EEG Response to Sedation Interruption Complements Behavioral Assessment After Severe Brain Injury
Funding: This study was funded by a McGill/Western Collaboration Grant from Healthy Brain for Healthy Lives and BrainsCAN (1a-5a-01) and by the Canadian Institutes of Health Research (CIHR, Project Grant 480995). C.M. is funded through Fonds de recherche du Québec–Santé (FRQS). This research is supported in part by the FRQNT Strategic Clusters Program (2020-RS4-265502—Centre UNIQUE—Union Neurosciences and Artificial Intelligence—Quebec) (awarded to C.M.). A.M.O. is supported by the Canadian Institutes of Health Research (CIHR, #408004) and is a Fellow of the CIFAR Brain, Mind, and Consciousness program. C.D. is funded through an FRQS Junior 1 Scholar Award, a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC), a CIFAR Global Scholars award, and institutional start-up funding from the Research Centre of the Centre intégré universitaire de santé et de services sociaux (CIUSSS) du Nord-l'Île-de-Montréal. S.B.-M. is funded by a Canada Research Chair (Tier II) and a NSERC Discovery Grant (RGPIN-2023-03619). Canada First Research Excellence Fund (CFREF).
This article has been uploaded to medRXiv preprint server: https://www.medrxiv.org/content/10.1101/2024.10.02.24314815v1.
ABSTRACT
Objective
Accurate assessment of the level of consciousness and potential to recover in patients with severe brain injury underpins crucial decisions in the intensive care unit but remains a major challenge for the clinical team. The neurological wake-up test is a widely used assessment tool. However, many patients' behavioral responses during a short interruption of sedation are ambiguous or absent, yielding little prognostic value. This study assesses the brain's electroencephalogram response during an interruption of propofol sedation to complement behavioral assessment during the neurological wake-up test to predict survival, recovery of consciousness, and long-term functional outcomes in patients with acute severe brain injury.
Methods
We recorded 128-channel EEG from 41 severely brain-injured patients during a clinically indicated neurological wake-up test. Behavioral assessment was performed before and after interruption of propofol sedation, using the Glasgow Coma Scale. Brain response to sedation interruption was quantified using EEG power, spatial ratios, and the spectral exponent.
Results
Recovery of responsiveness during the neurological wake-up test is reflected in participants' brain response to sedation interruption. Electrophysiological patterns can be decoupled from participant behavioral response, with some individuals demonstrating neurophysiological signs of waking up despite an absent behavioral response. Using the brain response to complement behavioral assessment improved prognostic value, distinguished patients according to survival and outperformed outcome predictions of the patients' attending physician.
Interpretation
EEG can complement behavioral assessment during the neurological wake-up test to improve prognostication, inform clinicians, family members, and caregivers, and to set realistic goals for treatment and therapy.
1 Introduction
The accurate assessment of levels of consciousness and potential for recovery in patients with severe brain injury underpins the most crucial clinical decisions in the intensive care unit (ICU), but remains a major challenge for the clinical team. Behavioral assessments, such as the Glasgow Coma Scale (GCS) [1], are widely used to evaluate patients. However, patients with acute brain injury patients often require continuous sedation [2, 3], which limits the clinical team's ability to conduct an accurate assessment [4]. It has therefore become routine practice to interrupt sedation at least once a day to allow patients to wake up for a neurocognitive assessment: this practice is known as the neurological wake-up test (NWT) [2-4]. The presence of behavioral responsiveness during the NWT is a key indicator for good long-term recovery [5, 6]. However, many patients show an absent or ambiguous behavioral response that has less diagnostic or prognostic value, as it may be caused by factors ranging from sensory-motor impairments to pain and fatigue [7-9].
A multitude of methods using electrophysiology and neuroimaging techniques have been developed to assess patients [10-13] and detect consciousness [14-16] without relying on a behavioral response. However, most methods require patients to tolerate an extended period without sedation and are therefore not suited for acute severely brain-injured patients, for whom even a short interruption of sedation may cause increased levels of stress and clinical risk [17, 18]. Our research group has previously shown that the brain's EEG response to propofol anesthesia induction can successfully be used for the assessment of unresponsive patients [19-22]. In this study, we propose a novel assessment technique, using the electroencephalographic (EEG) response to sedation interruption to complement the behavioral assessment and improve prognostication of patient survival and functional recovery.
We hypothesize that there will be a dynamic change in EEG power, spatial ratios, and spectral exponents [23-25] in the patients who regain responsiveness during the NWT. We further hypothesize that changes in these features can be decoupled from the patients' behavioral response, with some patients showing a dynamic EEG change despite an absent behavioral response. Finally, we hypothesize that the EEG response during the NWT improves prognostication for survival and functional outcome of patients who show absent or ambiguous behavioral responses.
EEG monitoring is routinely used to assess the potential for neurological recovery [26]. Our approach aims to propose a novel way of capturing dynamic and reactive electrophysiological features in an acute setting, while being resource- and time-efficient and thus highly translatable to clinical practice. In current practice, early prognostication of patients with brain injuries remains an “uncertain art and science” [27]; our study aims to support clinical decision making by providing a tool that complements behavioral assessment with neurological markers of return of consciousness.
