which may be important for development of an in vitro HSE model, and which were different with the study groups that contained neural or immune components. In comparison to the RNASeq results from less complex skin models (such as a keratinocyte model), the tissue models described herein identified more genes, likely due to the addition of important cell types of the skin including adipose, neural, and immune components. We speculate that such additional complexity and readouts could be useful for studies related to in vitro studies, such as insight into diseases like psoriasis.19
The skin is a highly organized and complex organ that maintains homeostasis. The neuro-immuno-cutaneous system refers to one of the interconnected systems of the skin, in which neural, immune, and dermal cells, including neuropeptides and cytokines, are in bidirectional communication with one another.11-13, 20 This system is key to many functions of the skin, including the response to stimuli and sensation, yet to our knowledge there has been no in vitro human model that combines these systems (immune, neural) for the study of human skin in vitro or in vivo.
In our prior study, we showed that a biomaterial for HSE dermis, silk-collagen protein composite hydrogel, was stable for long-term experimentation, avoiding collagen contraction and degradation issues that can result in collagen-only HSEs.6 Pro-inflammatory cytokine secretion was observed at higher levels from HSEs when a hypodermis was present, and these tissue models also expressed more proteins (associated with inflammation, cell adhesion, motility, and extracellular matrix formation).6 In the current study, a focus was on select proteins of interest related to the cell types present in the skin tissue model, including keratinocytes, fibroblasts, hiNSCs, adipocytes, and immune cells. The immune cells included macrophages or monocytes, to discern cell-specific contributions to the HSEs, including neuro-immuno-cutaneous components.
The objective of this work was to develop HSEs which could be used to selectively understand the neuro-immuno-cutaneous system. Through use of only primary human cells, this model tissue system can be used as an in vitro tool for skin research. The goal of this work was to define, via each key skin layer or component (e.g., neural cells), the relative contributions of corresponding genes, proteins, or pathways contributed by that layer. Therefore, by comparing HSE models with and without specific layers, important functional markers could be selected for future research questions. To accomplish this, a combination of imaging, protein secretion analysis, and RNASeq was performed on four different HSE types, with and without neural or hypodermal/immune components, to investigate the effect of the neuro-immuno-cutaneous system on HSE functions.
2 Results
2.1 System Design, Timing, and Sample Collection
Model construction methods are described in Cell Culture Conditions, Adipose Surgery, and Tissue Model Construction sections. The most complex HSE, SC3, contains all model components: hypodermis, dermis, epidermis, and a neuronal component using only primary, human cells or tissue (Figure 1). To assemble the model, first the hypodermis is created (described in Adipose Surgery section). We have previously defined that this lipoaspirate contains many cell populations, including adipocytes, pre-adipocytes, endothelial cells, smooth muscle pericytes, and immune cells (likely macrophages/monocytes).6, 21 Next, a silk-collagen protein composite hydrogel was used as the dermal material, containing primary human fibroblasts. The silk-collagen hydrogel is resistant to degradation and contraction, and combines the Arg-Gly-Asp (RGD) domains of collagen with the biocompatibility and tunability of silk.6, 22-24 Primary human keratinocytes are then seeded on the dermal gel and allowed to epithelialize for one week prior to the experiment start date. On the experiment start date, the entire model is constructed (as described in Tissue Model Construction section), with the hiNSC-containing collagen gel coating the hypodermis, which is placed underneath the dermal/epidermal gels, raised to air–liquid interface (ALI), and maintained over the experiment duration. Samples were collected for protein analysis (cell culture supernatant, Cell-Specific Assays section) at D1, D5, D7; samples for imaging were fixed (Histology and Immunohistochemistry section) at D1 and D7; samples for RNASeq were collected (RNA Extraction section) at D1 and D7.
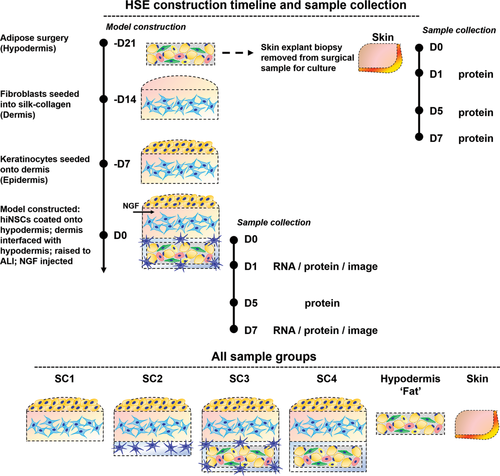
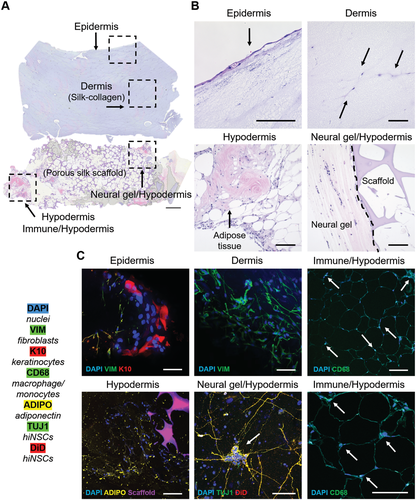
2.2 Histological and Immunohistological Imaging
Of the four HSE models constructed, only one study group contains all four cell/tissue components (epidermis, dermis, hypodermis, neural): SC3. All layers of SC3 are presented with hematoxylin and eosin (H&E) (Figure 2A,B) or histology and immunohistochemistry (IHC) staining (Figure 2C). IHC was performed to identify key cell type(s) of each layer. The epidermis was characterized by K10, a keratinocyte marker. The dermis was characterized with vimentin for fibroblasts. The hypodermis was characterized in four ways: to identify adipose tissue morphology (Figure 2B), adiponectin production by adipocytes in the hypodermis (Figure 2C), inherent-immune cells from the adipose tissue by CD68 staining (for macrophages/monocytes), and to visualize hiNSCs which surrounded the hypodermis with TUJ1 and Vybrant DiD Cell-Labeling Solution (DiD) staining.
