Pathway Analyses of Inherited Neuropathies Identify Putative Common Mechanisms of Axon Degeneration
Funding: The authors received no specific funding for this work.
ABSTRACT
Objective
Inherited neuropathies (IN) are associated with over 100 different genetic mutations presenting with a variety of phenotypes. This complexity suggests multiple pathways may converge onto a limited number of downstream pathways to effect axonal injury. Ontologic and protein–protein interaction (PPI) studies of the genes associated with inherited disorders of axon degeneration can identify these putative pathophysiologic mechanisms. Comparison with pathways in central disorders of axon degeneration may reveal universal pathways and, thus, therapeutic targets.
Methods
Pathway analyses of genes associated with IN with axonal, demyelinating, and intermediate phenotypes and hereditary spastic paraplegia (HSP) were performed using the Metascape resource. The resulting PPI and ontology networks were analyzed for common and shared versus disparate pathways. Pathways from IN were also compared to those of HSP.
Results
PPI networks demonstrated robust integration of the phenotype-specific networks, with over 20 hubs identified and highly interconnected networks of tRNA aminoacylation and intracellular trafficking. The voltage-gated sodium channel-associated axonal IN did not integrate with the rest of the network, prefiguring potentially different pathophysiologic processes. Ontologic analyses identified tRNA metabolism, axonal transport, and endomembrane/organelle trafficking to be common to all IN phenotypes. There was overlap of these IN ontologic processes with those identified in HSP, suggestive of common pathways involved in the development of inherited, length-dependent axonopathies.
Interpretation
Intracellular trafficking and endomembrane/organelle transport are common pathways associated with inherited axonopathies. Therapies targeting tRNA metabolism and intracellular trafficking are promising therapeutic targets given the convergent PPIs of these subnetworks.
1 Introduction
Since the early description of an inherited neuropathy by Charcot, Marie, and Tooth in the late 19th century [1, 2], greater than 100 genes have been associated with inherited neuropathies (IN) [3, 4]. Over the same period, the great clinical heterogeneity of these disorders has resulted in a variety of organizational frameworks. Early classification characterized the neuropathies by nerve conduction velocity, with a velocity < 25 m/s consistent with demyelination (hypertrophic nerves on pathology), while those over 45 m/s were considered neuronal (axon loss on pathology); an intermediate phenotype was defined by nerve conduction velocities between 25 and 45 m/s and demonstrated mixed pathology [5, 6]. Later work added an additional layer of classification based on inheritance patterns [7], especially with regard to the hereditary sensory and motor neuropathies (Charcot–Marie Tooth disease), with types one and two denoting dominant demyelinating and axonal phenotypes, respectively, and type four representing recessive phenotypes. Type three was historically used for severe, early onset inherited neuropathy. In addition to the hereditary sensory motor neuropathies, other families of IN were identified on a descriptive basis: hereditary sensory autonomic neuropathy, distal hereditary motor neuropathy, and even mitochondrial disease-associated neuropathy. With the advent of more affordable genomic sequencing, the complexity of this heterogeneous group of disorders increased considerably, with multiple mutations in the same gene associated with various phenotypes, and even the same mutation, at times, identified with different phenotypes and penetrance. While efforts have been made to change nomenclature to a phenotypic-inheritance-genomic paradigm [4], the sheer number of genes and variable phenotypes suggests a more global characterization of the mutations may be more clinically useful to characterize the inherited disorders. While the number of genes makes classification difficult, each associated gene is a hint at the basic, biologic underpinnings of neuropathy, especially length-dependent neuropathy such as many of the IN.
Pathway analysis of a genetic or protein data is the study of protein–protein interactions (PPIs) and how these interactions are associated with certain biologic processes. An extended list of genes, as in the case of IN, is an ideal substrate for pathway analysis to provide additional insight into the pathophysiology of IN and, at points of convergence or regulation, potential therapeutic targets. A similar strategy has previously been used to identify pathways associated with increased risk of developing an idiopathic, inflammatory myopathy [8] and identifying common mechanisms of human disease [9]. This analysis can be completed on a subset of IN, such as by axonal, demyelinating, or intermediate phenotypes as well as the full set of all three phenotypes. Furthermore, by extending ontologic pathways from disorders of peripheral nervous system axon degeneration into similar disorders of the central nervous system, such as hereditary spastic paraplegia (HSP), fundamental, common processes of axon degeneration may be elucidated.