2 Methods
2.1 Participants
This study was part of a larger protocol, previously published by Duclos et al. [28]. Patients were recruited from four different intensive care units in Canada and included if they were at least 18 years old, within 14 days of ICU admission, and continuously sedated following a brain injury (Supporting Information: Methods). All patients were unresponsive at the time of recruitment. Therefore, written informed consent for study participation was provided by the patient's legal representative family member, in accordance with the Declaration of Helsinki. Since 2020, a total of 1878 patients were screened; 183 patients were eligible for the study; consent was obtained for 53 patients; and EEG data were acquired from 47 patients. Six patients were excluded due to increased levels of agitation and head movement. The remaining 41 patients (28 male, 13 female), ranging from 21 to 88 years (mean 54.78, SD 19.30) were included in the analysis (18 patients with anoxic brain injury, 12 stroke patients, 8 TBI patients, 3 other etiologies, see Table 1). The time between admission to the ICU and EEG data acquisition ranged from 0 to 11 days (mean = 2.98, SD 2.04). The score on the GCS at the time of data acquisition ranged from 3T to 9T (mean 4.83, SD 2.06, see Tables 1 and 2). The APACHE-II score ranged from 8 to 44 (n = 38, mean 19.21, SD 8.26). All patients were intubated at the time of recording. The study was approved by the Research Ethics Board of the McGill University Health Centre (Project ID 2020-5972) and the Western University Health Science Research Ethics Board (Project ID 114303).
| ID | Age | Sex | Etiology | Days since admission | APACHE II score | Prop. dose (μg/kg/min) | GCS Phase 1 (E, V, M) | GCS Phase 3 (E, V, M) | Out 1 surv. | Out 2 resp. | Out 3 GOSE | Out 4 DRS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40–44 | M | Stroke | 3 | n/a | 30 | 4T (1, 1T, 2) | 4T (1, 1T, 2) | W | N | W | W |
| 2 | 40–44 | M | Stroke | 6 | 14 | 50 | 7T (1, 1T, 5) | 10T (4, 1T, 5) | S | Y | 3 | 13 |
| 3 | 60–64 | F | Stroke | 2 | 27 | 33 | 6T (1, 1T, 4) | 6T (1, 1T, 4) | D | Y | 1 | n/a |
| 4 | 65–70 | F | Stroke | 1 | 17 | 30 | 7T (1, 1T, 5) | 7T (1, 1T, 5) | D | Y | 1 | n/a |
| 5 | 55–59 | F | Anoxic | 5 | n/a | 33 | 3T (1, 1T, 1) | 3T (1, 1T, 1) | S | Y | — | — |
| 6 | 55–59 | M | Anoxic | 3 | 25 | 83 | 3T (1, 1T, 1) | 3T (1, 1T, 1) | S | Y | — | — |
| 7 | 45–49 | F | Anoxic | 1 | 24 | 16 | 3T (1, 1T, 1) | 6T (1, 1T, 4) | W | N | n/a | n/a |
| 8 | 65–69 | F | Stroke | 4 | 12 | 30 | 7T (2, 1T, 4) | 10T (3, 1T, 6) | S | Y | 6 | 1 |
| 9 | 75–79 | F | Other | 6 | 18 | 25 | 3T (1, 1T, 1) | 7T (2, 1T, 4) | S | Y | 8 | 0 |
| 10 | 40–45 | M | Stroke | 2 | 14 | 50 | 8T (2, 1T, 5) | 10T (3, 1T, 6) | S | Y | 7 | 2 |
| 11 | 50–54 | F | Stroke | 3 | 14 | 50 | 6T (1, 1T, 4) | 8T (2, 1T, 5) | S | Y | 3 | 8 |
| 12 | 50–54 | M | Anoxic | 2 | 34 | 50 | 3T (1, 1T, 1) | 4T (1, 1T, 2) | S | Y | 7 | 6 |
| 13 | 80–84 | M | Stroke | 5 | 17 | 20 | 6T (—, 1T, —) | 6T (—, 1T, —) | W | Y | n/a | n/a |
