Receptor-Targeted Drug Delivery and the (Many) Problems We Know of: The Case of CD44 and Hyaluronic Acid
Abstract
CD44 is best known for being the most common receptor of hyaluronic acid (HA). It is also a heterogeneous molecule: while its standard isoform (CD44s) is ubiquitously expressed, there are also a range of variants (CD44v), and both CD44s and CD44v undergo a variety of post-translational modifications. The signaling roles and frequent overexpression of both CD44s and CD44v in cancer (e.g., in cancer initiating cells/cancer stem cells) has raised interest in them for diagnostic applications, but also—and most importantly for this review—as possible molecular targets in tumor therapy, with their endocytic character being a clear advantage for the intracellular release of payloads. In this area, the most popular approach employs HA-based carriers. However, their rational design and therefore the success of HA-based therapies are hampered by the rather limited understanding of not only the identity, but also the dynamic properties of CD44. In this review, the reader is exposed to the full breadth of the challenges that HA carriers currently face, which start at the CD44 post-transcriptional and post-translational heterogeneity, and also include the understanding of receptor clustering phenomena (influencing also HA avidity), as well as the evaluation of off-target effects.
1 Introduction
CD44 is a type I (extracellular N terminus) transmembrane glycoprotein, identified in the 1980s as (the) lymphocyte homing receptor.1, 2 By the end of the century, it became predominantly recognized as a rather promiscuous receptor, acting primarily for glycosoaminoglycans (GAGs) such as hyaluronic acid (HA)3 and chondroitin sulfate,4 and for signaling proteins such as osteopontin5 and galectin-8,6 but being also capable to bind matrix proteins (collagen,7 fibronectin,8 and laminin9). The structural variety of CD44 ligands mirrors the diversity of functions ascribed to this receptor, which range from signaling (e.g., in the resolution of inflammation10) and ligand internalization (at least for HA,11 possibly also for osteopontin12) to cell anchoring and homing (e.g., in the bone marrow13). Although this set of functions14 can be summed up by a (maybe too simple) definition of CD44 as a mediator in the response of cells to their microenvironment, be it in homeostasis or in pathological processes,15 one has to bear in mind that an intricate regulation is required due to its complex and inter-connected role. In particular, these processes will exert control not only on the level of CD44 expression but also on its three levels of molecular heterogeneity, i.e., post-transcriptional (a number of isoforms from alternative splicing), post-translational (extensive modification, mainly with glycosides and glycosaminoglycans), and epigenetic (hypermethylation of the cd44 promoter region regulates CD44 silencing16).
1.1 The Therapeutic Value of CD44
- (1) Those that hijack the natural mechanism of CD44-mediated endocytosis; this is typically the case of HA-displaying nanocarriers, as well as side-chain-modified soluble HA derivatives, as shown in two recent reviews.23
- (2) Those designed to exhibit a specific affinity for either a specific isoform or all of them; for example, monoclonal antibodies common to all isoforms, such as RG7356,24 or peptides against CD44v6.25
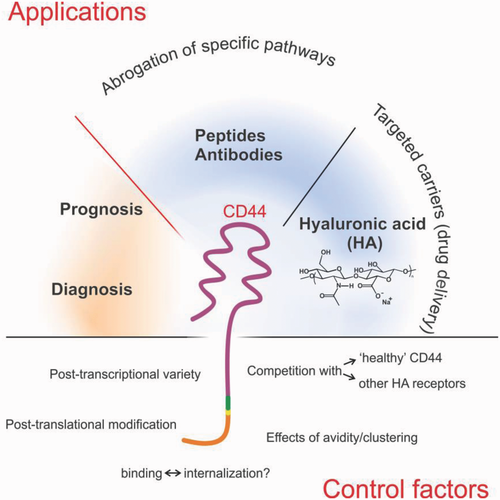
The advantages of HA-based carriers over, e.g., anti-CD44 antibodies are the lower cost, the easier combination with a variety of active pharmaceutical principles and a better (but not necessarily completely) known mechanism of binding and internalization. On the other hand, antibodies allow for targeted action precise to the molecular level, and therefore are more suitable for the abrogation of specific signaling pathways.
We here review our current level of understanding of (and control over) the factors that can modulate CD44/HA interactions (bottom, Figure 1).
1.2 The Problems of CD44 Molecular Identity
CD44 has been dubbed as “a molecule with a thousand faces”26; this definition reflects the multiplicity of its functions, but also its molecular variability. The latter can have a profound impact on targeting strategies; for example, some CD44v can also be produced under nonpathological conditions (e.g., CD44v6 in M2-like macrophages27). Even more worryingly, if on one hand CD44v can be considered a hallmark of cancer, on the other hand the shorter, standard isoform of the receptor (CD44s, also known as hematopoietic CD44H) is virtually ubiquitously present in mammalian cells already under homeostatic conditions14; this can lead to significant but unpredictable off-target effects, because the CD44v/CD44s expression ratio is not only cell- but also developmental stage-dependent, and an isoform-specific binding of CD44 ligands is far from demonstrated. Indeed we still lack clear if not quantitative relations between the post-transcriptional but also post-translational CD44 heterogeneity on one side, and its interactions with HA on the other. For instance, N-glycosylation appears to be essential for CD44 to bind HA,28 but not many studies elaborate on the receptor functional state in both healthy and diseased cellular models. Further, the accuracy of these very models can strongly depend on their CD44 match with real pathologies.
In this review, we therefore provide an overview of a) the intracellular processes responsible for the ubiquitous or restricted expression of CD44 isoforms in health and disease; b) the effects of CD44s/CD44v post-translational modifications on HA uptake; c) the resulting design strategies applicable to HA-based carriers.
