NMDAR-Antibody Encephalitis Diagnosed With Primary Central Nervous System Lymphoma: A Case Series
Funding: The authors received no specific funding for this work.
Kon Chu and Cynthea Prima contributed equally as co-first authors.
ABSTRACT
N-methyl-D-aspartate receptor-antibody encephalitis (NMDAR encephalitis) is one of the most common forms of autoimmune encephalitis, with a paraneoplastic relationship described in approximately 38%. Primary central nervous system lymphoma (PCNSL) is a rare hematologic malignancy that is not often considered as the underlying neoplasm in this autoimmune disease. We report three cases of suspected NMDAR encephalitis diagnosed in the context of PCNSL. Our cases highlight clinical features to suspect the coexistence of these two rare conditions and demonstrate the potential role PCNSL can play as the paraneoplastic source condition.
1 Introduction
N-methyl-D-aspartate receptor (NMDAR)-antibody encephalitis (NMDAR encephalitis) is one of the most common and serious forms of autoimmune encephalitis. Key symptoms include memory loss, psychosis, seizures, aphasia, altered mentality, dyskinesia, autonomic dysfunction, and central hypoventilation [1]. Association exists between NMDAR encephalitis and an underlying neoplastic condition, with approximately 38% of cases exhibiting this paraneoplastic relationship. Ovarian teratoma is most common, followed by rarer cases of small cell lung carcinoma and renal cell carcinoma [1-3]. There are few reports of NMDAR encephalitis with concurrent systemic B-cell lymphoma [4-6].
In this case series, we present three cases of patients with suspected NMDAR encephalitis who later proved to have concomitant primary central nervous system lymphoma (PCNSL). The presented cases demonstrate that PCNSL may coexist with NMDAR encephalitis and, in some cases, may potentially serve as a source for a paraneoplastic reaction. Moreover, the importance of adopting a multidisciplinary approach in diagnosis is underscored.
2 Subject and Methods
Patient characteristics are summarized in Table 1.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age/Sex | 71/Male | 55/Female | 34/Male |
| Clinical presentation | |||
| Presenting symptoms | Dizziness, gait disturbance, psychosis, altered mental status | Memory impairment, incoherent speech | Leg weakness, visual impairment, aphasia, abulia, irritability, cognitive impairment |
| Symptoms related to NMDARe | Psychosis, altered mental status, seizures | Cognitive impairment, speech disturbance | Aphasia, abulia, irritability, cognitive impairment |
| mRS at presentation/last follow-up | 5/5 | 3/2 | 3/4 |
| Laboratory findings | |||
| CSF cell count (mm3) | 15 | 10 | 14 |
| NMDAR antibody | Positive→not repeated | Positive→negative | Positive→negative |
| MRI findings | Diffuse infiltrative T2 high signal intensities in the bilateral subcortical white matter with diffusion restriction and subtle enhancement | Multifocal T2 high signal intensities in bilateral cerebral white matter with subtle patchy enhancement | Diffuse infiltrative T2 high signal intensities at right corona radiata, basal ganglia and thalamus, and at left basal ganglia, superior cerebellar peduncle, midbrain, both cerebellum and pineal gland |
| Lymphoma characteristics | |||
| Pathologic subtype | Diffuse large B-cell lymphoma | Diffuse large B-cell lymphoma | Diffuse large B-cell lymphoma |
| EBV ISH | Positive | Negative | Negative |
| Lymphoma NGS panel results | Negative | SNV/INDEL: Myd88, Irf4, Ezh2, Socs1, Tp53, Gna13 |
SNV/INDEL: Myd88, Pik3r1, Pim1, Ccnd3, Pten CNV: Myd88, Rhoa, Notch1, Crebbp |
| Treatment | |||
| Chemotherapy | Rituximab, methotrexate, vincristine, procarbazine | Rituximab, methotrexate, vincristine, procarbazine, hippocampal sparing whole brain radiotherapy | Rituximab, methotrexate, vincristine, procarbazine, autologous SCT |
| Immunotherapy | Steroid pulse, IVIG, rituximab, tocilizumab | Steroid pulse, IVIG | Steroid pulse, IVIG |
- Abbreviations: CNV, copy number variation; CSF, cerebrospinal fluid; EBV, Ebstein-Barr virus; EEG, electroencephalogram; INDEL, insertion–deletion; ISH, in situ hybridization; IVIG, intravenous immunoglobulin; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NGS, next generation sequencing; NMDARe, anti-N-methyl-D-aspartate receptor encephalitis; SCT, stem cell transplantation; SNV, single nucleotide variation.
In all three cases, patient samples were tested for anti-NMDAR antibodies with both rat hippocampal tissue indirect immunofluorescence assays and cell-based assays using commercial kits (Euroimmun, Lübeck, Germany).