| 14 | 80–84 | M | Stroke | 1 | 16 | 25 | 7T (1, 1T, 5) | 8T (1, 1T, 6) | S | Y | 3 | 7 |
| 15 | 30–34 | M | Anoxic | 1 | 44 | 66 | 3T (1, 1T, 1) | 3T (1, 1T, 1) | D | Y | 1 | — |
| 16 | 25–29 | M | TBI | 2 | 8 | 50 | 6T (1, 1T, 4) | 6T (1, 1T, 4) | S | Y | 7 | 1 |
| 17 | 60–64 | M | Anoxic | 2 | 21 | 66 | 3T (1, 1T, 1) | 10T (4,1T, 5) | S | Y | 8 | 0 |
| 18 | 60–64 | M | Anoxic | 2 | 28 | 50 | 3T (1, 1T, 1) | 11T (4, 1T, 6) | S | Y | 6 | 3 |
| 19 | 85–89 | M | TBI | 4 | 17 | 20 | 6T (1, 1T, 4) | 6T (1, 1T, 4) | W | N | n/a | n/a |
| 20 | 50–54 | F | Anoxic | 2 | 22 | 50 | 3T (1, 1T, 1) | 7T (3, 1T, 3) | S | Y | 3 | 6 |
| 21 | 20–24 | M | TBI | 4 | 12 | 40 | 7T (1, 1T, 5) | 6T (1, 1T, 4) | S | Y | 8 | 0 |
| 22 | 65–69 | M | Anoxic | 2 | 26 | 50 | 3T (1, 1T, 1) | 11T (4, 1T, 6) | S | Y | 5 | 4 |
| 23 | 35–39 | M | Stroke | 5 | 11 | 25 | 4T (1, 1T, 2) | 4T (1, 1T, 2) | D | N | 1 | — |
| 24 | 80–84 | M | Anoxic | 2 | 26 | 66 | 3T (1, 1T, 1) | 3T (1, 1T, 1) | D | N | 1 | — |
| 25 | 30–34 | M | Anoxic | 3 | 9 | 50 | 3T (1, 1T, 1) | 8T (4, 1T, 3) | W | N | n/a | n/a |
| 26 | 35–39 | M | Anoxic | 0 | 21 | 33 | 3T (1, 1T, 1) | 3T (1, 1T, 1) | W | N | n/a | n/a |
| 27 | 85–89 | M | TBI | 1 | 14 | 20 | 6T (1, 1T, 4) | 9T (3, 1T, 5) | D | N | 1 | — |
| 28 | 65–69 | F | Anoxic | 1 | 29 | 16 | 6T (2, 1T, 3) | 8T (2, 1T, 5) | D | Y | 1 | — |
| 29 | 35–39 | F | TBI | 3 | 9 | 60 | 9T (3, 1T, 5) | 11T (4, 1T, 6) | S | Y | 4 | 5 |
| 30 | 35–39 | M | TBI | 5 | 13 | 50 | 7T (1, 1T, 5) | 7T (1, 1T, 5) | S | Y | 3 | 5 |
| 31 | 65–69 | M | Anoxic | 2 | 18 | 33 | 3T (1, 1T, 1) | 3T (1, 1T, 1) | S | Y | — | — |
| 32 | 40–44 | M | Stroke | 7 | 20 | 35 | 3T (1, 1T, 1) | 4T (1, 1T, 2) | W | N | n/a | n/a |
| 33 | 75–79 | M | Anoxic | 2 | 23 | 33 | 3T (1, 1T, 1) | 3T (1, 1T, 1) | W | N | n/a | n/a |
| 34 | 60–64 | M | Anoxic | 1 | 37 | 33 | 3T (1, 1T, 1) | 11T (4, 1T, 6) | S | Y | — | — |
| 35 | 30–34 | F | Anoxic | 2 | n/a | 50 | 3T (1, 1T, 1) | 6T (—, 1T, —) | W | N | n/a | n/a |
| 36 | 85–89 | M | Stroke | 3 | 21 | 25 | 5T (1, 1T, 3) | 11T (4, 1T, 6) | D | Y | 1 | — |
| 37 | 55–59 | M | Other | 3 | 23 | 20 | 3T (1, 1T, 1) | 5T (3, 1T, 1) | S | Y | 8 | 6 |
| 38 | 80–84 | M | Other | 3 | 15 | 30 | 9T (3, 1T, 5) | 10T (3, 1T, 6) | W | Y | n/a | n/a |
| 39 | 40–44 | F | Anoxic | 3 | 13 | 40 | 3T (1, 1T, 1) | 9T (4, 1T, 4) | S | N | 2 | 20 |
| 40 | 35–39 | F | TBI | 2 | 8 | 50 | 6T (1, 1T, 4) | 9T (3, 1T, 5) | S | Y | 8 | 0 |
| 41 | 20–24 | M | TBI | 11 | 10 | 50 | 9T (4, 1T, 4) | 9T (4, 1T, 4) | S | Y | 3 | 8 |
- Note: ID: patient identifier; sex (M, male; F, female); Prop: propofol concentration in μg/kg/min, score of Glasgow Coma Scale (GCS) in Phase 1 (sedation baseline) and Phase 3 (sedation interruption) (E: eye opening, V: verbal response, M: motor response, T: intubated); Outcome 1: survival (W: withdrawn from life sustaining treatment, S: survived, D: deceased), Outcome 2: responsiveness (N: no—did not regain responsiveness, Y: yes—did regain responsiveness), Outcome 3: maximal score on Glasgow outcome scale-extended (GOS-E), Outcome 4: minimal score on Disability Rating Scale (DRS).