2 Molecular Structure of CD44
CD44 is encoded by a single gene, which is located on the short arm of chromosome 11, spans ≈50 kb of DNA,17, 29-31 and encompasses 20 exons (10 constants and 10 variables) and 19 introns29, 30 (Figure 2A). Despite the gene being highly conserved, the actual size of the receptor is highly variable; one common cause is that the CD44 transcripts are further edited to generate, e.g., CD44s (no variable exon, the isoform typical of blood cells) or CD44v (where variable exons are added typically in the stem region, in blue in Figure 2B), in a fashion that depends on the cell type, the stage of development, or the pathophysiological condition.1, 17, 29, 32-34
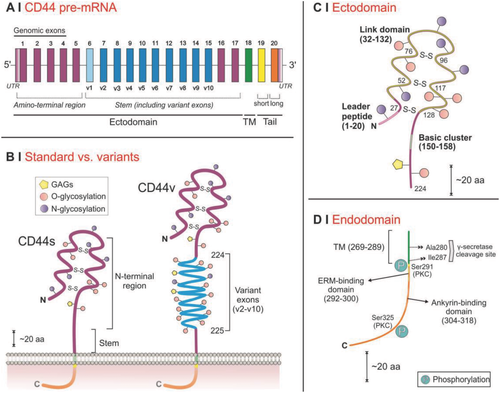
It is worth mentioning that size is not the only parameter differentiating CD44 isoforms; for example, they may also differ in turnaround time. CD44 is estimated to have a half-life of about 8 h according to Naor et al.26 or, possibly more likely, in the range of 12–48 h according to experimental data on normal and malignant cell lines35, 36; quantitative densitometry has shown a CD44s half-life of about 48 h in normal melanocytes, which is reduced to 20 h in MV3 metastatic melanoma cells35 (highly expressing CD44v5 and CD44v637). Experiments on clonal MCDK cell lines expressing human CD44s also reveal a reduced half-life of 5–8 h for the tail-less (lacking intracellular domain) CD44 isoform, as opposed to 16–18 h for wild-type CD44s.38 CD44 structural parameters are summarized in Table 1 and analyzed in Section 3 for CD44s, and in Section 4 for the hypervariable stem region, broadly following a recent review.39
| Protein region (exons) | Structure | |
|---|---|---|
| Ectodomain (Figure 2C) | Globular domain (exons 1–4) |
Disulfides from six highly conserved Cys residues stabilize globular domain and HA binding groove. HA-binding region (amino acids 20–169) composed of the LINK domain (amino acids 32–132) and a basic motif (amino acids 150–158). Two arginines and two tyrosines are critical for HA binding. |
| Stem region (exons 4–17; exons 6–15 are also referred to as variant exons v1–v10) |
Fixed region of 46 amino acids in CD44s; incorporation of a variable region in CD44v. Alternative splicing (variant exons skipping/inclusion) can lead to a 2–3-fold increase in size. Variant exons may contain GAG-binding motifs. |
|
| Transmembrane domain (exon 18) |
Highly conserved, 23 hydrophobic amino acids. Allows lipid raft association (upon palmitoylation) and receptor clustering. Cys286: CD44 dimerization. Ala280/Ile287: γ-secretase cleavage site. |
|
| Intracellular domain (exons 19/20) (Figure 2D) |
Alternatively spliced in long-tail or a less common short-tail variant. Long-tail CD44 contains a nuclear localization signal, which is involved in gene regulation. Ankyrin (amino acids 304–318) and ERM (amino acids 292–300) binding sites couple CD44 with cytoskeleton. C-terminus PZD-domain: putative phosphatase association and regulation (receptor signaling). |
|
Another major source of size variability is post-translational modification. CD44s is ubiquitously expressed in most vertebrate cells26, 31 and stems from the genomic exons 1–5, 16–18, and 20 giving rise to a 341 amino acid protein with an expected molar mass of 37 kDa18; however, the usual molar mass detected for CD44s is in the range of 85–95 kDa17, 31 the increase being mostly due to glycosylation. These effects are reviewed in Section 4.
2.1 Extracellular Domain
The extracellular domain (ectodomain) is where most CD44 interactions with the external environment occur; it is also its most variable part (Figure 2B), which indicates that also its interactions are likely to vary much in intensity. The first five genomic exons are reported to be constant and encode the N-terminal globular region (from the N terminus to the basic cluster in Figure 2C). Exon 1 comprises the 5′ untranslated region (UTR), the start codon and the leader peptide (amino acids 1–20).14 It is noteworthy that a specific variant, broadly homologous to CD44v5 but lacking the leader peptide, was seen in mature B cell malignancies40; probably also due to a unique C terminus, this variant is intracellular, and therefore almost impossible to target even if overexpressed.
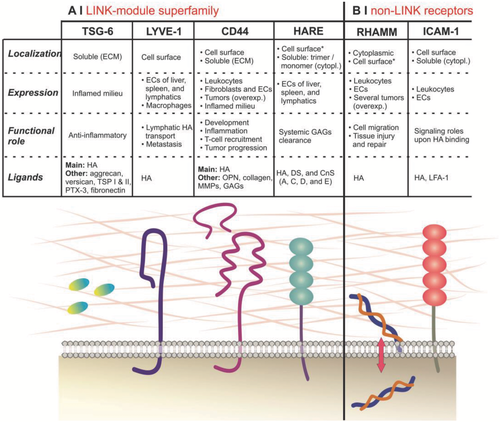
The CD44s ectodomain is normally subdivided in three regions21: the LINK domain (amino acids 32–132), the basic cluster (amino acids 150–158, mostly positively charged), and the stem structure (46 amino acids). The LINK domain is topologically similar to a C-type lectin domain,41 and has a ≈35–40% homology with similar domains in hyaladherins such as LYVE-142 and others, which are grouped in the so-called LINK-module hyaladherin superfamily (Figure 3). As it has been demonstrated for TSG-643 and CD44,44 HA binding requires the LINK domain to undergo a conformational transition that allows interactions with amino acidic residues normally not exposed (in TSG-6, two tyrosines involved in hydrogen bonding, that add to the electrostatic interactions provided by two basic residues). Differently from other components of the LINK-module superfamily, in CD44 the HA-binding region (amino acids 20–169,45 encoded by genomic exons 2–446) is not limited to the LINK domain, but involves also a basic cluster;47 both include a putative HA-binding BX7B motif, where B is a basic amino acid and X a generic (but not acidic) one, which is also present in non-LINK. The first motif (in the LINK region) reportedly has a higher affinity for HA,18 but NMR studies have shown that residues both within the LINK module (Arg58 and Tyr59) and in the BX7X basic cluster26 motif are critical to HA binding; this presence of multiple binding sites suggests that there may be a variety of factors modulating the overall strenght of the interactions. Importantly, three disulfide bonds (Cys27-Cys128, Cys52-Cys117, and Cys76-Cys96)1, 17 are necessary for the correct folding of the extracellular region,21 for the ensuing stability of the LINK module48, 49 and therefore also to obtain stable HA interactions.48 It is worth mentioning that CD44 possesses additional binding sites, contiguous to the HA binding region, for secondary ligands, such as osteopontin,5 collagen and laminin,9 fibronectin,8 selectins,50 serglycin/gp600,51 the major histocompatibility complex class II (MHC-II),18 or leukocytic receptors (e.g., CD62E and CD62L).52 We are not aware if any study has shown whether their occupancy affects either agonistically or antagonistically the strength of HA/CD44 interactions; on the other hand, evidence has been reported that binding to chondroitin sulfate, most likely at a site close to the basic cluster, reduces the binding.53, 54
The stem region (nonvariable in CD44s) is encoded by genomic exons 4, 5, 16, and 17, and connects the extracellular domain to the transmembrane domain. This stalk-like structure contains proteolytic sites for membrane-associated proteases.55 The insertion of variant exons due to alternative splicing increases the stem length and expand the diversity of its post-translational modifications.29, 56