As the field of bioinformatics has matured, an increasing number of tools are available for pathway analysis of datasets. In this study, published gene lists from IN and HSP were analyzed by freely available software, Metascape [10] and Cytoscape [11], to identify common and distinct ontologic processes within the axonal, demyelinating, and intermediate phenotypes of IN, as well as the combined gene list, to identify shared and distinct pathophysiologic mechanisms as well as therapeutic insights.
2 Methods
2.1 Inherited Neuropathy Gene Lists and Pathway Analyses
Gene lists were prepared for IN from the literature. Genes associated with Charcot–Marie–Tooth disease, hereditary sensory and autonomic neuropathies, mitochondrial neuropathies, and hereditary distal motor neuropathies were compiled from two recent review articles [3, 4], subdivided into axonal, demyelinating, and intermediate phenotypes [4]. These gene lists (Table 1) and a “combined” list of genes from all three phenotypes were uploaded to Metascape [10] for Express Analysis and visualized in Cytoscape [11].
| Axonal phenotype | ||||||
| AARS1 | DNAJB2 | HARS1 | MED25 | NEFL | RETREG1d | SPTLC2 |
| AIFM1 | DNM2 | HSPB1 | MFN2 | NGF | SCN11A | TRIM2 |
| ATL1 | DNMT1 | HSPB2 | MME | NTRK1 | SCN9A | TRPV4 |
| ATL3 | DST | HSPB3 | MORC2 | PDK3 | SETX | TWNK |
| ATP1A1 | DYNC1H1 | HSPB8 | MPV17 | PLEKHG5 | SIGMAR1 | VCP |
| ATP7A | ELP1a | IGHMBP2 | MPZ | POLG | SLC25A19 | WARS1 |
| BICD2 | FBXO38 | KIF1A | MT-ATP6 | PRDM12 | SLC5A7 | WNK1 |
| BSCL2 | GARS1 | LMNA | MT-ND5 | PRPS1 | SORD | |
| DCTN1 | GDAPb | LRSAM1 | NAGLU | RAB7A1c | SPG11 | |
| DHTKD1 | GDAP1b | MARS1 | NEFH | REEP1 | SPTLC1 | |
| Demyelinating phenotype | ||||||
| EGR2 | GDAP1 | MPZ | NEFL | RRM2B | SH3TC2 | |
| FGD4 | HK1 | MTMR2 | PMP22 | SBF1 | SURF1 | |
| FIG4 | LITAF | NDRG1 | PRX | SBF2 | TYMP | |
| Intermediate phenotype | ||||||
| COX6A1 | GDAP1 | GNB4 | KARS1 | NEFL | YARS1 | |
| DNM2 | GJB1 | INF2 | MPZ | PLEKHG5 | ||
- a Also known as IKBKAP.
- b Homologs.
- c Not matched for pathway analysis.
- d Also known as FAM134B.
Protein–protein-interaction (PPI) networks have been described previously [10], but, briefly, gene lists were matched to Entrez identities, then the gene protein product was annotated by STRING, BioGrid, OmniPath, and InWeb_IM databases to identify PPIs. Highly interconnected subnetworks were identified by the Molecular Complex Detection (MCODE) algorithm [12] in a subset of PPIs defined as a network of between three and 500 proteins.
Ontologic pathway analysis was prepared similarly [10] with Entrez gene identities annotated for ontologic pathways by the following sources: KEGG Pathway, GO Biological Processes, Reactome Gene Sets, Canonical Pathways, CORUM, WikiPathways, and PANTHER pathways. Genes were assigned an ontologic term identity; then, if p < 0.01 (hypergeometric distribution) and the term included at least three genes, they were assigned an ontologic cluster. The node with the lowest p-value was used to title the cluster. q-values as a correction for multiple comparisons were calculated by the Benjamini-Hochberg procedure [13].