2.3 Cell-Specific Protein Secretion
To elucidate cell-specific effects with respect to sample group, key markers were selected for further analyses which are related to specific cell functions (Table 1). Proteins produced by keratinocytes, fibroblasts, neurons, adipocytes/adipose tissue, and immune cells (macrophages, monocytes), or a combination thereof, were selected.
| Cell type(s) of interest | Cell-specific marker | Marker abbreviation | Description | References |
|---|---|---|---|---|
| Keratinocyte | Thymic stromal lymphopoietin | TSLP | Activated keratinocytes in response to trauma, infection, allergens; key in immune response | 25-27 |
| Fibroblast | Fibroblast activation protein | FAP | Activated fibroblasts, may have role in ECM remodeling/wound healing | 28 |
| Neural stem cells, keratinocytes, fibroblasts | Nerve growth factor | NGF | May mediate cutaneous re-innervation; released in high concentrations during inflammation | 29-32 |
| Adipocytes | Glycerol | Glycerol | Glycerol related to fat metabolism activity | 33 |
| Macrophages, keratinocytes, fibroblasts | Granulocyte macrophage colony-stimulating factor | GM-CSF | GMCSF can be heightened in response to inflammation. | 34 |
| Macrophages, keratinocytes, fibroblasts | Macrophage colony-stimulating factor | MCSF1 | MCSF detectable in steady state needed for homeostasis | 35-39 |
Differences in protein secretion were observed within and between groups (Figure 3A–F, Data Availability contains “between groups” significance information). TSLP secretion was highest from the skin explant and the fat (in vitro hypodermis). TSLP secretion from HSE groups was lower than the skin explant and fat, and was similar with time across all HSEs (Figure 3A). TSLP, FAP, and glycerol secretion significantly increased with time in the skin explant (Figure 3A,B,D). FAP secretion was highest in the skin explant, but lower and similar with time in the Fat and all HSE study groups (Figure 3B). Nerve growth factor (NGF) secretion was minimal from the skin explants and fat group (Figure 3C). Differences were noted between groups with and without neurons added (i.e., SC1 vs SC2, SC3), where groups with neurons at D1 secreted significantly more NGF than SC1 (Figure 3C) at all timepoints (P < 0.001). SC4, which does not have neurons added, but does contain hypodermis, had similar secretion levels to SC2 and SC3.
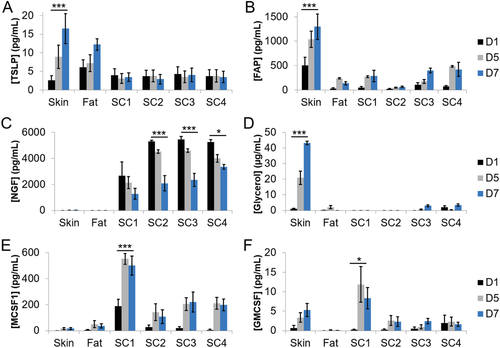
Glycerol secretion was highest in the skin explant, and increased with time, was negligible in SC1 and SC2 (without hypodermis) and was low but detectable in the fat, SC3, and SC4 study groups, which all contain an in vitro hypodermis (Figure 3D). MCSF1 secretion was lowest from the skin explant and fat group but was highest in SC1 and significantly increased with time (Figure 3E). All other HSE groups (SC2, SC3, and SC4) also secreted MCSF1, which increased with time, but SC1 secretion was almost three times higher than all other HSE groups. GMCSF secretion was detected in the skin explant, not in the fat, and was highest in SC1 (increasing with time), and low but stable secretion from all other HSE groups (Figure 3F).
2.4 Multigroup Analysis of Gene Expression via RNA-Seq
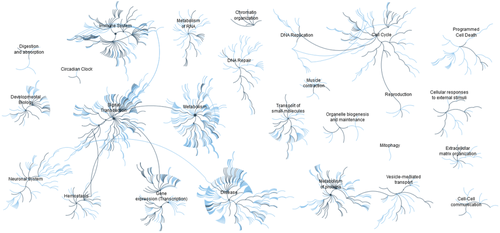
To further characterize the differences between the HSE groups, RNA-Seq was performed on all four study groups. As a visualization tool, Reactome pathway browser was used to view the most relevant genes from the RNA-Seq dataset (q-value, false discovery rate (FDR) = 0.1) (Figure 4). Of note, the immune system, signal transduction, protein metabolism, and gene expression (transcription) were among the most dominant pathways visualized by Reactome for the HSE systems overall.
Through Gene Set Enrichment Analysis (GSEA), specific pathways and the related genes were identified through application of the hallmark gene set with respect to HSE sample type (Figure 5A).40, 41 The complement pathway was the most upregulated in SC4, then SC3, and downregulated in SC2 and SC1 (both without hypodermis). Similar trends were observed for coagulation, glycolysis, adipogenesis, epithelial–mesenchymal transition, and apoptosis pathways, which overall were upregulated in SC3 and SC4 but downregulated in SC1 and SC2.
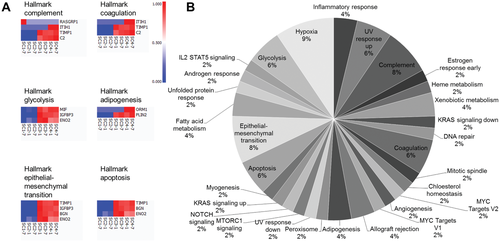
From the entire dataset at q = 0.1, all hallmark pathways with respect to number of matches per pathway were presented (Figure 5B). Hypoxia, complement pathway, and the epithelial–mesenchymal transition had the highest number of matches via this analysis tool. Other key pathways included the inflammatory response, angiogenesis, and Notch, KRAS, Interleukin-2 (IL2)/Signal transducer and activator of transcription 5 (STAT5), and mammalian target of rapamycin complex 1 (MTORC1) signaling.