Finally, it is important to note that the capacity of CD44 clustering is critical to HA binding; for example, multifunctional CD44 antibodies can induce it in cells that are constitutionally incapable of HA binding.57
2.2 Transmembrane Domain
The CD44 transmembrane region (encoded by genomic exon 18) is a single-pass domain of 23 hydrophobic amino acids that also provides a platform for protein interactions.39 A cysteine residue (Cys286) is involved in the dimerization of CD44 receptors on cell surfaces,58 while a γ-secretase cleavage site is located at Ala280/Ile287 and regulates intramembrane proteolysis.59 Of relevance is the fact that the transmembrane domain is critical for the localization of CD44 in lipid rafts60 and hence for its clustering,59, 60 where it associates to a variety of other receptors, such as ErbB261 or sphingosine 1-phosphate receptor62; of note, the disruption of lipid rafts leads to the loss of the signals induced by HA binding, similarly to what seen with CD44 blocking antibodies or CD44 silencing,62 and also appears to reduce the CD44 coupling (through its endodomain, see next section) to cytoskeletal elements.63
2.3 Cytoplasmic (Tail) Domain
The CD44 cytoplasmic domain is affected by post-transcriptional modification be encoded by genomic exons 19 or 20 depending on alternative splicing, respectively generating a rather uncommon short-tail or the more usual long-tail isoforms.17 CD44s, for example, is a long-tail isoform that lacks exon 19; it also contains a nuclear localization signal, whose nuclear translocation appears to support stemness factors in tumor cells64 and in general appears to be critical for forms of CD44/STAT3-mediated transcriptional reprogramming65 that may have important roles in fibrosis; finally, the CD44 intracellular part also contains a C-terminal PZD-binding domain which may regulate phosphorylation, as seen in other receptors.46 Motifs in intracellular regions are important for a membrane protein subcellular localization,14, 66 but also to allow its coupling to cytoskeletal components.14 Indeed, CD44 interacts with ankyrin (binding domain: amino acids 304–318)67 which in turn mediates contact with spectrin and participates in HA-dependent cell adhesion and motility.17, 68 Additionally, a basic motif in the tail of CD44 is responsible for its interaction with ezrin, radixin and moesin (ERM) proteins (binding domain: amino acids 292–300),69 linking in turn to the actin cytoskeleton to CD44. This action has important consequences in the regulation of cell migration, protein sorting, and lipid raft targeting.70 Another important cytoplasmic partner is the ERM-related tumor suppressor merlin, which is able to inhibit cell growth through its interaction with CD44.14, 71 The merlin-CD44 complex can be seen as a molecular switch to control either cell growth arrest or proliferation depending on the participation of other ERM-proteins, on the phosphorylation state of merlin, and also on the HA molecular weight. When bound, high molecular weight HA can effectively crosslink several CD44 receptors, favoring thus the release of merlin, which in turn can be activated via dephosphorylation to function as an antiproliferation agent.72
At the same time, it has been demonstrated that while post-translational modification of the cytoplasmic domain (via phosphorylation) does not affect HA binding,38 the complete abrogation of the cytoplasmic domain reduces it strongly,38, 73 and this can be recovered only by clustering CD44 with multifunctional antibodies,73 or by creating dimers of the “tail-less” CD44.74 It has, however, been also shown that the induction of additional cytoplasmic clustering, e.g., by replacing the cytoplasmic CD44 domain with that of β5 integrin, does not significantly improve HA binding.75
Therefore, CD44 clustering/oligomerization may play a role as important as the receptors own molecular structure in controlling HA binding, and “changes in the distribution of CD44 on the cell surface, induced by molecular interactions either from within the cell or from outside, may regulate its role as a receptor.”74
3 Post-Transcriptional Regulation: Alternative Splicing
The expression of alternatively spliced isoforms of CD44 has attracted great attention because of their participation in the progression of several solid tumors,26 with important roles in the formation and maintenance of CSCs and (pre)metastatic niches.39 Here we separately discuss the isoforms differing in extracellular domains (Section 3.1), which can influence HA binding, and those in intracellular ones (Section 3.2).
3.1 Ectodomain Isoforms (CD44vx, CD44vx–x)
The alternative splicing of CD44 transcripts can lead to the insertion of individual (CD44vx) or multiple (CD44vx–x) variant exons between amino acids 224 and 225.14, 76 This addition translates into a significant elongation of the molecule. Most articles report a maximum value up to new 381 amino acids; we have been unable to find the original reference, but it seems reasonable to assume it to be the keratinocyte CD44v (CD44v3–10), which is the largest human form of the receptor with a molecular weight in the range of 250 kDa (180 kDa after deglycosylaton77). This equates to an almost 3-fold increase in size in comparison to CD44s and it seems more than likely that this may affect HA binding, either in terms of steric hindrance (lower binding) or higher distance from the cell surface (higher binding due to better exposure). In terms of nomenclature, e.g., CD44v4 is the isoform obtained through the insertion of exon v4 only, whereas in CD44v8–10 the insertion involves the variant exons v8, v9, and v10. Importantly, CD44v8–10 corresponds to both the epithelial form of CD44 (CD44E), which does not bind to HA, but also to the CD44R1 isoform, typically associated to metastatic tumors, which differs from CD44E in three mutated amino acidic residues and is capable of HA binding.78 A further difference may come from intron removal: it has been shown that the v8–v9 is removed more efficiently in CD44R1 than in CD44s,79 and this may apply also to the comparison with CD44E.
In theory, at least 800 splice variants of CD44 could be produced17, 80 and there is a general understanding that high CD44v expression often correlates with poor prognosis in cancer.26 A reality check, however, shows that despite the number (vast but below 800) of isoforms indeed observed experimentally,17, 33, 81-83 only twelve appear to be commonly linked to disease progression.39 For example, whereas CD44v3,v8–10 is related to breast cancer progression84 the larger CD44v3–10 is the usual form expressed by nontumoral keratinocytes.77 To further complicate the landscape, not only CD44v fingerprinting (i.e. the specific pattern of CD44v expression) but also its biological effects can be very disease-specific: for example, while the expression of CD44v3 and CD44v6 positively correlates with poor outcome in nasopharyngeal cancer85 (as much as in lupus erythematosus86) that of CD44v5 in the latter had no correlation; however CD44v5 is a prognostic factor in thymic neoplasms.87
In Table 2, we report the expression of these isoforms both in normal and malignant cells.