| N | N | N | N | GOS-E | DRS | |
|---|---|---|---|---|---|---|
| Regained responsiveness | Did not regain responsiveness | Follow-up calls | ||||
| Group A (n = 9) | ||||||
| Survivors | 7 | 7 | 0 | 6 (1 missed) |
Mean: 5.16 SD: 1.34 Range: 3–7 |
Mean: 3.67 SD: 1.97 Range: 1–7 |
| Non-survivors | 1 | 1 | 0 | — | GOSE = 1 | N.A. |
| WLST | 1 | 1 | 0 | — | — | — |
| Group B (n = 13) | ||||||
| Survivors | 8 | 7 | 1 | 8 |
Mean: 5.37 SD: 2.64 Range: 2–8 |
Mean: 6.63 SD: 6.65 Range: 0–20 |
| Non-survivors | 2 | 1 | 1 | — | GOSE = 1 | N.A. |
| WLST | 3 | 0 | 3 (excluded) | — | — | — |
| Group C (n = 19) | ||||||
| Survivors | 8 | 8 | 0 | 5 (3 missed) |
Mean: 5.6 SD: 2.15 Range: 3–8 |
Mean: 4.0 SD: 3.03 Range: 0–8 |
| Non-survivors | 5 | 3 | 2 | — | GOSE = 1 | N.A. |
| WLST | 6 | 1 | 5 (excluded) | — | — | — |
- Abbreviations: DRS, Disability Rating Scale; GOS-E, Glasgow Outcome Scale-Extended; N, number of participants; WLST, withdrawal of life-sustaining treatment.
2.2 Electroencephalography Data and Anesthetic Protocol
EEG data were recorded in five phases: (1) sedation baseline (i.e., during continuous sedation); (2) decreasing sedation level (i.e., during the transition after interruption of sedation); (3) sedation interruption; (4) increasing sedation level (i.e., during the transition after re-initiation of sedation) and (5) sedation reinstated (i.e., after resumption of continuous sedation) (see Figure 1 for schematic illustration of the recording protocol). Weaning of sedation was clinically indicated either with the aim to completely stop sedation or to perform a neurophysiological assessment. Decisions about the length of sedation interruption or necessity to reinitiate sedation were made by the attending nurse based on standard-of-care risk assessment and were not affected by study enrollment and EEG data acquisition. Thus, only the length of Phase 1 (sedation baseline) was standardized at 10 min. The total length of the protocol and dose of sedation varied between patients according to standard of care (Supporting Information: Methods). EEG data were recorded using a high-density 128-channel saline net and an Amps 400 amplifier (Electrical Geodesic Inc., USA). Data were recorded at a sampling rate of 1 kHz and were referenced to the vertex; electrode impedances were reduced to below 5 kΩ prior to recording. Preprocessing was performed using MNE python [29] (Supporting Information: Methods).
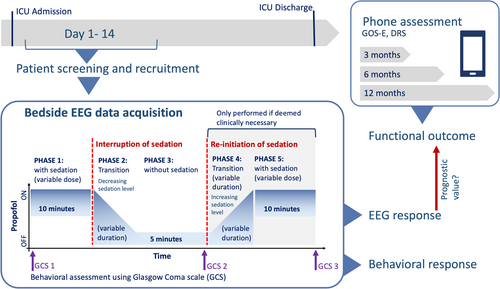
Patient responsiveness was assessed by the attending nurse using the GCS [1] three times during the protocol: before Phase 1 (sedation baseline); at the end of Phase 3 (sedation interruption); and at the end of Phase 5 (sedation reinstated) (Figure 1). Analysis was performed separately on the interruption of sedation (i.e., Phase 1–3, n = 41) and the re-initialization of propofol (i.e., Phase 3–5, n = 21, see Supporting Information: Methods). In addition to the EEG analysis, the raw EEG traces were evaluated by a trained neurologist according to the ACNS 2021 guidelines [30]. Ratings are provided in the Supporting Information: Methods (Table S2).
2.3 Feature Extraction
The brain response to the interruption of sedation was estimated using three families of EEG markers: (1) spectral power; (2) spatial ratios; and (3) the spectral exponent. Relative power was calculated in five frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), low beta (13–20 Hz) and high beta (20–30 Hz). Total EEG power was estimated from 1 to 45 Hz. Power spectral density between 1 and 45 Hz was estimated using the Welch algorithm with a 2 s window and 50% overlap. Aperiodic component and offset of the power spectral density were estimated using the “fitting oscillation and one over f” algorithm [31] in the 1–45 Hz range (min_peak_height = 0.1, max_n_peaks = 3, aperiodic_mode = “fixed”).
All features were calculated on each 10 s epoch and channel individually. We then summarized features by extracting: (1) the space-averaged feature strength, (2) the standard deviation of the feature over space, and (3) the posterior–anterior ratio [23].
2.4 EEG Dynamic Change
The EEG dynamic change was defined as the amount of change, weighted by a certainty score. The amount of change was defined as the difference between each feature's Phase 1 (sedation baseline) average and Phase 3 (sedation interruption) average (i.e., Phase 1–3; negative values indicating an increase in response to sedation interruption) (see Figure 2, amount of change). The certainty score was defined by the proportion of datapoints post-interruption (i.e., Phase 2 and 3) that were one standard deviation above or below the Phase 1 (sedation baseline) features (see Figure 2, certainty of change). The resulting value indicates whether differences in the EEG feature were induced by the interruption of sedation or by spontaneous, endogenous fluctuations (Supporting Information: Methods).