GO gene sets of specific interest to the HSE systems (hallmark adipogenesis, immune system process, inflammatory response, nervous system development, and sensory perception) were applied to the dataset at q = 0.1 (Figure 6A). Using this approach, the HSE groups and their key processes could be visualized. For example, SC3 and SC4 (both containing hypodermis) upregulate several adipogenesis-specific genes Enoyl-CoA Hydratase, Short Chain 1 (ECHS1), Perilipin 2 (PLIN2), and Adiponectin, C1Q And Collagen Domain Containing (ADIPOQ), whereas these genes are downregulated in SC1 and SC2 (both without hypodermis). SC1, which contains only keratinocytes and fibroblasts, highly upregulates several genes related to skin development Hornerin (HRNR), Loricrin (LOR), and Keratin-71 (KRT71) which were downregulated in all other study groups. Other genes related to skin development SRY-Box 18 (SOX18) and Insulin Like Growth Factor Binding Protein 5 (IGFBP5) were upregulated in SC3 and SC4 but downregulated in SC1 and SC2. Certain genes related to sensory perception Myosin IIIA (MYO3A), ATP Binding Cassette Subfamily A Member 4 (ABCA4), and Myosin VIIA (MYO7A) were only upregulated in SC1. Two genes related to immune system processes and inflammatory responses were upregulated in SC3 and SC4, but downregulated in SC1 and SC2 (BCL2 Interacting Protein 3 (BNIP3) and Complement-2 (C2)), and Orosomucoid 1 (ORM1) and Forkhead Box P3 (FOXP3) were upregulated only in SC4. SC1 upregulated three genes related to immune system processes and inflammatory responses: Apolipoprotein A4 (APOA4), Growth Hormone Secretagogue Receptor (GHSR), and Interleukin 31 Receptor A (IL31RA). The only gene for nervous system development upregulated in both SC2 and SC3 (both groups contain hiNSCs) was SRY-Box 11 (SOX11). SC3 also upregulated Hes Related Family BHLH Transcription Factor with YRPW Motif-Like (HEYL) and Contactin 4 (CNTN4), downregulated Cholinergic Receptor Muscarinic 2 (CHRM2) (which SC1 and SC2 upregulated), and downregulated Protocadherin Alpha 5 (PCDHA5) and Nuclear Receptor Subfamily 2 Group E Member 1 (NR2E1) which only SC1 upregulated.
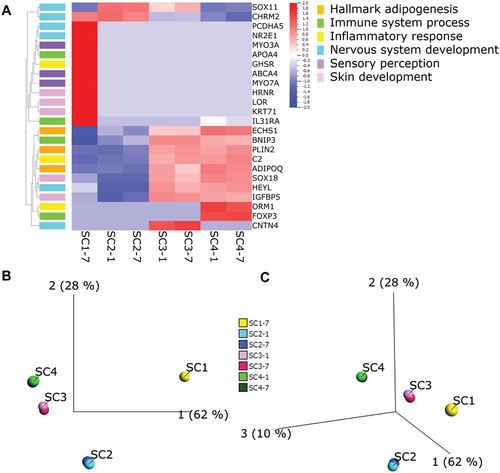
With the GO sets applied, principal component analysis (PCA) confirms in 2D (Figure 6B) and 3D (Figure 6C) that all groups were distinct from one another. SC3 and SC4 are the most similar groups in this instance, and SC1 is the least similar from all other groups. SC3 and SC2 are more similar than SC2 and SC4. SC1 is more related to SC2 than any other group. Time points for all samples aligned with their respective group (Figure 6B,C).
2.5 Two-Group Analysis of Gene Expression via RNA-Seq
Two-group analysis was performed to identify key genes of interest with respect to sample group (Figure 7A). SC1 had 222 genes uniquely up/downregulated, SC2 had 31, and both SC3 and SC4 had 23 genes of interest total. Selected genes from each sample group were selected for further analysis (Figure 7B), which are defined with further detail in Table 2: Bet1 Golgi Vesicular Membrane Trafficking Protein Like (BET1L), Contactin 4 (CNTN4), Chemokine (C-X-C Motif) Receptor 4 (CXCR4), Fibroblast Growth Factor-19 (FGF19), Keratin-71 (KRT71), Macrophage Stimulating 1 Like (MST1P9), Orosomucoid 1 (ORM1), and Sp4 Transcription Factor (SP4).

| SC1 | ||||
|---|---|---|---|---|
| Human gene | Abbreviation | Regulation | Function | GO set |
| Chemokine (C-X-C Motif) Receptor 4 | CXCR4 | Down | Chemokine receptor | Inflammatory response |
| Amyloid Beta Precursor Protein Binding Family A Member 2 | APBA2 | Down | Neuronal adapter protein | Nervous system development |
| Mucin 21, cell surface associated | MUC21 | Up | Protective mucous barriers/epithelial | |
| Mucin 13, cell surface associated | MUC13 | Up | Epithelial mucin | |
| Keratin 71 | KRT71 | Up | Epithelial keratins | Skin development |
| Myosin IIIA | MYO3A | Up | Actin-dependent motor protein | Sensory perception |
| Interleukin 31 Receptor A | IL31RA | Up | Cytokine receptor | Immune system process |
| SC2 | ||||
| Bet1 Golgi Vesicular Membrane Trafficking Protein Like | BET1L | Down | Golgi vesicular membrane trafficking | |
| Solute Carrier Family 16 Member 13 | SLC16A13 | Down | Monocarboxylate transporter | |
| POU Class 6 Homeobox 2 | POU6F2 | Up | DNA binding transcription factor activity | Sensory perception |
| Fibroblast Growth Factor 19 | FGF19 | Up | Growth factor activity | Nervous system development |
| Cell Adhesion Molecule 1 | CADM1 | Up | Cell–cell adhesion via Ca(2+) | Immune system response |
| Immunity Related GTPase M | IRGM | Up | May regulate autophagy/pro-inflammatory cytokine production | |
| Adhesion G Protein-Coupled Receptor B3 | BAI3 | Up | A p53-target gene; synaptogenesis | |
| SC3 | ||||
| Contactin 4 | CNTN4 | Up | Synaptogenesis | Nervous system development |
| Nitric Oxide Synthase 2 | NOS2 | Up | Toll-like receptor signaling pathway | |
| Macrophage Stimulating 1 Like | MST1P9 | Up | Macrophage stimulating | |
| V-Set And Transmembrane Domain Containing 5 (C11orf90) | VSTM5 | Up | Neuronal morphogenesis; synaptogenesis | |
| Dermatan Sulfate Proteoglycan 3 | EPYC | Up | Fibrillogenesis; proteoglycan family | |
| Histone Cluster 1 H4 Family Member I | HIST1H4I | Up | Histone family | |
| Sulfotransferase Family 1C Member 2 | SULT1C2 | Up | Sulfotransferase family | |
| SC4 | ||||
| Sp4 Transcription Factor | SP4 | Down | Transcription factor | |
| Orosomucoid 1 | ORM1 | Up | Acute-phase reactant | Inflammatory response |
| Forkhead Box P3 | FOXP3 | Up | Transcriptional regulator, associated with immune system homeostasis | Immune system process |
| Guanylate Cyclase 2D, Retinal | GUCY2D | Up | Retina-specific guanylate cyclase | Sensory perception |
| Inter-Alpha-Trypsin Inhibitor Heavy Chain 2 | ITIH2 | Up | Inter-alpha-trypsin inhibitor family; hyaluronan carrier | |
| Leukocyte Immunoglobulin Like Receptor A3 | LILRA3 | Up | Encodes immuno-receptors; soluble receptor for class I histocompatibility complex antigens | |
| Killer Cell Lectin Like Receptor B1 | KLRB1 | Up | Regulates natural killer cells | |
Additional genes identified through two-group comparison are described in further detail in Table 2.