Transcription factor regulators were identified by enrichment (as above) of a given transcription factor target genes as identified through the TTRUST database [14, 15].
The top 20 ontologic clusters, by statistical significance, were then visualized as a network with nodes representing enriched ontologic terms, the color describing the cluster identity, and node size indicative of the percent of genes in the uploaded list included within all nodal genes. The network was prepared by connecting terms with a > 0.3 similarity.
Analyses were performed in March 2023 (Intermediate gene list), September 2023 (Demyelinating gene list), and December 2023 (axonal and combined gene lists).
2.2 Hereditary Spastic Paraplegia Pathway Analysis
Genes associated with HSP were identified from a review [16] and analyzed by Metascape [10], as above. Ontologic network, PPI, and transcription factor analyses were performed and visualized as for the IN. Analysis was performed December 2024.
Overlap of the HSP PPI and ontologic analyses with the combined inherited neuropathy list analyses was performed manually.
3 Results
In an effort to identify common pathways and networks underlying IN, gene lists were prepared from the literature [3, 4] for axonal, demyelinating, and intermediate phenotypes, as well as a combined list of all three phenotypes (Table 1) and uploaded to Metascape [10], a freely available pathway analysis tool. This division of phenotype was designed to capture possible fundamentally different pathophysiology between primary disorders of myelination versus the axon. The first step in analysis was the matching of gene name to Entrez gene identity for database queries. In this matching, there were 67 unique genes within the axonal phenotype. A list of 69 genes was uploaded for the axonal phenotype to account for the IKBKAP alias of ELP1 and the FAM134B alias of RETREG1. In total, 65 of the 69 genes were matched to Entrez gene identifiers for the axonal phenotype, after the aliases were combined, GDAP associated with GDAP1 as a homolog, and RAB7A1 not matched. For the demyelinating phenotype, 18 genes were uploaded and all matched. Similarly, all 11 of the unique intermediate genes were successfully matched. For the combined list, of 96 genes identified in the literature, 88 were identified as unique, and 90 were uploaded to account for the aliases as in the axonal phenotype. Following the same culling and failures to match as in the axonal phenotype, a total of 86 of the 90 genes were matched and analyzed.
An Express Analysis in Metascape was used to determine PPI networks as well as ontologic clusters. No canonical pathways were identified in these analyses.
3.1 Protein–Protein Interaction Networks
Protein–protein interaction networks were visualized in the axonal, demyelinating, intermediate, and combined phenotype using Cytoscape [11]. The combined and axonal inherited neuropathy networks demonstrated marked interconnectivity, while the demyelinating and intermediate networks were more fragmented or limited, respectively (Figure 1). Interestingly, in the combined phenotype network, the demyelinating networks fully integrated into the axonal phenotype network through EGR2:DNM2 and MTMR2:NEFL interactions as well as multiple interactions through HK1. Of note, the interaction of the voltage-gated sodium channels SCN9A and SCN11A was separate from the other networks, suggesting a fundamentally different pathophysiologic process than the other causes of inherited neuropathy.
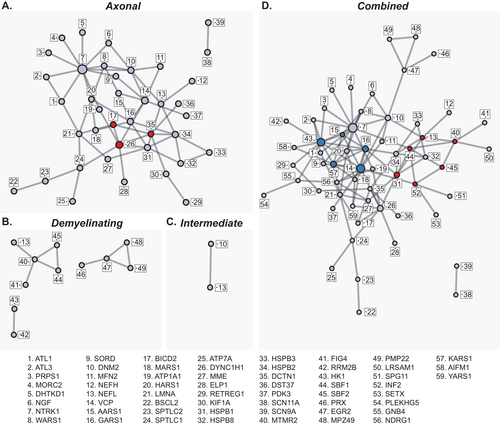
PPI networks are especially helpful in determining common nodes of integration among different genes that are supportive of a common pathologic pathway. Central nodes (“hubs”) were identified as having four or more PPIs. In the axonal phenotype inherited neuropathy PPI network (Figure 1A), there were 13 hubs (number of interactions): NTRK1 (11), DYNC1H1 (7), VCP (7), DNM2 (6), GARS1 (6), HSPB1 (6), HSPB2 (6), DCTN1 (5), HARS1 (5), LNMA (5), NEFL (4), SPTLC1 (4), and WARS1 (4). The demyelinating phenotype inherited neuropathy PPI network (Figure 1B) had a single hub at MTMR2, while the intermediate phenotype PPI (Figure 1C) had no hubs. The combined phenotype PPI (Figure 1D), which integrated all networks, had 22 hubs: NTRK1 (13), VCP (12), HK1 (11), KARS1 (8), GARS1 (8), DYNC1H1 (8), HSPB1 (7), DNM2 (7), LNMA (6), HSPB2 (6), HARS1 (6), SBF1 (5), NEFL (5), MTMR2 (5), INF2 (5), DCTN1 (5), AARS1 (5), WARS1 (4), SPTLC1 (4), NDRG1 (4), MARS1 (4), and EGR2 (4).