| CD44 isoform | (Over)expressed in | Relevance | |
|---|---|---|---|
| Physiological | Pathological primary/lines | ||
| Absence |
Protoplasmic astrocytes.88 Platelets.15 |
Jurkat cell line (Leukemia T lymphocyte cells).89 Some forms of OSCC.90 Some forms of HNSCC.91 |
Unknown. |
| CD44s |
Lymphohematopoietic cells92: lymphocytes, macrophages, dendritic cells, granulocytes and erythrocytes. Epithelial cells92: epidermis, tonsils and pharynx, salivary glands, pancreas, thyroid follicles. Glial and neuronal cells88: very high expression in “fibrous”-like astrocytes. |
Carcinoma: renal,92 pancreatic,93-95 colorectal,96 and hepatocellular.97, 98 HCC,97 NSCLC,99 osteosarcoma,100 biliary tract cancer.101 Common human cancer cell lines24: PANC-1, AsPC-1, PC-3, SKOV-3, MOLM-13, HL60, THP-1, U87-MG, SK-Hep-1, Kasumi-1, Calu-6, A549, HEL 92.1.7, EOL-1. |
Associated with cell migration, invasion and survival.26, 99, 100 Negative prognostic factor: primary colorectal carcinomas,98 HCC,99 NSCLC.96, 97, 101 Positive prognostic factor: myxofibrosarcoma.102 Acquisition of mesenchymal phenotype and anchorage independent survival.98 |
| CD44v2 | Normal urothelium.103 | Colorectal carcinoma,104 pancreatic cancer,105 breast cancer,94 ESCC.106 |
Marker for detection of transitional cell carcinoma.103 ESCC marker (indicates adjuvant therapy in patients with no lymph node metastasis).106 |
| CD44v2–10 |
Normal colonic crypt epithelium, predominantly in the crypt base.107 |
Breast solid tumors and cancer cell lines (e.g., MDA-MB-231, MCF-7).108 | Positive steroid receptor status, low proliferation and luminal A subtype.108 |
| CD44v3 |
Apical ectodermal ridge cells.109 Polymorphonuclear leukocytes.110 |
OSCC,111 HNSCC,112 breast,113 and endometrial114 carcinomas. |
CSC marker for HNSCC, increases proliferation and enhances cisplatin resistance.111 Marker for cell migration.112 |
| CD44v3,v8–10 | Not reported in healthy tissues. | Colorectal adenomas and carcinomas.115, 116 | Overexpressed in >70% of colorectal liver metastases.115 |
| CD44v3–10 | Keratinocytes.17 |
Synovial fluid cells in arthritis,6 HNSCC.112 Cancer cell lines24: MKN45, HCC1937, KPL4, JIMT-1, NCI-N87, FaDu, Detroit-562, Cal-27, NCI-H520, NCI-H1993, Colo205. |
Considered as a marker for tumor progression and aggressiveness.112 |
| CD44v4 |
Choroid plexus cells.117 Neurons.88 |
Breast carcinoma and breast cancer cell lines118: MDA-MB-231, MDA-MB-435, MDA-MB-468. | Incorporation of E-selectin ligand facilitates tumor cell migration.118 |
| CD44v5 | Choroid plexus cells, Purkinje cells.117 |
Renal119 and breast120 carcinoma, osteosarcoma.100 Cervix cancer cell lines: HeLa.121 |
Associated with increased metastatic behavior.100 |
| CD44v6 |
Choroid plexus cells.117 Mammary epithelial cells, nonproliferating ductal epithelium.122 THP-1 M2-like macrophages.27 |
ESCC,123 OSCC,124 HNSCC125 and HPSCC,126 tongue,127 pancreatic95 and cervical carcinoma.128 Colorectal CSCs,129 osteosarcoma,100 prostate,130 biliary tract101 and breast cancer.131 |
Pancreatic carcinoma metastasis and progression95 (CD446+/CD44s− is an independent survival factor). Involved in c-Met signaling.132 Downregulation promotes metastasis in OSCC.124 Useful marker of tumor invasion and metastasis.133 |
| CD44v8–10 (CD44E, or CD44R1) | Very low expression in normal tissue.134 |
Hepatocellular,98 gastric and colorectal carcinomas134-136 Cancer cell lines134-136: BT-20, AGS, KATOIII, HCT-116, HT-29. |
Human gastric CSC marker.134 Promotes GSH synthesis135 (enhanced oxidative defense). EGFR signaling preferentially cooperates with CD44v8–10.108 |
| CD44v9 | Normal endometrial glandular cell membrane.137 |
Endocervical adenocarcinoma,138 pancreatic carcinoma,95 cervical precancerous lesions,139 OSCC124; gastric,140, 141 esophageal,141 colon,142 prostate143 and ovarian cancer144; colorectal CSCs.145 |
Pancreatic carcinoma metastasis and progression.95 Associated with proliferative activity, GSK-3β activity, EMT and inhibition of apoptosis.146 Downregulation promotes metastasis in OSCC.124 Most likely candidate CSC marker147 |
| CD44v10 |
Choroid plexus cells.117 Bone marrow stromal cells.148 |
Hepatocellular carcinoma.98 Prostate149 and pancreatic cancer.150 | Allows differentiation between metastatic and nonmetastatic prostate cancer cells. Putatively involved in counteracting metastases in vivo.150 |
- HCC, hepatocellular carcinoma; NSCLC, non–small cell lung cancer; ESCC, esophageal squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; HPSCC, hypopharyngeal squamous cell carcinoma; OSCC, oral squamous cell carcinoma; GSK-3β, glycogen synthase kinase-3β; Table 2 does not include the variant exon 1: this is expressed in rats and mice,32 but due to the presence of an early termination codon it remains silent in humans.143, 151 However, point mutations may trigger the expression of isoform CD44v1 in human cells, as described for fibroblasts isolated from rejecting cardiac allografts, even though its functional implications remain unknown.152
To date the regulation of CD44 alternative splicing has not been completely elucidated due to its complex genomic organization, to the variety of cell specific cis- and trans-acting mechanisms involved in the process, but also to the possibility of single mutations (e.g., distinguishing CD44E from CD44R1). A number of carcinogenesis-promoting genes can modulate CD44 expression, often in a mitogenic signal-dependent fashion,14 and spliced isoforms can therefore be up or down-regulated upon stimulation with cytokines and growth factors. For example, interleukin-1 induces the expression of v3 and v6 isoforms in a process mediated by the early growth response element-1.153 Secondary ligands of CD44 may also regulate alternative splicing. For instance, osteopontin favors the expression of variants v6 and v9 in breast cancer cells.154 A list of the most frequent alternative splicing regulators is enclosed in Table 3. Please note that the use of these regulators or the genetic modification of related pathways may be useful to change the CD44v/CD44s expression ratio of cancer cell lines, obtaining a CD44-fingerprint relevant for specific in vitro and in vivo experiments. For instance, Hu et al. modified the CD44v/CD44s ratio of breast cancer cells by regulating the expression of epithelial splicing regulatory protein 1 (ESRP1), thereby demonstrating the higher capacity of lung metastasis of CD44slowCD44vhigh breast CSC with respect to CD44shighCD44low ones.155
| Type | Effector | Impact on alternative splicing/target CD44 mRNA | Cell line or tissue |
|---|---|---|---|
| Up-regulators | TPA, IGF1, PDFG156 | Exons v3, v5, v6, v7, v8 and v9. | SK-N-SH |
| MAPK/ERK157 | Exon v5. | LB-17 | |
| IL-1153 | Exons v3 and v6. | ECV304 | |
|
SRm160121 Sam68158 ASPP1159 |
Exon v5 (in a Ras-dependent manner). | HeLa and 293T,121 T-lymphoma,158 HEK293159 | |
| BaP160 | Exon v10. | HeLa | |
| bFGF161 | Exons v3, v4, and v5–10. | HUVEC | |
| OPN146, 154 | Exons v6 and v9. | 21NT | |
| TNF-α162 | Exons v3 and v6 (via JNK pathway). | MCF-7 | |
| CD44s and exons v3 and v6 (via p38 pathway). | MDA-MB-231 | ||