2.1 Case 1
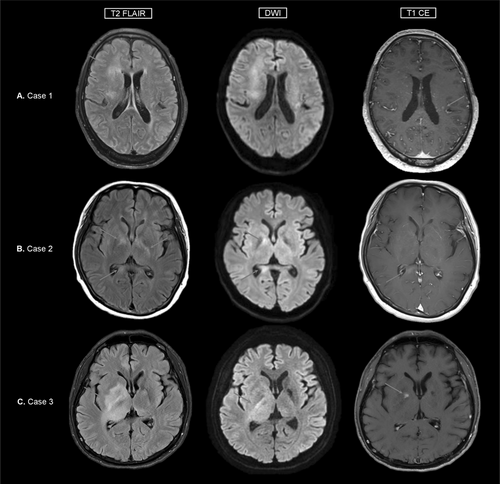
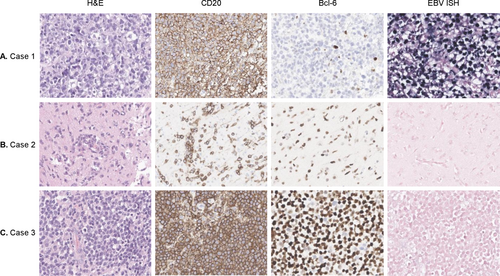
A 71-year-old male presented with progressive symptoms of dizziness, gait disturbance, psychosis, and altered mental status over 3 months. Initial testing for autoimmune antibodies proved negative, and the patient was treated with intravenous (IV) methylprednisolone, which only showed a temporary effect. On referral, he was drowsy and only capable of making incomprehensible sounds. Brain magnetic resonance imaging (MRI) revealed diffuse infiltrative T2 high signal intensities in the bilateral subcortical white matter with diffusion restriction and subtle enhancement (Figure 1A). Cerebrospinal fluid (CSF) findings showed mild lympho-dominant pleocytosis with protein elevation (15 leukocytes/μL, protein 145 mg/dL) and cytology was negative for malignant cells. Fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET) showed decreased uptake values in the lesion. Re-testing for autoimmune antibodies proved positive for NMDAR in CSF and serum, and the patient was treated sequentially with IV immunoglobulin (IVIG) and two cycles of rituximab and tocilizumab each. Despite intensive treatment, symptoms did not improve, and on follow-up imaging, multiple rim enhancing mass-like lesions newly appeared. Stereotactic brain biopsy results were consistent with Ebstein-Barr virus (EBV)-positive diffuse large B-cell lymphoma (DLBCL) (Figure 2A) and no abnormalities were found in the lymphoma next generation sequencing (NGS) panel. The patient was transferred to medical oncology for chemotherapy and received four doses of rituximab, methotrexate, vincristine, and procarbazine (R-MVP). Autoantibody testing was not repeated after transfer. On last follow-up, brain MRI showed a decrease in lesion size, but no improvement of symptoms was observed with a final modified Rankin Scale (mRS) score of 5.
2.2 Case 2
A 55-year-old female presented with symptoms of memory impairment and incoherent speech over a month. She was initially diagnosed with encephalitis and treated with IV methylprednisolone and IVIG without improvement of symptoms. The patient was admitted to our institution for positive anti-NMDAR antibodies in serum and CSF testing. Initial examination showed inattention and impaired cognition. In the serum complete blood count, bicytopenia was present. CSF findings showed 10 leukocytes/μL and protein elevation of 57 mg/dL. Brain MRI showed multifocal T2 high signal intensities in bilateral cerebral white matter with subtle patchy enhancement (Figure 1B). Fluorine-18 FDG PET imaging of the brain showed multifocal hypermetabolic lesions. A bone marrow biopsy, skin punch biopsy, and brain biopsy via mini-craniotomy were performed. Among these, only the brain biopsy was consistent with EBV-negative DLBCL (Figure 2B), leading to a diagnosis of PCNSL. Lymphoma NGS panel showed variants in Myd88, Klhl6, Irf4, Pim1, Ezh2, Socs1, Tp53, and Gna13 genes. Upon transfer to medical oncology, the patient received 6 doses of R-MVP, after which complete resolution and negative seroconversion of NMDAR antibody were achieved. The patient is currently receiving intraocular methotrexate due to ocular relapse of lymphoma, but otherwise has no problems with memory and speech, with an mRS score of 2.
2.3 Case 3
A 34-year-old male presented with leg weakness, visual impairment, abulia, aphasia, irritability, and cognitive decline over a month. Brain MRI showed diffuse T2 high signal intensities with swelling at the right frontal area, internal capsule, basal ganglia, thalamus, midbrain, and both cerebella (Figure 1C). Fluorine-18 FDG PET imaging of the brain showed hypermetabolic lesions. Vitreous biopsy showed a few atypical cells with positive IgH gene rearrangement tests. CSF findings showed 14 leukocytes/μL, protein 31 mg/dL, and were positive for IgH gene rearrangement, with some of the monoclonal peaks identical to vitreous fluid. CSF and serum anti-NMDAR antibody proved positive. Brain biopsy was not feasible at this point, so the patient was first treated with IVIG and steroid and followed up. Over 3 months, symptoms progressively worsened, and repeat brain MRI showed an increase in diffuse infiltrative T2 high signal intensity lesions. Subsequent brain biopsy was consistent with EBV negative DLBCL (Figure 2C). Lymphoma NGS panel showed variants in Myd88, Pik3r1, Pim1, Ccnd3, Pten, Rhoa, Notch1, and Crebbp genes. The patient was transferred to medical oncology and treated with 6 doses of R-MVP, after which he achieved complete resolution. For NMDAR encephalitis, the patient received 3 doses of IVIG monthly until the re-test for anti-NMDAR antibody was negative. Although the patient is wheelchair bound with a mRS score of 4, his cognition and dysarthria showed marked improvement. He is currently undergoing autologous stem cell transplant for consolidation therapy.