3 Discussion
3.1 Distinctions between HSE Groups
In our previous paper, we discussed the utility of a HSE with neurons and immune system components as a physiologically relevant, yet complex in vitro tissue model system.6 In the present work, the cell culture conditions were modified, and the experimental groups were expanded to better discern the contributions of specific cell types to the skin tissue functions. Of note, keratinocytes, fibroblasts, and hiNSC cell seeding densities were increased to enhance cell–cell communication, and the HSE model size was reduced to compensate for the lower contraction of silk-collagen hydrogels (vs collagen-only hydrogels).6
A parametric system was designed to analyze the HSEs (SC1, SC2, SC3, and SC4) (Figure 1, Table 3). SC1, a keratinocyte and fibroblast model, is most similar to HSEs commonly used today, containing only these cell types, but the biomaterial scaffold is a silk fibroin-collagen hydrogel. Tissue models using only fibroblasts and keratinocytes are used for numerous applications: briefly, as an emerging technique, they can be 3D printed layer-by-layer with precision as reconstructed skin,1-3 can support the study of 3D printing of large-scale objects,4 added to biomaterial substrates as tissue-engineered alternatives to human skin for study of pharmacological, biological, or mechanical processes,5 utilized as a tissue-engineered substitutes to compare suitability versus human skin for permeability applications which necessitates consideration to the complexity of the barrier layer.6-8 The application of these systems extends to many areas of study, and while there have been many promising results to date, they rely on two cell types, and therefore may be omitting key factors relevant to skin equivalent models.
| Sample name | Description | Components |
|---|---|---|
| Skin | Human skin biopsy | Epidermis, dermis, hypodermis |
| Hypodermis (“Fat”) | In vitro lipoaspirate-seeded porous silk scaffold | In vitro hypodermis |
| SC1 | In vitro skin | Epidermis, dermis (keratinocytes, fibroblasts) |
| SC2 | In vitro innervated skin | Epidermis, dermis, hiNSCsa) (SC1 + hiNSCs) |
| SC3 | In vitro innervated, immuno-competent skin | Epidermis, dermis, hypodermis, hiNSCs (SC2 + inherent immune cells from hypodermis) |
| SC4 | In vitro immuno-competent skin |
Epidermis, dermis, hypodermis (SC1 + inherent immune cells from hypodermis) |
- a) hiNSC = human induced neural stem cell.
SC2, which includes hiNSCs in addition to keratinocytes and fibroblasts, is more similar to other innervation tissue models that include neurons.1, 2 Through research of tissue models of this type, keratinocytes were found to play a key role in neural outgrowth, which can be used to understand skin conditions such as eczema.1 Further, neural cells (porcine dorsal root ganglia) influenced epidermal morphology, a key to skin homeostasis. This tissue model could potentially serve as a tool to observe interactions between nerve and skin in normal versus disease states.2 However, there are known interactions between neurons with other cell types, especially immune cells, with the nervous system in skin that are involved in numerous conditions, including psoriasis and atopic dermatitis.14 Tissue models that only include keratinocytes/fibroblasts and neural cells may therefore be lacking important components to understand these diseases.
SC4 is composed of keratinocytes, fibroblasts, and a hypodermis, which is similar to previously reported constructs, however the hypodermis in this study was composed of human adipose-derived stem cells (hASCs).3 The objective of that model was to use the HSE as an in vitro tool to study obesity or diabetes.3 In the current study, exchanging hASCs for lipoaspirate alters the tissue model and may be better suited to study obesity, for example, because the entire adipose tissue is exploited, including the immune component. The silk scaffold with lipoaspirate was used as a patient-specific model.16 The main advantage of using lipoaspirate, as opposed to a single-cell source such as hASCs, is that numerous primary, human cell types are contributed from the lipoaspirate (adipocytes, preadipocytes, endothelial cells, and smooth muscle cells, immune cells including macrophages)17, 18 which results in a more physiologically relevant system than a stem-cell-only model.43 However, the disadvantages include patient variability16-18 and variability in cell-type composition which may differ from patient-to-patient, or even in different samples from the same patient source.43
We previously reported that this system (SC4) had complex secretion of pro-inflammatory cytokines, several of which have been implicated in obesity research.6, 44, 45 However, as SC4 lacked a nervous component it may be missing key factors to serve as an obesity or skin tissue model, as the nervous system has a key role not only in cutaneous inflammation but also in adipose tissue functions.46 For instance, adipocytes are known to secrete pigment epithelium-derived factor, an adipokine with neurotrophic function, at very high amounts, as a potential protein of interest for diabetes and obesity research. However, the link to the nervous system for this protein has not been fully explored.47 Also, it has been reported that sympathetic neurons can influence adipocytes by neuropeptide Y (NPY) secretion, and that neurons are in turn also influenced by adipocyte-derived soluble factor or insulin secretion from adipocytes in coculture.48
SC3 is composed of keratinocytes, fibroblasts, hiNSCs, and a hypodermis to contribute inherent immune cells from the lipoaspirate6, 49 (Figure 2C, and Figure S1, Supporting Information). To our knowledge, no HSE model exists that combines all of these components beyond our initial publication.6 We hypothesize that this model is the most relevant to studying the neuro-immuno-cutaneous system, which is known to be important for skin homeostasis and many key functions of the skin.12, 13 Of all the in vitro tissue models explored in this work, SC3 would be most representative of the cell types and tissue layers of natural human skin, including the epidermis, dermis, hypodermis/immune, and neural components.8
Each HSE group studied (SC1, SC2, SC3, SC4) has potential to contribute useful information to skin research, as each has a focus: skin only (SC1), skin/nerve interactions (SC2), skin/nerve/adipose/immune interactions (SC3), and skin/adipose/immune interactions (SC4), respectively. When directly compared, the differences in functions between the study groups were elucidated based on mRNA and protein analyses. Through the improvements in HSEs presented here, new information and insights into the respective components of the skin (e.g., neural, adipose, and immune) can be gained to better understand the neuro-immuno-cutaneous system in the context of homeostasis, damage, disease, and regeneration.