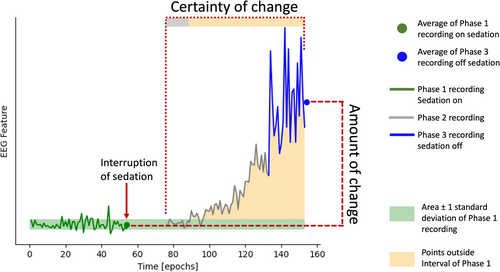
EEG dynamic change was calculated individually on the mean, standard deviation, and posterior–anterior ratio of each feature (i.e., total power, delta, theta, alpha, low beta, high beta, exponent and offset), yielding a total of 24 features (i.e., eight mean, eight standard deviation, eight posterior–anterior ratio).
2.5 Grouping According to Behavioral Response
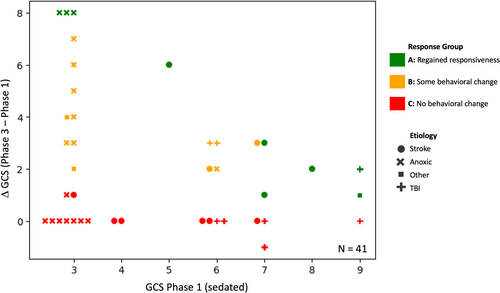
Participants' change in behavioral responsiveness (ΔGCS) during the NWT was defined as the difference between GCS score off sedation and pre-interruption of sedation: ΔGCS = GCSPhase3 − GCSPhase1 (Figure 1). ΔGCS and GCSPhase3 were used to categorize patients into three groups: Nine patients regained ability to follow commands during the NWT and were assigned to “Group A: regained responsiveness” (see Figure 3, green markers; see Table 1). Thirteen patients showed increased levels of behavior during interruption of sedation (i.e., ΔGCS ≥ 2), despite not following commands and were categorized as “Group B: Some behavioral change” (see Figure 3, orange markers). Nineteen patients did not show any or only minimal behavioral change during NWT (i.e., ΔGCS < 2) and were assigned to “Group C: No behavioral change” (see Figure 3, red markers).
2.6 Machine Learning Analysis
We first trained a Linear Ridge regression [32] to predict the ΔGCS of Group A participants based on their EEG dynamic change. For the training, participants' age and propofol concentration were combined with the 24 EEG features. Model fit was evaluated using mean absolute error between true and predicted ΔGCS. The optimal regularization parameter was determined using a leave-one participant out cross-validation. A good model performance indicates that the model has learned to identify EEG patterns that are related to behavioral responsiveness during the NWT.
We then used the model to predict the change in GCS (ΔGCSpredicted) during the NWT in participants who showed ambiguous or absent behavioral response (Group B and C). Behavioral responsiveness can be dissociated from consciousness (i.e., covert consciousness) [7-9]. We therefore did not expect to see a high prediction accuracy for Group B and C. Instead, a high deviation between ΔGCS and ΔGCSpredicted could indicate that participants show electrophysiological signs of waking up despite absent or ambiguous behavioral responsiveness.
2.7 Outcome Assessment and Prognostic Analysis
Patient functional outcome was assessed using a follow-up phone assessment at 3, 6, and 12 months post-recording. Functional outcome was quantified as the maximal Glasgow Outcome scale-extended (GOS-E) [33] score and the minimal Disability Rating Scale (DRS) [34] reached within 1 year post-EEG (Supporting Information: Methods). As this study aimed to investigate the value of EEG in patients with absent or ambiguous behavioral responses, the analysis of prognostic value was only performed on participants from Group B and C (n = 32).
Prognostic analysis was performed based on three outcome criteria: (1) survival (n = 23); (2) recovery of responsiveness (n = 24); and (3) functional outcome (i.e., GOS-E and DRS, n = 20). Nine patients had withdrawal of life sustaining treatment and were excluded from this analysis (see Table 2, see Supporting Information: Methods for more detail). To compare our proposed tool to bedside clinical practice, we additionally collected patients' APACHE score [35] and clinicians' prognostication of patient functional recovery (Supporting Information: Methods).
2.8 Statistical Analysis
The prognostic value of the EEG model to predict survival and recovery of responsiveness was performed using a Python implementation of the one-sided Mann–Whitney U test [36], with the expectation of a larger ΔGCSpredicted for patients with a favorable outcome. The performance of the machine learning model was evaluated using the area under the receiver-operating curve (AUC). It is important to note that the machine learning model only predicted an expected ΔGCS, but not patient outcome. The purpose of the AUC is solely to evaluate how clinically informative the EEG-predicted response was, compared to the observed behavioral response. The difference between AUCs was assessed using a bootstrapping approach, using 100 randomly chosen bootstrap samples (i.e., random selection with replacement from the original dataset), followed by a Wilcoxon signed-rank test. The prognostic value for functional outcome was assessed using a Spearman-rank test.
3 Results
3.1 Changes in Behavior Are Reflected in the Dynamic Change of Participant EEG
We first analyzed the dynamic changes in EEG of only the participants who regained responsiveness during an interruption of sedation (i.e., Group A, see Figure 3).