3 Discussion
In this case series, we present three cases of suspected NMDAR encephalitis with pathologically confirmed PCNSL. Our cases demonstrate that PCNSL may be an alternative diagnosis hidden behind positive anti-NMDAR antibodies, potentially serving as a paraneoplastic source. The importance of taking a comprehensive approach in interpreting autoantibodies is underscored.
Detection of anti-NMDAR antibodies, especially in CSF, is crucial to diagnosing NMDAR encephalitis [7, 8]. These antibodies are thought to be pathogenic by binding to the GluN1 subunit of NMDA receptors and causing alterations in their synaptic dynamics [7]. In our presented cases, it is unclear to which extent the antibodies contributed to the patients' symptoms. Indeed, the antibodies might have been completely irrelevant, as neuropsychiatric manifestations are common in PCNSL [9]; or, the autoantibodies could have been capable of causing or aggravating symptoms. Whichever the case, it is important to keep in mind that an alternative, neoplastic etiology may be present in patients with positive antibodies and suspected NMDAR encephalitis, especially when atypical features are encountered.
Anti-NMDAR antibodies in patients with confirmed CNS malignancies have already been reported by previous studies. Lu et al. reported a 67-year-old man presenting with partial seizures, who was diagnosed with astrocytoma and anti-NMDAR antibodies [10]. Mariotto et al. reported a 54-year-old woman presenting with cognitive impairment, who had positive antibodies but was diagnosed as lymphomatosis cerebri [11]. The mechanism behind autoantibody production in these cases may be neuronal release of cryptic autoantigens due to tissue damage by tumor cell infiltration, similar to the mechanism in post-herpes simplex NMDAR encephalitis [12].
There are also reports of concomitant anti-NMDAR antibodies in patients diagnosed with lymphoma, confined to the CNS or not [4-6]. In the few cases where lymphoma was limited to the CNS, the pathologic subtype was DLBCL [4, 5]. In the literature, there are studies that suggest a potential association between DLBCL and autoimmune diseases [13]. The reprogramming of B cells that occurs in DLBCL oncogenesis may potentially lead to immune escape of autoantibody-producing B cells. This leads us to speculate that the anti-NMDAR antibodies in our patients could be a result of a paraneoplastic reaction with PCNSL as the source, although there is a possibility that the two conditions coexisted purely by chance. While it is well-studied that numerous genes encoded by EBV can cause immortalization and immune escape of B cells, each leading to oncogenesis and autoantibody production [14], the implication of EBV is unclear in our cases, there being only one EBV-positive case.
When there is a hidden neoplastic condition, it is important to never miss diagnosis, since the prognosis and treatment can be entirely different. While the subacute and nonspecific symptoms of PCNSL can make it difficult to differentiate from NMDAR encephalitis, prognosis differs significantly, with a low 5-year survival rate of 30.1% in PCNSL [15] as compared to mortality of 5%–10% in NMDAR encephalitis [1]. Moreover, PCNSL requires anti-cancer treatment while the mainstay of NMDAR encephalitis treatment is immunotherapy [7]. Thus, even when autoantibody results turn out positive, they need to be interpreted with caution, taking the total clinical picture into account. Our cases illustrate some atypical features that should raise red flags. Older age at presentation may provide a clue as PCNSL is increasingly diagnosed in elderly patients. None of our patients had dyskinesia nor autonomic dysfunction commonly found in NMDAR encephalitis patients. Clinical deterioration (symptoms, imaging) despite immunotherapy may also provide insight. Ancillary test results can also be helpful. In patients with visual impairment, an ophthalmologic exam can be a non-invasive way of discovering intraocular lymphoma involvement. Atypical findings in imaging, such as diffuse infiltrative lesions with diffusion restriction in brain MRI, should prompt further investigation and be utilized in target selection for brain biopsy, which is essential for diagnosis and treatment decision based on histological subtype.
In conclusion, PCNSL can infrequently coexist in patients with suspected NMDAR encephalitis, and should be listed as a differential diagnosis when atypical features are encountered. Additional diagnostic measures such as brain biopsy are essential to confirm its presence.
Author Contributions
The author takes full responsibility for this article.
Acknowledgments
The authors have nothing to report.
Disclosure
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