3.2 Protein Analysis
TSLP is primarily produced by epithelial and dendritic cells, and is understood to be increased during inflammation.25-27 The skin explant secreted the highest amount of TSLP which increased with time. The fat scaffold had higher levels of TSLP compared to the HSEs, and this also increased with time, whereas the HSEs secreted low but stable levels of the protein suggesting that the keratinocytes in the HSEs had some activity but may have been less active than other groups.
FAP, a fibroblast-associated protein,50 is typically expressed by stromal fibroblasts of inflamed tissue when compared to normal tissue.28 The skin explant group had elevated levels of both TSLP and FAP compared to the in vitro HSEs. The FAP level in the HSEs overall increased with time, suggesting that while levels were lower compared to an explant, that the fibroblasts within the HSEs were increasingly active with time.
Glycerol concentration was also highest from the skin explant. Glycerol is known to exist on the skin surface for hydration and barrier properties,51, 52 and is an important molecule for adipose metabolism.33 SC3 and SC4, both with an in vitro hypodermis, had a glycerol concentration equivalent to the fat group (in vitro hypodermis alone), suggesting that these groups can maintain the function of the adipose component. SC1 and SC2, which do not have a hypodermis component, do not secrete glycerol.
NGF can be produced by a variety of skin cells, including keratinocytes,31, 53 fibroblasts,32, 54 and neurons;29 adipocytes can also produce NGF.55 NGF was injected into the subepidermal space of all HSEs to encourage upward migration of hiNSCs. However, from imaging (Figure 2C), hiNSCs remained in the location where they were initially seeded. Directing migration of the hiNSCs should be considered in subsequent tissue models. Despite equal amounts of NGF being injected into the HSEs, there were differences in secretion between groups. The half-life of human recombinant NGF has been reported as ≈45 min or less; we therefore speculate that the NGF was produced by the HSEs, and was not represented by residual NGF following the initial injection.56, 57 Samples SC2 and SC3 both have added hiNSCs, but SC4 does not, however each group has equivalent starting concentrations of NGF. The NGF concentration decreased with time from all HSE groups, with SC4 having the highest remaining concentration by D7, which may be due to contributions from the adipose tissue. SC1, with no hiNSCs or adipose tissue, had the lowest concentration of NGF with time, initially about half that of the other HSE groups. Release of NGF from the skin explant and fat group was negligible. While HSEs were injected with NGF at D0, the differences noted between groups suggested that there was an added contribution from both hiNSCs and the adipose tissue, but only when interfaced with in vitro skin tissue.
Proliferating fibroblasts can produce MCSF1,36 which could be the main reason that SC1, composed of only fibroblasts and keratinocytes, had the highest MCSF1 concentration of all sample groups, and especially for HSE groups. Since SC1 has only two cell types and layers, compared to up to four tissue layers with numerous cell types (from the adipose tissue), the fibroblasts in SC1's dermal layer may be more proliferative, and in turn, secrete more MCSF1.
Keratinocytes are known to produce GM-CSF, which is multifunctional and known to be involved in dendritic cell formation, inflammation, and production/differentiation of immune cells including macrophages and neutrophils.58-62 Fibroblasts can also produce GM-CSF.34 SC1 had the highest concentration of GM-CSF, which may be an effect from the fibroblasts and keratinocytes combined. SC1 having the highest signal could also be a result of lack of signaling from other skin cell types that are present in the other HSEs which may be regulating their GM-CSF levels.
Overall, from all proteins studied with respect to the HSEs, all groups functioned as expected (e.g., all HSEs expressed similar levels of TSLP and FAP, SC1 had the lowest NGF level, samples without hypodermis had negligible glycerol levels) except for SC1, which had elevated levels of GM-CSF and MCSF1, unlike any other HSE study group. As previously stated, many of the proteins explored can be secreted by multiple cell types of the skin utilized in this study. This approach has limitations that emerging experimental techniques (such as combinations of proteomics/genomics readouts from single cells) are attempting to address by providing fully integrated information on cell populations.63, 64 Further, it is possible that differences between sample groups could be due to cell count or tissue density, especially in the case of the human explant compared to HSEs. This presents a limitation in direct comparison to human explants and to the understanding of whether the HSE tissues were activated (in the case of MCSF1, for example) or not. However, we speculate that these HSE tissue models could be a very useful system with which to study inflammation. For example, the high values of MCSF1 from SC1 (the model most similar to many commercial skin models), when compared to the other study groups, could identify potential overexpression of inflammatory signals from simpler HSEs versus more complex ones since MCSF1 secretion decreases as more cell types and layers are added. As a drug screening tool, these models could provide an upper and lower bound for inflammatory signals from in vitro models which could be translated for downstream applications.