The best model performance was reached with a regularization parameter of 10, yielding a mean absolute validation error of 1.36. The features that were most important for the prediction of ΔGCS were the change in low beta, alpha, and theta power, and the spectral exponent.
We performed a partial Spearman rank test (using covariates of age and propofol concentration) as a post-hoc analysis to investigate the features' correlation with ΔGCS in Group A. The analysis confirmed that change in responsiveness in Group A was significantly correlated to the change in low beta power (ρ = −0.90, p < 0.01), alpha power (ρ = −0.81, p < 0.05) and the spectral exponent (ρ = 0.89, p < 0.01). A larger increase in responsiveness was accompanied by a stronger increase in alpha and low beta activity, and a stronger flattening of the spectral exponent following interruption of sedation (i.e., negative values indicating an increase in response to sedation interruption).
This demonstrates that behavioral changes following interruption of sedation are accompanied by changes in the participant's EEG response. In addition, the EEG response following interruption of sedation can be used to predict changes in the GCS following interruption of sedation.
3.2 EEG Features Can Be Decoupled From Participant Behavioral Response
Next, we analyzed the dynamic EEG change of participants who showed an ambiguous (i.e., Group B) or absent behavioral response during an interruption of sedation (i.e., Group C, see Figure 3).
Patients' ΔGCS was predicted with a mean absolute error of 2.06 for group B and 2.27 for group C, with predicted values ranging from −0.18 to 7.05. High values of ΔGCSpredicted indicate the presence of electrophysiological patterns of waking up, similar to participants with high ΔGCS in Group A. Negative values indicate EEG dynamic changes that were even weaker than the training example with the lowest ΔGCS.
Figure 4 illustrates the predicted and observed ΔGCS. Although the predicted difference of most Group B participants matched the observed difference, some participants' ΔGCSpredicted were highly under- or over-estimated. We use four case examples of the largest under- and over-estimated ΔGCSpredicted to elucidate the different types of brain responses during the NWT. Cases 1 and 2 (Patient 33 and 11 in Table 1) showed an absent (ΔGCS = 0) or ambiguous (ΔGCS = 2) behavioral response during the NWT. However, based on the EEG reaction to the interruption of sedation, the model generated a ΔGCSpredicted of 6.56 and 7.06, respectively. In contrast, Cases 3 and 4 (Patient 4 and 7 in Table 1) showed an absent (ΔGCS = 0) or ambiguous (ΔGCS = 3) response during the NWT. In both cases, the model generated a ΔGCSpredicted of −0.18 and 0.04, respectively.
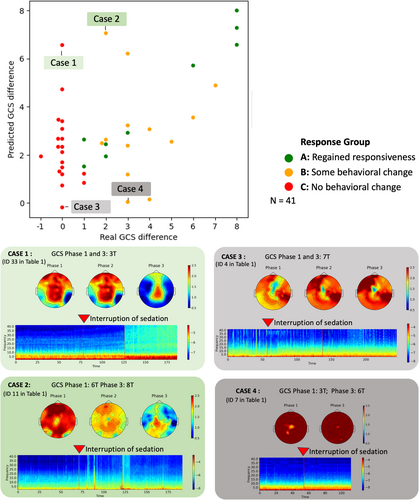
The time-resolved spectrogram and topographic maps of spectral exponent for the four highlighted cases reveal the electrophysiological patterns underpinning the prediction (Figure 4). While Cases 1 and 2 show a prominent increase in global power and flattening of the spectral exponent following sedation interruption, no such effect was observed for Cases 3 and 4.
Altogether, these cases demonstrate that dynamic EEG changes reveal heterogeneity in the group of participants whose response to the NWT was ambiguous or absent. Despite remaining completely unresponsive, some participants showed EEG patterns that are associated with an increased behavioral response. Our results indicate that EEG features can be decoupled from behavioral responsiveness during the NWT.
3.3 EEG Response to Interruption of Sedation Has Prognostic Value for Participants With Absent or Ambiguous Behavioral Responsiveness
Of the 23 participants included in the prognostic analysis of survival, only 11 had an observed ΔGCS above zero during the NWT. The observed ΔGCS had low predictive value for survival, with an AUC of 0.64 (Figure 5A). Participants' maximum observed GCS score was also poorly predictive of survival, with an AUC of 0.62 (Figure 5A). In contrast to the observed behavioral changes, the ΔGCSpredicted had higher prognostic value, with an AUC of 0.75 (t-statistic = 1136.5, p < 0.001) (Figure 5B). Observed GCS scores did not significantly differentiate participants according to their outcome; in contrast, the EEG model generated significantly higher ΔGCSpredicted for participants who survived (U = 84, p < 0.05) (Figure 5B). Figure S1 visualizes this effect for each response group individually.