In addition to the identification of hubs and common pathways, PPI analysis was extended to identify highly interconnected PPIs using the MCODE algorithm within Metascape [12].
In the axonal phenotype PPI, MCODE identified Golgi-ER transport microtubule organization as a highly connected network comprising DYNC1H1, BICD2, and DCTN1. In the combined dataset PPI, two such networks were identified, with the previous MCODE network identified in the axonal only dataset no longer significant. In this network, phosphoinositide metabolism and intracellular trafficking were an MCODE subnetwork consisting of NEFL, MTMR2, SBF2, INF2, SBF1, and HSPB1, while a second subnetwork of tRNA aminoacylation was composed of PPIs between HK1, AARS1, KARS1, VCP, and GARS1.
3.2 Gene-Ontology Identities and Networks
Ontologic pathway analysis was prepared similarly to PPI pathway analysis, with Entrez gene identities annotated for ontologic pathways by the following sources: KEGG Pathway, GO Biological Processes, Reactome Gene Sets, Canonical Pathways, CORUM, WikiPathways, and PANTHER pathways. The 20 most statistically significant ontologic clusters for the axonal, demyelinating, and intermediate phenotypes (Table 2) as well as the combined analyses (Table 3) demonstrated several distinct, as well as several shared, themes. The greatest ontologic heterogeneity was seen in the axonal phenotype, with the most prominent unique ontologic themes of organelle organization, stress response, ATP metabolism, and tRNA processing, while the distinct themes in the demyelinating phenotype included development and, unsurprisingly, myelination. Interestingly, intracellular trafficking and mitochondrial organization were common ontologic themes in both the axonal and demyelinating phenotypes. Intermediate phenotypes were identified only with intracellular trafficking, and the combined analysis had similar findings as to the individual phenotype analyses. The full list of ontologic clusters (rather than only the top 20), as well as information about the number of matched genes in each cluster, is available as supplemental information (Table S1).
| Description | Log10(p)a | Log10(q)b |
|---|---|---|
| Axonal | ||
| Intracellular trafficking proteins involved in CMT neuropathy | −15.21 | −10.86 |
| Cytosolic tRNA aminoacylation | −8.80 | −4.83 |
| Response to temperature stimulus | −8.70 | −4.83 |
| Sensory perception of pain | −8.24 | −4.50 |
| Response to unfolded protein | −8.11 | −4.46 |
| Axodendritic transport | −7.97 | −4.40 |
| Establishment of organelle localization | −7.80 | −4.30 |
| Regulation of neuron apoptotic process | −7.30 | −4.10 |
| Amyotrophic lateral sclerosis | −7.08 | −3.96 |
| Mitochondrion organization | −6.47 | −3.52 |
| Positive regulation of cellular component biogenesis | −5.87 | −3.03 |
| Endoplasmic reticulum organization | −5.65 | −2.87 |
| Nuclear migration | −4.73 | −2.09 |
| Positive regulation of macroautophagy | −4.68 | −2.05 |
| Mitochondrial genome maintenance | −4.63 | −2.02 |
| Cerebellar Purkinje cell layer development | −4.53 | −1.95 |
| Autophagy | −4.26 | −1.76 |
| Establishment of protein localization to organelle | −3.99 | −1.55 |
| ATP metabolic process | −3.41 | −1.15 |
| DNA duplex unwinding | −3.24 | −1.00 |
| Demyelinating | ||
| Intracellular trafficking proteins involved in CMT neuropathy | −33.59 | −29.24 |
| Myelination | −17.15 | −13.34 |
| Peripheral nervous system development | −9.05 | −5.41 |
| Regulation of myelination | −7.89 | −4.45 |
| Mitochondrion organization | −3.90 | −0.97 |
| Intermediate | ||
| Intracellular trafficking proteins involved in CMT neuropathy | −9.78 | −5.43 |
- a p-Value in log10.