3.3 Multigroup Analysis
Numerous key pathways were first visualized (Figure 4) then identified (Figure 5) of the HSEs, including: adipogenesis, complement, coagulation, glycolysis, epithelial–mesenchymal transition, hypoxia, apoptosis, and UV response. Overall, GSEA pathway analysis identified a split in the HSEs, where SC1/SC2 behaved similarly as did SC3/SC4. The SC3/SC4 group upregulated genes of several of the groups mentioned (adipogenesis, glycolysis, for example) whereas SC1/SC2 downregulated them. The addition of the hypodermis to HSEs appears to greatly influence the pathways activated or deactivated. Without a hypodermis (SC1, SC2), the HSEs downregulated pathways that are known to be important to skin function, such as glycolysis, whereas it was upregulated in the study groups containing hypodermis (SC3, SC4).65
Several biological processes GO sets were selected for their pertinence to skin homeostasis and function (Table 4): hallmark adipogenesis, immune system process, inflammatory response, nervous system development, sensory perception, skin development.13, 15, 55, 62, 66-68 The HSE groups remained distinct both with (Figure 5B,C) and without (Figure S2, Supporting Information) GO sets applied.
| Standard name | GO set | Systematic name |
|---|---|---|
| Hallmark adipogenesis | N/A | M5905 |
| Skin development | N/A | M15889 |
| Immune system process | GO:0006955 | M19817 |
| Inflammatory response | GO:0006954 | M10617 |
| Nervous system development | GO:0007399 | M7312 |
| Sensory perception | GO:0007600 | M14699 |
Only groups with a hypodermis (SC3, SC4) upregulated adipogenesis genes (ECHS1, PLIN2, ADIPOQ), and only SC1 upregulated sensory development genes at q-value (0.1); SC4 upregulated Guanylate Cyclase 2D, Retinal (GUCY2D), a sensory gene, at q-value (0.2) (Figure S3, Supporting Information). However, for all other GO sets studied there was no clear distinction between which group up or downregulated genes belonging to a certain set (i.e., SC2 was not singularly upregulating all nervous system processes). Certain genes, such as LOR (a skin development gene), were upregulated only in SC1, in agreement with research that classifies positive LOR expression as a key developmental marker of healthy skin.19, 69 In comparison to another cultured keratinocyte-only HSE model, SC1 had several overlapping genes (including KRT71, and several similar gene classes between both datasets including C#orf# genes, TRIM#, and LOC# genes), but numerous others which were not mentioned in that analysis.70 It would be difficult to directly compare these models, as many components are different: SC1 is a 3D HSE with a dermis composed of a silk-collagen hydrogel material, and also contains dermal fibroblasts, which could influence the RNASeq results. However, SC1 may be a more accurate representation of skin than the cultured keratinocyte model due to its 3D architecture and inclusion of dermal fibroblasts. Comparisons between the HSE systems (SC1–SC4) with similar models, or commercial models, are presented in Table 5.
| HSE model | Gene expression highlights | GO sets upregulated | Selected similar model | Similarities | Differences | Similar commercial model? |
|---|---|---|---|---|---|---|
| SC1 |
|
|
71 |
Primary keratinocytes and fibroblasts used; Full thickness model |
Use of silk-collagen hydrogel for dermis | T-Skin (Episkin), EpiDermFT (MatTek) |
| SC2 |
|
|
2 |
Contain neural, dermal, epidermal components; Full thickness model with neural and dermal components initially seeded in separate layers |
Porcine dorsal root ganglion cells as neural component (versus hiNSCs); Collagen gel as dermal matrix (vs silk-collagen); Not fully human model |
No |
| SC3 |
|
|
None | No | ||
| SC4 |
|
|
3 | Includes hypodermis component with porous silk scaffold | Utilizes hASCs vs human lipoaspirate | No |
Samples with hiNSCs added, SC2 and SC3, both upregulate SOX11 for nervous system development which regulates neuronal proliferation, axon growth, and promote peripheral nerve regeneration. This result was consistent with previous findings of hiNSCs gene expression.72 The SC3 group also upregulates HEYL and CNTN4. HEYL has been implicated in tumor suppression and the p53 pathway but is also a neuronal differentiation mediator. CNTN4 is an axon-related gene relevant to the development of the nervous system.
SC3 is the most complex group with function in every GO set studied (with exception of sensory development); SC4 similarly has upregulation of every GO set (with exception of sensory development), but SC3 upregulates nervous system development genes (CNTN4, SOX11) that are downregulated in SC4. The other main difference between SC3 and SC4 is FOXP3 (immune) and ORM1 (inflammatory) expression in SC4. We speculate that the addition of hiNSCs could be implicated in why there were these differences between groups. For ORM1, overexpression has been linked to inflammatory skin conditions like atopic dermatitis.73 This could explain why SC3, which has most of the components of human skin, did not overexpress this gene. It could be possible to utilize these HSE systems to investigate ORM1 in “healthy” (perhaps SC3) versus “diseased” (perhaps SC4) human skin.
In terms of the immune and inflammatory response of the HSEs, SC1 and SC4 uniquely upregulated several genes (APOA4, IL31RA, GHSR; FOXP3 and ORM1, respectively). Complement-2 (C2) was downregulated in SC1/SC2 but upregulated in SC3/SC4, likely as a result of the presence of the hypodermis. However, SC3 downregulates FOXP3 (immune) and ORM1 (inflammatory) which were upregulated by SC4, which may be due to either the addition of hiNSCs in SC3 or because of the total interaction of cells/components within SC3.
3.4 Two-Group Analysis
Through two-group analysis, several uniquely expressed genes with respect to sample type were identified. Multiple genes were selected for each sample type to classify groups further. One emerging difference between groups was that SC1 had numerous genes relevant to epithelialization or skin development uniquely expressed. Descriptions of genes and their related functions are in Table 2. The classifications of these genes (e.g., several neuronal system related genes were present in SC2 and SC3 groups), aligned to the relevant groups, but the approach was not exhaustive.
3.5 Future Directions of HSE Assessment
Tissue-engineered systems, including HSEs, are in the age of an information renaissance due to the advancements in genomics and proteomics. RNASeq is uniquely suited to study novel genes and identify pathways that microarrays alone may not; the larger datasets from RNASeq provide a means to identify specific genes and relevant pathways for certain skin diseases such as psoriasis.74, 75 However, standard RNASeq of a tissue sample or model cannot selectively identify gene expression from individual cell types.17 Alternative techniques which potentially can pair differential expression with cell type include single-cell RNASeq, or fluorescent-activated cell sorting (FACS) followed by RNASeq (FACS-Seq). FACS-Seq has been used on human skin cells, by first isolating only certain skin cells of interest and then performing RNASeq with respect to individual cell populations. This methodology allowed for analysis of individual cell contributions of the skin, which solidifies the technique as a powerful tool.17
For future application, the HSEs could be utilized as an in vitro diagnostic to connect specific cell types of the skin with expressed genes and pathways and implicate them for therapeutics, or to simply better understand the full complexity and interconnected nature of cells of the human skin.