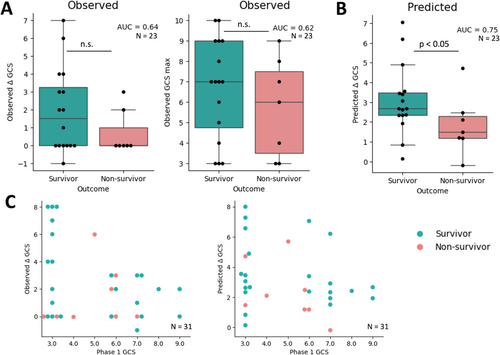
The model-predicted ΔGCS only minimally increased prognostic value for recovery of responsiveness (Figure S2). No significant correlation was identified between observed or predicted ΔGCS and functional outcome (i.e., GOS-E and DRS score). Figure 5C illustrates the improvements offered by a combined neuro-behavioral assessment during the wake-up test.
Altogether, we demonstrated that the EEG response following interruption of sedation has prognostic value for the survival of participants with absent or ambiguous behavioral responsiveness during the NWT. Based on the EEG response, participants who survived showed a higher ΔGCSpredicted compared to participants who did not.
To compare the effect of sedation interruption (i.e., Phase 1–3) to sedation induction (i.e., Phase 3–5), we performed the same analysis on Phase 3–5 and compared the results in the Supporting Information: Methods (Figure S3). We also performed the analysis on Phase 1 (sedation baseline) and Phase 3 (sedation interruption) individually, without considering the contrast between states (Figure S3). Brain activity during Phase 3 alone (sedation interruption) showed a trend towards separating patients according to the recovery of responsiveness (Figure S3). Our results indicate that both the behavioral and EEG responses to propofol interruption should be considered for patient assessment.
3.4 Prognostic Value of the EEG Response to Interruption of Sedation Can Improve Physician Prediction of Outcome
Physicians' predicted GOS-E score at 6 months positively correlated with participants' GCSPhase3 (ρ(28) = 0.39, p < 0.05, partial correlation corrected by age). As expected, a patient's behavioral responsiveness strongly influenced the physician's prediction of long-term functional outcome, with more responsive patients correlated with predictions of better recovery.
Physicians rated their predictions with a confidence of 1.82 ± 0.71, with 1 indicating “slightly confident” and 2 being “fairly confident”. The lowest average confidence score (1.8 ± 0.35) appeared in Group B, who showed an ambiguous behavioral response. Overall, there was a high level of uncertainty in the functional outcome of unresponsive patients, which was maximal when patients show an ambiguous behavioral response.
The absolute distance between the real and the physician-predicted GOS-E score was 1.80 ± 1.42 (Supporting Information: Methods; Figure S4). However, when binarizing patient outcomes according to survival (GOS-E > 1) or recovery of responsiveness (GOS-E > 2), physician predictions could not separate good from poor outcomes (Supporting Information: Methods; Figure S5). Similarly, the APACHE II score achieved a maximal prognostic AUC of 0.64 for recovery of responsiveness (Supporting Information: Methods; Figure S5). Our results confirm that prognostication after brain injury in the study population is accompanied by an overall high uncertainty, which illustrates the need for additional clinical assessment tools for this population.
4 Discussion
In this study, we demonstrated that the EEG dynamic change during the NWT can improve prognostication of severely brain injured patients who show an absent or ambiguous behavioral response to interruption of sedation. Despite behavioral unresponsiveness, some patients' EEG response to interruption of sedation showed electrophysiological signs of waking up. Moreover, the EEG dynamic change during the NWT predicted patient survival better than behavioral responses alone.
The NWT is a widely used and standard-of-care assessment. Complementing the NWT with EEG monitoring has the potential to improve clinical prognostication and to provide clinicians with a perspective on “waking up” beyond its behavioral component. The attending physician's outcome prediction is highly dependent on the patient's observed behavior. Assessing covert neurophysiological function alongside behavior can improve clinician confidence in neuroprognostication and decision-making in acute care settings for severely brain injured patients.
Of the four case examples highlighted in the results, the EEG of two changed dynamically in response to interruption of sedation despite the absence of a behavioral response. The first patient suffered an anoxic brain injury and showed a strong EEG dynamic change during the NWT, while his GCS remained constant at 3T (Table 1). However, these results are inconclusive, as this patient was withdrawn from life-sustaining treatment and the natural course of his recovery remains unknown. The second case patient suffered a stroke and had an ambiguous behavioral response during the NWT. Her EEG had strong neurophysiological signs of waking up, which aligned with her survival and recovery of consciousness. The EEG of the third and fourth case examples remained static during the NWT. The third case was a stroke patient who passed away in palliative care but regained the capacity to follow commands in the ICU; the fourth suffered an anoxic brain injury and was withdrawn from life-sustaining treatment. This case series illustrates the potential benefits, but also the limitations of the proposed tool. Although the EEG response during the NWT may capture the brain's capacity for recovery, patient survival and long-term functional outcome are also determined by factors including age, organ function, treatment availability, social support, and the occurrence of secondary medical events unrelated to the original brain injury. The technique proposed in this study is thus not intended to be used in isolation, but rather to complement current state-of-the-art medical assessments to generate a better-informed prognosis.