- b Multi-test adjusted p-value in log10.
| Description | Log10(p)a | Log10(q)b |
|---|---|---|
| Combined | ||
| Intracellular trafficking proteins involved in CMT neuropathy | −37.11 | −32.77 |
| Cytosolic tRNA aminoacylation | −12.41 | −8.37 |
| Mitochondrion organization | −8.47 | −5.16 |
| Amyotrophic lateral sclerosis | −8.15 | −4.92 |
| Cellular anatomical entity morphogenesis | −7.79 | −4.62 |
| Response to temperature stimulus | −7.72 | −4.58 |
| Sensory perception of pain | −7.50 | −4.39 |
| Organophosphate biosynthetic process | −7.33 | −4.24 |
| Response to unfolded protein | −7.25 | −4.19 |
| Axodendritic transport | −7.23 | −4.19 |
| Establishment of organelle localization | −6.61 | −3.66 |
| Regulation of neuron apoptotic process | −6.34 | −3.45 |
| Endomembrane system organization | −6.30 | −3.44 |
| EGR2 and SOX10-mediated initiation of Schwann cell myelination | −5.93 | −3.14 |
| Response to oxygen levels | −5.24 | −2.59 |
| Regulation of myelination | −5.08 | −2.46 |
| Monoatomic cation transport | −4.88 | −2.30 |
| Positive regulation of cellular component biogenesis | −4.84 | −2.28 |
| Response to inorganic substances | −4.73 | −2.19 |
| Autophagy | −4.52 | −2.03 |
- a p-Value in log10.
- b Multi-test adjusted p-value in log10.
Network analysis of the terms within each ontologic cluster was completed for the axonal, demyelinating, intermediate, and combined gene lists. Within the axonal phenotype, all ontologic terms were connected, although the members of the cytosolic tRNA aminoacylation and ATP metabolic processing were most distinct (Figure S1A). Similarly, the demyelinating phenotype ontologic networks were connected with the exception of the mitochondrial organization cluster, which was not connected to the other clusters (Figure S1B). The intermediate phenotype had a single node/term, so a network could not be prepared (Figure S1C). Interestingly, the combined gene list network had all clusters connected with the exception of the cytosolic tRNA aminoacylation cluster, which did not connect to the other clusters (Figure S1D). Within the larger, combined network, the mitochondrion organization cluster, as well as a myelination and intracellular trafficking combined cluster, were most distinct from a larger network of all other clusters.
Following the association of ontologic terms and clusters, a transcription factor target study was completed of the demyelinating, axonal, and intermediate (Table 4) as well as the combined (Table 5) gene lists. In this analysis, the gene list is compared to transcription factor target gene lists that were identified in the TTRUST reference database, and enriched transcription factor target genes identified as per the ontologic analysis [14, 15]. Regulating (proximal to the known gene) transcription factors are possible therapeutic targets, as modulation of the regulator may affect downstream compensation and/or overcome reduced protein efficiency. Interestingly, in the axonal phenotype, 20 enriched transcription factor targets were identified, and the most significantly enriched transcription factor, MEF2D, is associated with myocyte differentiation and regulated by histone deacetylase (class two). The demyelinating phenotype had eight transcription factor targets with HFH4 the most enriched, a transcription factor associated with cilia and cell motility. No transcription factors were identified in the intermediate gene set, likely due to the small gene list. The gene list of the combined axonal, demyelinating, and intermediate phenotypes was also associated with 20 transcription factor targets, with the most enriched target, ZIC1, associated with nervous system development and cell projection. Interestingly, the second highest enriched, RSRFC4 (MEF2A), is associated with myocyte growth as well as neuronal differentiation and cell survival.