| DARPP-32163 | Exons v8–10. | AGS, MKN-45 | |
| AGO1 and AGO2164 | Variant exons (through spliceosome recruitment and modulation of RNA polymerase II elongation rate). | HeLa S3 | |
| YB-1165 | Activation of exon v5 keeping via multiple CAUC motifs. | MDA-MB-231 | |
|
PP2Cγ166 p72 RNA helicase167 |
Inclusion of exons v4 and v5 (coregulated by YB-1). | HeLa | |
| SRp55168 | Depletion of SRp55 favors inclusion of exon v7. | U2OS | |
| SRp40169 | Exons v2, v3, v5, and v6. | Breast cancer | |
| Tra2-β1170 | Exons v4 and v5 (synergy with YB-1). | Breast cancer | |
| HIF-1α171 | Up-regulation of CD44s and isoforms (CD44v6, CD44v7/8) mature mRNA under hypoxic conditions. | MDA-MB-231, SUM-149 | |
| HGF, EGF172 | General alternative splicing. | HeLa | |
| ESRP1155, 173 | ESRP1 depletion triggers a switch from CD44v to CD44s. |
4T1173 MCF10CA1h155 |
|
| Down-regulators | Mitomycin-C168 | CD44s mRNA and mRNA spanning v6 exon. | U2OSE6AS |
| Exons v7 and v10. | |||
| ESRP2174 | ESRP1/2 depletion down-regulates CD44v8–10 in favor of CD44s. | PNT2 | |
| WNT5A175 | Exons v4, v5, and v6. | MDA-MB-231, MDA-MB-4T1 | |
| Inhibitors | AR176 | General CD44 splicing (p68 enhanced). | LNCaP |
| PCBP1177 | Exons v3, v5, v6, v8, and v10. | HepG2 | |
| THAP11178 | Exons v3, v6, and v8 | HepG2 | |
| Silibinin179 | ≈90% total mRNA and ≈70% decrease in CD44v7–10 mRNA. | PC-3M | |
| RBM3180 | CD44v8–10 mRNA (in favor of CD44s mRNA). | PC-3 | |
| NS5A (HCV)181 | CD44v6 mRNA. | SB-HCV Molt-4 |
- TPA, 12-O-tetradecanoyl phorbol-13-acetate; IGF1, insulin-like growth factor-1; PDGF, platelet-derived growth factor; IL-1, interleukin 1; PCBP1, poly(rC) binding protein 1; THPA11, Thanatos-associated protein 11; SRm160, serine/arginine-related nuclear matrix protein 160; Sam68, Src-associated in mitosis 68; ASPP1, apoptosis-stimulating of p53 protein 1; RBM3, RNA-binding motif protein 3; WNT5A, wingless-type MMTV integration site family, member 5A; BaP, benzo(a)pyrene; bFGF, basic fibroblast growth factor; ESRP1 and ESRP2, epithelial splicing regulatory protein 1 and 2; OPN, osteopontin; DARPP-32, dopamine- and cAMP-regulated neuronal phosphoprotein; SRp55, serine/arginine-rich protein 55; AGO1 and AGO2, argonaute proteins 1 and 2; YB-1, Y box-binding protein 1; NS5A, nonstructural 5A protein (HCV); AR, androgen receptor; HIF-1α, hypoxia-inducible factor 1-α.
3.2 Intracellular Isoforms (CD44-st, CD44-lt)
Most literature dealing with CD44 mRNA processing has focused on ectodomain variants, i.e., those traditionally referred to as CD44v, although the exons encoding for the cytoplasmic tail domain are also susceptible to alternative splicing.182 The two existing CD44 tail isoforms share three arginine residues encoded by exon 1817, 29 and differ in the presence of exon 19. If this exon is spliced out, the amino acids codified by exon 18/exon 20 give rise to the long-tail CD44 isoform (CD44-lt, tail domain 72 amino acids long).18 On the contrary, when exon 19 is included there is an in-frame termination signal that causes the short-tail or “tail-less” CD44 isoform (CD44-st, tail domain 3 amino acids long18 terminating at Arg294),1 characterized by a drastically reduced half-life.38 Reverse transcription polymerase chain reaction (RT-PCR) studies reveal that the CD44-lt transcript of the hematopoietic form of the receptor (CD44H, i.e., CD44s) is much more abundant than its truncated counterpart.182 CD44-st has only been identified as a dominant-negative receptor in chondrocytes,183 and its inhibition seems to enhance HA internalization.182 Importantly, the DNA sequence of the 3ʹ-UTR for the short-tail isoform carries poly-(A+T) tracts,29 which suggests the expression of CD44-st may be strictly downregulated by means of mRNA rapid turnover.
3.3 MicroRNAs
MicroRNAs can target the 3ʹ-UTR of CD44 and strongly reduce its expression at the protein level. For example, down-regulation of miR-328 due to oxidative stress in the tumor microenvironment upregulates CD44 expression and promotes cancer cells growth and drug resistance.184
4 Post-Translational Modifications
CD44 heterogeneity is further augmented through a variety of post-translational modifications.185-188 In this section, we address: 1) the main post-translational modifications reported in literature for CD44, 2) their functional role at the cellular level, and 3) the impact of these modifications on the uptake of HA. All points are summarized in Table 4.
| Post-translational modification | Effect on CD44/HA interactions | Proposed explanation |
|---|---|---|
| N-glycosylation | Negative236-238 | Interferes with basic amino acids clusters and/or prevents HA binding by steric hindrance. Possible effect on CD44 mobility and clustering. |
| O-glycosylation |
Negative28 |
Effects on HA interactions strongly depend on the cell type and growth conditions, and need to be evaluated in a case-by-case basis. |
| Sialylation | Negative51, 186, 201 | Possibly interferes with HA binding by increasing CD44 overall negative charge (HA-CD44 repulsion, interactions with basic cluster). |
| Chondroitin sulfate addition | Negative53, 54, 238 | CnS-associated proteins may bind to/near the HA binding site, cause a conformational change in the ectodomain that blocks HA binding, or simply increase the negative charge (see sialylation). |
| Carbohydrate sulfation | Positive187, 199 | It may facilitate CD44 clustering, both enhancing the affinity for HA and increasing avidity. |
| Keratan sulfate addition | Negative206 | KS may modulate adhesion to HA via changes in protein conformation (LINK domain). |
| Palmitoylation | None211 | It is relevant for lipid raft targeting and HA internalization, but does not influence binding. |
| Phosphorylation | None38 | Phosphorylation-defective mutants show that HA binding and internalization are both phosphorylation-independent processes. |
| Proteolytic cleavage | Competition223, 225 | CD44sol has the ability to compete with membrane-anchored CD44s/CD44v for HA binding. Alternative (synergic) scenarios are also possible, see Section 5.3. |
4.1 Glycosylation
CD44s can undergo N-glycosylation at six sites (Asn25, Asn57, Asn100, Asn110, Asn120, and Asn255, the first five located within the HA binding domain189) and O-glycosylation at seven sites (Ser/Thr residues in the extracellular region proximal to the membrane).17, 18 CD44 glycosylation is considered to be a regulatory mechanism of CD44 binding to HA,14, 28 which would appear to hinge on three states with different activation: 1) an active form that constitutively binds HA, 2) an inducible form which binds HA upon cell stimulation, and 3) an inactive state which does not (or weakly) bind HA.18 Typically, the active HA-binding form of CD44 is poorly glycosylated, whereas inactive receptors are characterized by an extensive glycosylation185; it has been suggested that this effect is due to conformational changes in the extracellular domain caused by N-glycosylation,66 although saccharidic side-chains may also reduce the extent of receptor clustering, or, when negatively charged, interact with the positively charged residues critical to HA binding.