3.6 Limitations
A major limitation of the current HSEs presented herein is the epidermal layer, and lack of similarity to native human skin in terms of stratification of epidermis, and accurate differentiation of the keratinocytes. Questions remain on the necessary level of differentiation of the epidermis, and the barrier function, that are needed for an optimal clinical HSE for patient use.9, 76 In native skin, both keratinocytes and fibroblasts maintain the barrier layer of the skin, specifically the stratum corneum of the skin, and when it is broken the wound healing process initiates and transepidermal moisture loss and percutaneous absorption are compromised.10, 11, 77, 78 With age, and as the skin has reduced ability to replace lipids, the barrier function of the skin is compromised.12, 79 Most in vitro HSEs do not recapitulate the barrier function of human skin,13 as they are typically composed only of keratinocytes and fibroblasts. Barrier function may not be a relevant concern since the model does not include blood vessels, hair follicles, or sweat glands of the native skin, and as a result, the barrier function reported would be impaired and not necessarily transferable to native skin.13 Other groups have suggested that including blood vessels and/or providing a method to allow for transfer of immune cells (i.e., microfluidic device or embedded channels in tissue-based HSEs) would facilitate more relevant barrier function results.9, 13 Therefore, another aspect to make this model more relevant to native skin and improve barrier function simultaneously could be to include such devices to mimic functional components of the native skin.
We establish a methodology for a complex in vitro HSE that includes numerous cell types and layers, including components of the neuro-immuno-cutaneous system. This model provides the ability to study complex skin pathologies using only primary, human cells, in an in vitro setting. However, beyond the epidermal layer, there are other considerations. Due to the complexity of the tissue model, optimizing a coculture medium is important, currently, the model uses a skin-specific media formulation. Through additional characterization of the lipoaspirate and the specific immune cell type(s) and ratio of cell populations, a new media composition could be developed to improve other aspects of the tissue model including epidermal morphology. Histological analysis also suggests that the layers peel apart, which is due to the complex construction of the model and different biomaterial interfaces. Improvements on construction and composition could also be considered to develop a model that is structurally cohesive. Through improvements the tissue model could become useful as a complete in vitro model for interpretation of the skin.
4 Conclusions
To conclude, we have developed a system of silk-collagen-based HSEs, from simple keratinocyte/fibroblast-only models to systems that contain numerous cell types and tissue layers including the epidermis (keratinocytes), dermis (fibroblasts), hypodermis (human lipoaspirate including inherent immune cells), and hiNSCs (neural component). These HSE systems utilize only primary, human cells. We demonstrate that each HSE type (SC1, SC2, SC3, SC4) is distinct, and by RNASeq we could define gene expression with respect to each study group. Only SC3 upregulated genes from major GO sets of interest: skin development, hallmark adipogenesis, immune system process, inflammatory response, and nervous system development. All other groups lacked positive expression in at least one of these areas. While HSE models in general are becoming more complex by adding additional cell types (to the standard keratinocyte plus fibroblast systems), to approach the full complexity of human skin for downstream use, the neuro-immuno-cutaneous system should be included. We present an HSE system that has functions in all these areas (neuro-immuno-cutaneous) that potentially could be used as an in vitro diagnostic tool for the skin.
5 Experimental Section
Materials: Cell culture materials and HSE construction materials were previously described6 and full details are included in Tables S1, S2, S4, and S5 (Supporting Information): cell culture media conditions, HSE media conditions, dermal hydrogel construction, and neural hydrogel construction, respectively. All assays and antibodies were purchased from Abcam (Cambridge, MA) unless otherwise noted. Silk was prepared either to 30 min (for porous silk scaffolds) or 60 min (HSE dermal component) extraction time, as previously described.6, 80 Collagen was purchased from Advanced Biomatrix (San Diego, CA). Horseradish peroxidase (HRP) and hydrogen peroxide were purchased from Sigma (St. Louis, MO).
Model: Cell Culture Conditions: Cell culture conditions were previously described6 and detailed information is located in Tables S1 and S2 (Supporting Information). Changes in the present work included primary human keratinocyte sourcing (C0205C, ThermoFisher), addition of 1% antibiotic-antimycotic (15240112, ThermoFisher) to Epithelialization 1, Epithelialization 2, and Cornification media, and the removal of NGF from the cell culture medium (Table S2, Supporting Information). Primary human fibroblasts were purchased from Lonza (CC-2511), and hiNSCs were obtained from Tufts University.72
Adipose Surgery: Human abdominoplasty samples were obtained with institutional review board approval (Protocol No. 0906007) at the Lahey Clinic (Burlington, MA) and used to generate the lipoaspirate. The details were previously described6 and include liquification of dissected adipose tissue which was then incubated with prewarmed scaffolds which was soaked in maintenance media (Table S1, Supporting Information) for 24 h prior to surgery. Then, the liquefied tissue excess was removed from scaffolds which were incubated for an additional 1–2 h prior to addition of maintenance media for the remainder of culture. The only significant change from the previously described experiment6 was size reduction of the porous, silk scaffolds to 10 mm diameter × 1 mm thickness. Adipose surgery was typically performed three weeks (-D21) prior to experiment start date (D0).
Tissue Model Construction: Tissue model construction, including details on scaffold preparation and timing can be found in a prior work6 with full details in Tables S1–S5 (Supporting Information).
Briefly, the dermal component was prepared by mixing hydrogel components (10× EMEM, Glutamax, fetal bovine serum), then silk and collagen were added. The solution was titrated with sodium bicarbonate, then HRP and fibroblasts (1.3 × 107 cells mL−1) were added, mixed, and finally hydrogen peroxide was added to initiate crosslinking of the tyrosine bonds in silk and collagen.24 The dermal component was prepared two weeks (-D14) prior to experiment start date (D0).
Prior to seeding the keratinocytes, the dermal gels were aspirated of their media, and 0.3 mL/insert of coating matrix solution (R-011-K, ThermoFisher; Table S1, Supporting Information) was added to the surface of the gels to aid keratinocyte attachment. After about 20 min the matrix coating solution was removed, then primary keratinocytes (≈2000 cells/12 mm insert in EPI1 media (Table S2, Supporting Information)) were seeded onto the dermal gel. This epidermal component was prepared one week (-D7) prior to experiment start date (D0).