| Gene set ID | Description | Log10(p)a | Log10(q)b |
|---|---|---|---|
| Axonal | |||
| M30063 | MEF2D target genes | −5.00 | −3.00 |
| M40764 | BPTF target genes | −3.60 | −1.80 |
| M40758 | MEF2C target genes | −3.50 | −1.80 |
| M30272 | ZNF197 target genes | −3.30 | −1.60 |
| M40702 | ZBED4 target genes | −3.30 | −1.60 |
| M613 | TGANNYRGCA TCF11MAFG 01 | −3.30 | −1.60 |
| M16022 | CTAWWWATA RSRFC4 Q2 | −2.90 | −1.30 |
| M12891 | GGAANCGGAANY UNKNOWN | −2.80 | −1.10 |
| M15440 | FOXJ2 02 | −2.70 | −1.10 |
| M29944 | DMRT1 TARGET GENES | −2.70 | −1.10 |
| M16079 | P53 DECAMER Q2 | −2.60 | −1.00 |
| M9431 | AP1 Q6 | −2.60 | −1.00 |
| M6969 | BACH1 01 | −2.60 | −0.98 |
| M12460 | AP1 Q4 01 | −2.60 | −0.97 |
| M5987 | ATF6 01 | −2.60 | −0.97 |
| M13482 | ZIC1 01 | −2.60 | −0.96 |
| M13237 | BACH2 01 | −2.50 | −0.93 |
| M7477 | AP1 Q2 01 | −2.50 | −0.92 |
| M7165 | ACTAYRNNNCCCR UNKNOWN | −2.50 | −0.88 |
| M29991 | HDAC4 TARGET GENES | −2.50 | −0.86 |
| Demyelinating | |||
| M6517 | AAAYWAACM HFH4 01 | −3.30 | −0.87 |
| M14948 | NRF2 01 | −3.30 | −0.82 |
| M7038 | GR Q6 | −3.30 | −0.82 |
| M2459 | EGR Q6 | −3.20 | −0.80 |
| M30110 | PAX7 target genes | −3.20 | −0.71 |
| M17420 | WGGAATGY TEF1 Q6 | −2.80 | −0.41 |
| M13849 | TGACATY UNKNOWN | −2.20 | 0.00 |
| M9645 | GGGYGTGNY UNKNOWN | −2.10 | 0.00 |
| Intermediate | |||
| None | |||
- a p-Value in log10.
- b Multi-test adjusted p-value in log10.
| Gene set ID | Description | Log10(p)a | Log10(q)b |
|---|---|---|---|
| Combined | |||
| M13482 | ZIC1 01 | −5.00 | −3.10 |
| M16022 | CTAWWWATA RSRFC4 Q2 | −4.10 | −2.30 |
| M30063 | MEF2D target genes | −4.00 | −2.20 |
| M30110 | PAX7 target genes | −3.70 | −2.00 |
| M822 | YTATTTTNR MEF2 02 | −3.70 | −2.00 |
| M40764 | BPTF target genes | −3.40 | −1.70 |
| M5987 | ATF6 01 | −3.30 | −1.70 |
| M30272 | ZNF197 target genes | −3.30 | −1.60 |
| M15389 | PTF1BETA Q6 | −3.20 | −1.50 |
| M7622 | ZIC3 01 | −3.10 | −1.40 |
| M9986 | AHR 01 | −3.10 | −1.40 |
| M16079 | P53 DECAMER Q2 | −3.10 | −1.40 |
| M17000 | CP2 01 | −3.10 | −1.40 |
| M6969 | BACH1 01 | −3.00 | −1.40 |
| M12460 | AP1 Q4 01 | −3.00 | −1.40 |
| M16213 | ATF 01 | −3.00 | −1.40 |
| M13237 | BACH2 01 | −3.00 | −1.30 |
| M29944 | DMRT1 target genes | −3.00 | −1.30 |
| M40702 | ZBED4 target genes | −2.80 | −1.20 |
| M613 | TGANNYRGCA TCF11MAFG 01 | −2.70 | −1.10 |
- a p-Value in log10.