The CD44 variable region contains four additional N-glycosylation sites and a large number of O-glycosylation sites in the case of exons v2, v8, v9, and v10, rich in Ser/Thr moieties.17 The decoration of CD44v with additional sugar chains is usually considered to reduce HA binding,28, 83, 190 although others have reported the opposite.191 Noteworthy, certain glycosylation patterns of CD44v are frequently linked to malignant processes in cancer. For instance, tumor hypoxia promotes the decoration of CD44v with the sialyl-LewisX carbohydrate antigen (sLeX), a common ligand of selectins with a role in metastasis of estrogen receptor-positive tumors to the bone,192 which indeed renders CD44v a ligand for endothelial E-selectins during metastasis.118, 193 This effect is not reported with CD44s.
It is noteworthy that glycosylation of CD44v has a number of roles in addition to the modulation of HA binding; for example, it is also involved in the quenching of intracellular reactive oxygen species (ROS) in CSCs, associated with the survival of preneoplastic foci and with radio/chemotherapy resistance mechanisms.194 CSC maintain low ROS levels due to the high expression of glutathione (GSH),195 synthesized in a process mediated by the cysteine/glutamate transporter XCT.135 This enzyme is key for tumor development196 and is docked and stabilized by N-glycosylated CD44v,135, 197 which upregulates GSH.198
A further source of post-translational heterogeneity is provided by the modification of CD44-associated carbohydrates. For example, sulfation typically occurs upon stimulation with inflammatory cytokines and seems to increase the affinity of CD44 for HA.187, 199 Probably even more common is sialylation200; all human forms of CD44 are negatively charged at physiological pH (isoelectric point between pH 4.2 and 5.8), which is typically ascribed to terminal sialic acid residues.18, 200 High sialylation appears to go hand-in-hand with reduced HA binding, possibly due to electrostatic repulsion,51, 186, 201 and indeed it has been speculated that sialylation/desialylation of CD44 may function as a switch to modulate the ability of CD44 to act as an HA-anchoring receptor. For example, hypersialylation of an N-glycosylation site within the HA-binding domain has been associated with an increased cell adhesion to selectins and metastatic behavior (in SW1990 pancreatic cancer cells).202
4.2 Glycosaminoglycanation
The region of CD44 proximal to the cellular membrane contains a chondroitin sulfate (CnS) binding site (Ser-Gly motif) common to all isoforms.17, 18, 203 CnS attachment occurs at Ser180 and may negatively regulate HA binding53, 54; this could be due to an induced structural change in the conformation of the ectodomain, to direct competition of CnS-modified proteins with HA for the binding to CD44, or to electrostatic repulsion.4, 51
CD44v can be extensively modified through the attachment of GAGs, which allows to recruit growth factors and cytokines18, 53; their presentation on the cell surface in turn enables autocrine or paracrine signaling.204 CD44 isoforms including exon v3 contain a double serine–glycine motif (SGSG), i.e., an attachment site for CnS, and preferential sites for heparan sulfate (HS) that make CD44 a heparan-sulfated proteoglycan,205 which implies a role of CD44v in inflammatory processes.204 Additional binding sites within the variable region have also been described for dermatan sulfate (DS),205 keratan sulfate (KS)206 and HS.203 Exon v6 adds a site each for hepatocyte growth factor (HGF) and a vascular endothelial growth factor207; additionally, it also increases GAG binding probably through a coordinated binding that involves the HA-binding N-terminal region, as also reported for v7-containing variants.82 The attachment of GAGs to CD44v causes the receptor to interact with Met signaling, which in turn activates a broad range of cellular pathways involved in cell proliferation, motility, migration, and invasion.132, 208 Of special importance is the role of CD44v6 as in the presentation of HGF and the formation of a ternary complex between CD44v6, HGF, and Met.132 Besides, the intracellular tail links c-Met signaling to the actin cytoskeleton by means of partner proteins in order to recruit the guanine nucleotide exchange factor son of sevenless, which amplifies MAPK/ERK signaling.209 A feedback up-regulation of CD44v6 in melanoma cells after HGF binding has also been described.210
In summary, the variant-dependent presence of GAGs adds another level of modulation to the interactions of CD44 with its ligands (not only HA).
4.3 Palmitoylation
CD44 can undergo reversible palmitoylation (formation of thioesters between palmitate and cysteine residues) at the highly conserved Cys286 (within the transmembrane domain) and Cys295 (within the proximal cytoplasmic domain).211 This modification not only seems to enhance the binding of ankyrin and possibly of ERM proteins,46 but also interferes with CD3-mediated signaling on human T-lymphocytes212 and influences CD44 raft targeting.46, 211 What is more, depalmitoylated and palmytoilation-defective CD44 mutants appear not to be sufficiently hydrophobic for their association with lipid rafts and as a result the internalization of both HA and the receptor itself are inhibited.211 It may therefore seem reasonable to assume the effect of palmitoylation to be similar to the other (membrane and intracellular) factors regulating CD44 clustering (Sections 2.2 and 2.3).
4.4 Phosphorylation
The cytoplasmic tail of CD44 contains six potential phosphorylation sites,17, 213 out of which Ser303 and Ser305 (mainly) have been shown to be the two most highly phosphorylated residues in vivo,18 although Ser323 is also possibly necessary for the phosphorylation of CD44 in vivo214; in cultured resting cells CD44 is also phosphorylated at Ser32546, 215 in a process mediated by protein kinase II (CAMKII).216 Interestingly, the activation of protein kinase C (PKC) switches off Ser325 phosphorylation and leads to phosphorylation of Ser291, which in turn modulates the interaction of CD44 with ERM proteins and is consequently paramount to cell motility.215 The activation of downstream Ser316 kinases by PKC has proved essential for CD44-mediated chemotaxis toward a phorbol ester gradient, which also suggests a role of this receptor in directional cell migration.213, 217 The presence of certain variant domains such as CD44v4–7 may enhance phosphorylation of the cytoplasmic tail17 and thus influence the interaction of the receptor with other intracellular proteins. For example, Rho-kinase A has been reported to promote the interaction of isoform CD44v3,v8–10 with ankyrin, stimulating tumor cell migration in metastatic breast lines.84 However, despite of this rich signaling behavior, mutations in all the above mentioned Ser residues have shown that HA binding is an essentially phosphorylation-independent process.38
4.5 Proteolytic Cleavage (Production of CD44sol)
CD44 can exist therefore not only as a transmembrane cell surface receptor, but also as a soluble molecule (soluble CD44, CD44sol), which can additionally be sequestered and become an integral component of extracellular matrices.218 CD44sol is predominantly produced through the proteolytical cleavage of the extracellular domain, which is followed by a second cleavage that liberates the intracellular domain in the cytoplasm219; CD44sol can also be produced de novo as an isoform lacking the cytoplasmic and transmembrane domains,220 although this pathway is believed to be considerably less common.