On the experiment start date, hiNSC-containing collagen hydrogels (Table S5, Supporting Information) were applied to hypodermis scaffolds, fully coated, and allowed to begin gelation in the incubator for about 1 h. Then, the dermal/epidermal silk-collagen construct was placed on top of the hiNSC-coated hypodermis, and the construct was raised to the ALI for the remainder of experimentation. The resultant construct, with all components, is referred to as SC3 (Table 3). For SC1, the hypodermal and neural steps were omitted; in SC2, the hypodermal step was omitted; in SC4, the neural coating of the hypodermis was omitted and replaced with an acellular collagen gel coating (Table 3).
Changes in the present work included a reduction in the tissue size by ≈50% to match a 12-well-insert transwell (Corning) format, a seeding density of keratinocytes (≈2000 cells/12 mm insert), fibroblasts (≈317 000 cells/12 mm insert, Table S4, Supporting Information), and hiNSCs (≈648 000 cells/12 mm insert, Table S5, Supporting Information) all increased from a prior work,6 and 1.5 µg/insert NGF (in 0.1% BSA) was also injected into the subepidermal space at day zero to encourage migration of hiNSCs toward the epidermis (added for all HSE groups: SC1, SC2, SC3, and SC4).
Cell-Specific Assays: Cell culture supernatant was collected at predetermined time points and stored at −80 °C until analysis. Assays were conducted according to product instructions (Table S6, Supporting Information).
Histology and Immunohistochemistry: Histological staining (hematoxylin and eosin) was performed on 6 µm thick, paraffin-embedded sections. Histology images were taken using a Keyence All-in-One Fluorescent Microscope (BZ-X710) and 20× air objective. HSEs were fixed for immunostaining by immersing them in 10% phosphate buffered formalin for ≈24 hours. Antibodies/molecules used for IHC are in Table S7 (Supporting Information). Samples were thoroughly washed with phosphate buffered saline (PBS) prior to proceeding to staining. Next, samples were permeabilized using a 1:1 (v/v) methanol:acetone solution, rinsed with PBS, and a subsequent permeabilization step of a 1% Tween (in PBS) solution was performed three times. HSEs were then blocked using 10% goat serum (ThermoFisher) for 1 h, primary antibodies were incubated overnight at 4 °C, rinsed, and secondary antibodies were added for 1–2 h at room temperature. Samples were rinsed in PBS prior to imaging. IHC images of bulk HSEs or 6 µm thick sections were taken with a Leica TCS SP8 confocal microscope with a 20× air, 25× water, or 40× water objective.
RNA Extraction: Samples were stored in RNAlater (ThermoFisher Scientific) at −80 °C until use according to manufacturer protocol. Total RNA was isolated using the RNeasy Mini kit (Qiagen, Germantown, MD).
RNA Sequencing: Purified RNA samples were quantified with Advanced Analytical Fragment Analyzer (High Sensitivity RNA Analysis Kit, 15 nt). After quantification, 100–200 ng of total RNA was used as input for library preparation using Illumina TruSeq stranded mRNA Sample Preparation Kit. The prepared libraries were then checked and quantified on Fragment Analyzer (High Sensitivity NGS Analysis Kit) and pooled equal molar. The pooled libraries were then quantified once more before loading onto one lane of the Illumina HiSeq 2500 and sequenced with High Output V4 chemistry and single read 50 bases format. The raw data were processed with bcl2fastq for base calling and sample demultiplexed and resulted in compressed fastq files.
RNA Sequencing Analysis: RNASeq analysis was conducted using Qlucore Omics Explorer (version 3.3) (Lund, Sweden). The compressed fastq files were imported into Qlucore as FPKM files, thresholded to 0.1 and logz transformed prior to the start of analysis. Transformed data were sigma-normalized (mean = 0; variance = 1), were filtered to a q-value of 0.1, and analyzed by averaged hierarchical clustering by gene. Filtered gene data of all groups at q = 0.1 were exported to Reactome (version 64, accessed June 11, 2018) and mapped to the human genome for visualization purposes.81 Gene Ontology (GO) sets (Table 4) available from Molecular Signatures Database (MSigDB, v6.1) were imported and filtering was applied.82, 83 Separately, GSEA was performed using Qlucore Omics Explorer, utilizing the H collection: hallmark gene set from Molecular Signatures Database (MSigDB, v6.1). Heat maps, PCA graphs, and box plots were prepared using Qlucore Omics Explorer. The term q-value, or q, represents the FDR in this setting, and the terminology was used interchangeably. All RNASeq data can be found in Mendeley data including raw (.fastq or FKPM) files, Qlucore files (.gedata file), GSEA results, detailed information on GO sets, and Reactome inputs (Data Availability).
Statistics: Statistics were conducted using OriginPro (version 8.6). Assays were performed in triplicate, presented as average from at least two independent experiments (two patient sources of lipoaspirate) with error bar ± SEM (N = 6). One-way ANOVA with Tukey post hoc test was performed to determine statistical significance, where P-values of P < 0.001 = ***; P < 0.01 = **; P< 0.05 = *. Images were presented as representative. RNASeq data were representative of samples from one independent experiment with lipoaspirate used from the same patient.
Acknowledgements
Research for this paper was conducted with grant support: P41EB002520 from the NIH, FA9550-11-C-0028 from the Department of Defense, the Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a. The authors thank Albert Tai for genomics assistance, Tufts Medical Center for histology, Yana Stackpole for assistance with Qlucore, Tina McKay for useful discussions and for assistance with hiNSCs. S.E.V.Y. and D.L.K. designed the experiments. S.E.V.Y. executed the experiments, analyzed the data, and prepared the manuscript. K.A.T. and H.N. contributed to experiments (assays) in addition to literature review. D.M.C. provided hiNSCs. D.L.K. supervised research efforts and edited the manuscript. All authors edited and approved the manuscript. Large datasets (RNA-Seq, protein data) are made available by the authors at Mendeley Data, citable via: Vidal Yucha, Sarah (2018), “RNASeq of in vitro tissue engineered skin with and without neural and immune components”, Mendeley Data, v1 https://doi.org/10.17632/7xbbhr35v6.1.
Conflict of Interest
The authors declare no conflict of interest.