Altered holistic spectral properties have been previously proposed to represent varying levels of thalamocortical integrity following brain injury [37]. The resulting “ABCD framework” is increasingly used to track the level of consciousness following brain injury [38-40]. The ABCD model has even been applied to EEG collected from sedated patients in a disorder of consciousness [38]. However, medication remains a confounding factor for spectral analysis which can not be entirely controlled in many cases. Thus, we propose the EEG-reactivity to anesthesia as a complementary method for the assessment of patients with an acute brain injury. Previous research from our group demonstrated the value of a short administration of propofol sedation to evaluate unresponsive patient's potential to recover [20]. This method relies only on EEG and propofol anesthesia which are both widely available in intensive care units. However, continuous sedation which is common during early treatment of severely brain-injured patients does not allow the recording of a non-sedated resting-state required for the application of this method. Developing a method which could identify prognostic value in the EEG response to both induction and interruption of sedation would be key to a more accessible clinical tool that is applicable across sedation status. The presence of higher-order cortical function despite behavioral unresponsiveness has been previously assessed and successfully identified in patients with acute severe brain injury [14-16]. However, the results of this study do not support the identification of cognitive-motor dissociation; further research is required to assess the relation between the brain response to anesthesia and the presence of cognitive-motor dissociation in unresponsive patients.
The results of this study need to be considered in light of several limitations. First, while all EEG was recorded during a complete interruption of propofol, other medications including morphine, fentanyl (four patients), midazolam (three patients), Keppra (two patients) and haloperidol (two patients) were not discontinued during the NWT. Second, before the NWT, patients were continuously sedated for durations ranging from hours to days and were exposed to different concentrations of propofol. We accounted for this potential confound in our analysis by adding the concentration of propofol as a feature to the linear regression. No significant difference was found between the concentration of propofol and participant survival or recovery of responsiveness. Third, EEG markers of consciousness are highly variable across etiologies [23, 41], and this study analyzed brain-injured participants from anoxic, traumatic, and stroke etiologies as a single group. However, our study investigated the propofol-induced relative change in EEG features, using a within-subject comparison to account for the specifics in each participant's brain injury. Fourth, the effect of anesthesia on the brain and behavior differs across sex and age [42-44]. We accounted for age-related differences by including age as a feature in the machine learning model, however, a larger dataset is necessary to identify relevant EEG features in different populations. Fifth, we only extracted features based on fixed EEG bandwidths using a 128-channel EEG system. The analysis of change using a clinical EEG and the whole spectrogram over time could be implemented on a larger sample of patients. Sixth, whereas this paper focused solely on anesthesia-induced changes in spectral properties, more advanced features and neural markers of consciousness such as complexity and criticality [45] should be considered for the development of future clinical tools. Seventh, to ensure patient safety, the necessity and time to re-initiate sedation was determined by the attending nurse. To account for the resulting variability, we evaluated the time-resolved reactivity of brain activity to sedation interruption, rather than the magnitude of change between states. However, results from patients who did not show a neurophysiological response to sedation interruption have limited interpretability. We cannot exclude the possibility that such response would have evolved after a longer duration without sedation. Beyond the total duration of sedation interruption, factors such as patient's metabolic state and liver function might have influenced the patient's brain response to a change in anesthetic level. Further research is required to dynamically map individuals' expected brain response to sedation interruption and to be able to use this method on an individual-subject level.
Author Contributions
C.M.: conceptualization, formal analysis, software, writing – original draft, visualization, resources, data curation, writing – original draft, writing – review and editing, project administration. L.N.: resources, data curation, writing – review and editing, project administration, funding acquisition. C.D.: resources, data curation, writing – review and editing, project administration, funding acquisition. M.H.: resources, data curation, writing – review and editing. K.D.: resources, data curation, writing – review and editing. G.L.: resources, data curation, writing – review and editing. A.F.: resources, data curation, writing – review and editing. X.W.: resources, data curation, writing – review and editing. H.A.-H.: resources, data curation, writing – review and editing. T.Z.: resources, data curation, writing – review and editing. R.L.: resources, data curation, writing – review and editing, project administration. A.M.O.: conceptualization, supervision, project administration, funding acquisition, writing – review and editing. S.B.-M.: conceptualization, supervision, project administration, funding acquisition, writing – review and editing.
Acknowledgments
This study was funded by a McGill/Western Collaboration Grant from Healthy Brain for Healthy Lives and BrainsCAN (1a-5a-01) and by the Canadian Institutes of Health Research (CIHR, Project Grant no. 480995). C.M. is funded through Fonds de recherche du Québec–Santé (FRQS). This research is supported in part by the FRQNT Strategic Clusters Program (2020-RS4-265502—Centre UNIQUE—Union Neurosciences and Artificial Intelligence—Quebec) (awarded to C.M.). A.M.O. is supported by the Canadian Institutes of Health Research (CIHR, #408004) and is a Fellow of the CIFAR Brain, Mind, and Consciousness program. C.D. is funded through an FRQS Junior 1 Scholar Award, a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC), a CIFAR Global Scholars award, and institutional start-up funding from the Research Centre of the Centre Intégré Universitaire de Santé et de Services Sociaux (CIUSSS) du Nord-l'île-de-Montréal. S.B.-M. is funded by a Canada Research Chair (Tier II) and a NSERC Discovery Grant (RGPIN-2023-03619).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data available on request due to privacy/ethical restrictions.