Because of the increased CD44sol levels reported in several cell lines55 and human tumors,221 it has been suggested that CD44sol could be a useful biomarker for tumor growth and invasion222, 223; however, it should be noted that CD44sol is also produced during homeostatic phenomena of migration, e.g., by thymocytes.224 Importantly, both the soluble and matrix-bound forms can compete with the membrane-anchored form for HA binding225; this disruption of HA cellular signaling is likely at the basis of the antitumoral activity observed by both by CD44sol,223, 226 and a BX7B-enriched peptide.227
Mechanistically, the cleavage requires the intervention of metalloproteases (MMPs),55 in particular MT1-MMP (also known as MMP14) and MT3-MMP (MMP16).228 However, the shedding of CD44 is probably a multicomponent process, which has been associated to the expression of cytokines such as oncostatin M and transforming growth factor-β1,225 as well as the epithelial growth factor (EGF),229 and possibly also involving interactions with matrix elements such as laminin-5 gamma 2 chains.230 The C-terminus remains embedded in the plasma membrane, until it is further processed by a presenilin-1/γ-secretase (cleavage of Ala280/Ile287); this process is known as regulated intramembranous proteolysis,59 and results in the secretion of a CD44β-like peptide and of an intracellular domain fragment (CD44-ICD), which translocates to the nucleus and promotes the transcription of target genes (including cd44 itself) via a phorbol ester response element231 (see also Section 2.3).
It is noteworthy that the interplay between MMPs and CD44 is not limited to cleavage events; for example, CD44 has been shown not only to be cleaved by MT1-MMP, but in some cases to complex it, anchoring it to the cytoskeleton (full-length CD44 is necessary for that).232 Other reports have also shown colocalization with (but not cleavage by) other MMPs, e.g., MMP-2,233 MMP-9233, 234 and MMP-7 complexes.235
5 Considerations for the Design of HA-Based Targeted Therapies
It is a general concept that HA-CD44 interactions can be employed for some forms of targeted therapy. This has led to the development of a large number of HA-based formulations, e.g., HA-decorated liposomes241 or nanoparticles,242 or HA nanoparticles obtained via hydrophobically driven self-assembly243 or polyelectrolyte complexation.244 However, a number of parameters that are probably critical for the biodistribution of such species are still either only partially known or substantially out of control.
Here, we present an analysis of the factors that at a systemic, tissue and cellular level can (detrimentally) affect the efficiency of a targeting strategy based on CD44/HA interactions.
5.1 Systemic Effects: Off-Target versus On-Target CD44/HA Interactions
The efficiency of a potentially tumor-targeting carrier can be significantly reduced from the physiological expression and function of CD44 in body areas that do not harbor malignancies. In this regard, macrophages are seen as the most likely off-target destination both within and outside tumoral microenvironments, due to the relatively high CD44 expression and the macrophage major role in HA uptake and degradation.245 A discussion based on the level of CD44 expression, however, is rather baseless, because CD44s affinity for HA is quite variable and dependent on a vast range of factors including the presence of an intact cytoplasmic domain,73, 246 the degree of receptor clustering,57 and, as previously discussed, the tissue-specific expression of variant isoforms and their post-translational modifications. In short, there is substantial evidence that a number of “healthy” CD44 forms have a low HA affinity, either constitutively such as CD44E78 or because of glycosylation,185, 186, 247 and there is anecdotal evidence of a higher binding of the tumor-associated forms. Nevertheless, literature offers several examples designed to improve the tumor targeting ability of HA by conjugating it with ancillary targeting ligands, such as folate248 or EGFR-targeting peptides249; as also evidenced by our group, the presentation of additional ligands, such as RGD250 or mannose,251 allows for synergic and more-than-additive targeting effects.
5.2 Systemic Effects: HA Binding to Other Receptors
- (1) TSG-6 is generally not constitutively expressed in most adult tissues, where it appears as a result of inflammation, with the few exceptions (epidermis,253 and Langerhans islets,254 astrocytes255) still probably related to high-metabolic activity and potentially inflammatory cells. Further, its role is at least partially complementary to that of CD44, for example, by crosslinking HA for a better presentation to CD44,256 and it is noteworthy that in the CNS it is produced only by CD44(+) adult rat astrocytes.255 Therefore, it could be concluded that if any interaction with TSG-6 occurs, it is likely to be beneficial for CD44 targeting.
- (2) LYVE-1 is structurally, but also functionally related to CD44, since it is endocytic257 and there is more than a parallel relationship between CD44/HA-mediated leukocyte extravasation from blood vessels and the LYVE-1/HA-mediated dendritic cells migration into lymphatic capillaries.258 LYVE-1 is found at the junctions between endothelial cells in small lymphatic capillaries, and its specificity is so high that it has also been found in lymph endothelial-mimetic macrophages.259 However, it has also been found in liver blood sinusoids (although being apparently absent in liver angiogenic capillaries and liver tumors),260 which may lead to think that LYVE-1 may provide a significant contribution to the liver accumulation of HA-displaying systems. On the other hand, it has been observed that in lymphatics LYVE-1 is downregulated under inflammatory conditions, while HA uptake is unaffected,261 suggesting therefore that LYVE-1 should have a minor influence on HA biodistribution.
- (3) HARE is expressed in endothelial cells in liver blood sinusoids, as well as lymph nodes and in the spleen, and it is geared toward HA endocytosis and eventually catabolism. Possibly because of its rapid recycling (10–15 min,262 whereas CD44 is typically not recycled,263 as also demonstrated by the plateau reached after a few hours in uptake experiments264), HARE is responsible for the physiological turnover of about one-third of the total body HA per day265; indeed its blocking with monoclonal antibodies determines a strong reduction of the liver HA clearance266 and most of the latter is indeed to be ascribed to HARE rather than to CD44 and ICAM-1.267 Importantly, to our knowledge no pathological association of HARE expression has been reported.
- (4) RHAMM is an HA receptor originally reported as a promoter of tumor cell mobility,268 which is present both extra- and intracellularly (isoforms such as RHAMMv4 are only intracellular), and in the latter case RHAMM associates to both HA and microtubules.269 Although there are reports of RHAMM-mediated mechanism of action for HA-based nanocarriers, i.e. in the treatment of tumor cells such as LnCaP,270 most literature implicitly or explicitly271 tends to discount a direct role of RHAMM in the extracellular uptake of HA, and tends to highlight a predominantly intracellular (signaling) role of RHAMM.272
- (5) ICAM-1 is present on endothelial cells in small amounts in homeostasis, but upregulated in inflammation, and has been recognized as an HA receptor since the mid-1990s.273 However, earlier reports of its endocytic character were based on the HARE misidentification as ICAM-1,273, 274 and to our knowledge no conclusive evidence of ICAM-1-mediated significant uptake of HA-based material has been reported.
In summary, HARE expression in the liver is likely to be the most important cause of off-target effects. As a possible solution, it has been suggested that the preadministration of soluble HA275 may suffice to saturate HARE and mitigate against potential side effects of a systemic HA therapy; however, the rapid HARE turnover will always be a significant hurdle.
5.3 Tissue-level Effects: HA Capture by Soluble/Vesicular CD44
Although we lack sufficient experimental evidence, soluble CD44 may fulfill a schizophrenic role. Firstly, it can simply compete with membrane receptors; alternatively, it may act as a platform for the enhanced presentation of HA to them, similarly to TSG-6. The latter interacts with HA with higher affinity (Kd = (0.2–2) × 10−6 m)276 than that of CD44 (Kd = (10–100) × 10−6 m44; please note that this refers to surface plasmon resonance measurements on receptors embedded in lipid layers, and therefore does not include avidity contributions), and delivers it in a crosslinked form that has an enhanced CD44 binding,277 possibly due to a higher avidity. Indeed, CD44RC, a soluble form of CD44 generated by alternative splicing and comprising a unique C-terminus that spans two HA-binding [B(X6)B] motifs,278 may enhance ligand interactions by binding to CnS side chains attached to membrane-anchored CD44, first by generating a multivalent complex with increased avidity for HA, but also possibly by cross-linking CnS moieties attached to different CD44 receptors, and thereby inducing their clustering.279 In our opinion, these mechanisms are particularly plausible for CD44 isoforms spanning additional CnS binding-sites, such as CD44v3 (Figure 4A).
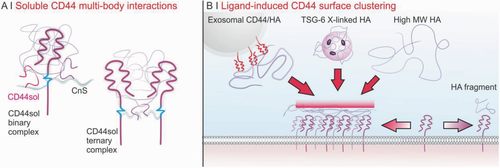
The same duality can also be presented by full-length CD44 forms embedded in vesicular structures, which have been recently reported: exosomes derived from ovarian cancer cells are enriched in CD44 and are able to transfer CD44 receptors to human peritoneal mesothelial cells, promoting metastasis.280
5.4 Cellular Effects: Avidity versus Affinity
HA and CD44 are likely to bind through multibody interactions. First, there is a point of receptor proximity: in cell membranes CD44 is virtually never molecularly dispersed, but instead it associates with lipid rafts,60, 281 with an association that seems to be strongly influenced by the expression of CD44v,28, 66, 279, 282 but not of CD44s.28 At the same time, the binding site accommodates a relative short saccharidic sequence (8 or 10 units show the same affinity44), which would therefore allow a large HA structure to bind to several CD44 receptors, i.e., to increase the avidity of the complex. Indeed, FRET experiments have confirmed that native high molecular weight HA (≈104 kDa) stimulates CD44 clustering, probably due to the multitude of possible binding sites, whereas HA oligosaccharides, which may only have 1 or 2 binding sites, ablate it (on CD44-transfected COS-7 cells, HK-2, and BT549 cells).283 This can explain why, despite the low affinity of the individual interactions (see Section 5.3), the CD44/HA complexes have a remarkably high tensile strength,284 or the high binding strength of TSG-6 crosslinked HA.
It seems reasonable to assume that the degree of CD44 clustering will increase with the size of the HA structure presented to the receptors, and also with the surface density of HA chains. Having said this, controlling the CD44 clustering induced by an HA nanocarrier structure would be very difficult, since it would require a fine control over both a) the size distribution: above a given maximal dimension, similar to the lateral size of a lipid raft, no increase in binding would be observed; b) the exposure of HA, which includes not only the accessibility of oligosaccharidic sequences, but also their surface density: an HA nanoparticle will offer a likely much higher local density of binding sites than a freely coiled HA in solution.
5.5 Cellular Effects: Unclear Link Between CD44/HA Binding and Internalization
A last, but not less important and often neglected aspect is the role that (clustered) CD44 may have in the internalization of an HA-based nanocarrier. For example, in a recent study we have reported a negative correlation between HA internalization rate and CD44 expression in differently polarized THP-1 macrophages: while the binding of HA (soluble or on nanoparticles) increased with increasing CD44 levels from M0 to M1 macrophages, the rate of endocytosis decreased.27 A possible cause may be the more difficult internalization with increasing degree of clustering, and this hypothesis may also explain why the rate of CD44 internalization in chondrocytes decreases upon binding of native HA.285 Although these events may easily be cell-specific, they highlight that if and when an intracellular delivery is required, high binding strength may be good for targeting, but not necessarily to rapidly achieve a pharmacological effect.
6 Conclusion
CD44 is an intensively studied receptor. Yet, despite the detailed knowledge accumulated on its structure, on its post-transcriptional variability and also on its post-translational modifications, we still lack a full understanding on how these parameters influence three probably very inter-related phenomena: a) the association of CD44 to additional membrane proteins, b) the CD44 clustering, and c) the binding to HA-presenting materials. On the top of this, we have also only rudimental knowledge of the possible off-target effects (binding to other receptors or to “healthy” CD44 forms) and to what happens during and after the CD44/HA binding (control over clustering and endocytosis mechanisms).
A first conclusion is that, due to our still rudimental understanding of the dynamics of the CD44/HA interactions in both pericellular and membrane-bound locations, there is a need for both cellular and acellular models that account for multibody interactions. A second point is that cellular models need also to be optimized to yield physiologically (pathologically) relevant CD44 scenarios; since a basic parameter such as the CD44s/CD44v expression ratio can dramatically vary depending on culture conditions, any sophisticated in vitro data can easily lose any in vivo relevance. Third, but most relevant for nanomaterial scientists, specific attention needs to be paid to preparative methods, since critical parameters such as size distribution and ligand exposure are controlled not thermodynamically, but by the process conditions.
Acknowledgements
J.M.R.d.l.R. is indebted to EPSRC for a Ph.D. studentship (part of the North-West Nanoscience (NoWNano) Doctoral Training Centre, EPSRC grant EP/G03737X/1).
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Julio M. Rios de la Rosa obtained his B.Sc. in biotechnology at Universidad Pablo de Olavide in 2013. In the same year, he joined the University of Manchester as part of the NoWNANO Doctoral Training Centre, recently completing his Ph.D. thesis. Dr. Rios de la Rosa is now Research Associate at NoWCADD, a translational center based at the University of Manchester and funded through AstraZeneca. His current research focuses on the characterization of colloidal and macromolecular carriers.

Annalisa Tirella has been working for the design and fabrication of engineered in vitro systems for the investigation of cell-material interaction in healthy and pathological states since her Ph.D. In 2014, she joined the University of Manchester and she is currently a lecturer in pharmaceutics in the Division of Pharmacy and Optometry. Her research focuses on physicochemical and mechanical properties of biomaterials, with particular interest on the role played by material stiffness and 3D architecture in cancer development. The ultimate goal of her research is to develop in vitro models able to better recapitulate the tumor microenvironment at different stages of development, and speed up the preclinical phase for chemotherapeutics.

Nicola Tirelli graduated in chemistry in 1992 at the University of Pisa (Italy) and holds a Ph.D. in industrial chemistry from the same institution. After a few years at the ETH Zurich, he joined the University of Manchester in 2003, becoming Full Professor in 2005. He currently holds a joint position with the Italian Institute of Technology in Genova. His research bridges polymer chemistry with drug delivery and regenerative medicine, with a strong focus on molecular mechanisms.