- b Multi-test adjusted p-value in log10.
3.3 Generalization to Other Hereditary Length-Dependent Axonopathies
Ontologic analysis can provide insights into generalized pathophysiologic mechanisms despite different primary genetic causes. For example, while the network analysis was completed on hereditary neuropathies that affect the peripheral nervous system, the major ontologic insights are predicted to be shared by other inherited length-dependent axonopathies. To test this hypothesis, a PPI and ontologic pathway analysis was performed on a list of genes from the literature [16] for HSP, a length-dependent axonopathy of the central nervous system. In total, 79 genes (Table 6) were uploaded to Metascape, 77 of which were successfully matched to Entrez gene identities; neither SPG40 nor C19ORF65R were matched, so they were not included in the analysis. The PPI network (Figure 2) was less interconnected than the inherited neuropathy networks (axonal and combined phenotypes), although it still had hubs at AP4M1 (5), AP5Z1 (5), AP4B1 (5), AP4S1 (5), and AP4E1 (4) as well as highlighted interconnected networks identified by MCODE for membrane trafficking, dense core vesicle transport, and nucleotide metabolism. Notably, the four interactions with AP4E1 are from four AP4 proteins within the same protein complex.
| ALDH18A1 | BSCL2 | ERLIN2 | MAG | SLC33A1 | SPG29 | USP8 |
| AMPD2 | C10orf12 | FA2H | MARS1 | SPART | SPG32 | VPS37A |
| AP4B1 | C19ORF65Ra | FARS2 | NIPA1 | SPAST | SPG34 | WASHC5 |
| AP4E1 | CAPN1 | FLRT1 | NT5C2 | SPG7 | SPG36 | WDR48 |
| AP4M1 | CPT1C | GBA2 | PGAP1 | SPG11 | SPG37 | ZFR |
| AP4S1 | CYP2U1 | GJC2 | PLP1 | SPG14 | SPG38 | ZFYVE26 |
| AP5Z1 | CYP7B1 | HSPD1 | PNPLA6 | SPG16 | SPG40a | ZFYVE27 |
| ARL6IP1 | DDH2 | IBA57 | RAB3GAP2 | SPG19 | SPG41 | |
| ARSI | DDHD1 | KIF1A | REEP1 | SPG21 | TECPR2 | |
| ATL1 | DSTYK | KIF1C | REEP2 | SPG24 | TFG | |
| ATP13A | ENTPD1 | KIF5A | RTN2 | SPG25 | UBAP1 | |
| B4GALNT1 | ERLIN1 | L1CAM | SLC16A2 | SPG27 | UCHL1 |
- Note: Bolded entries shared with combined phenotype inherited neuropathy analysis.
- a Not matched for pathway analysis.
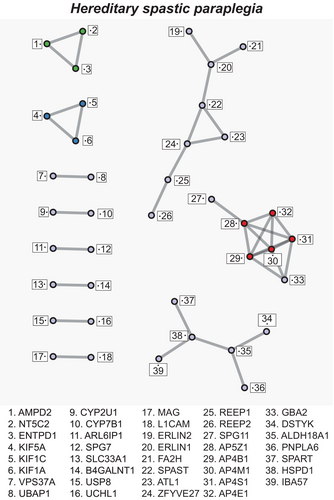
The gene ontology analysis of the HSP gene list was distinct from the inherited neuropathy associations. More specifically, the top 20 ontology clusters in the HSP list demonstrated a predominance of vesicle sorting and endoplasmic reticulum network organization; although, interestingly, central nervous system myelination was also identified as a dominant cluster (Table 7). Nucleotide metabolism, endomembrane organization, and mitochondrial physiology were also identified as significant clusters, similar to some of the clusters in the combined inherited neuropathy analysis. A table of all significant ontologic clusters with associated cluster coverage is available as supplemental data (Table S2). When the ontologic clusters were visualized as a network of significant ontologic terms (Figure S2), there were more isolated clusters than in the IN. However, two major clusters of nucleotide metabolism and membrane processing (central nervous system myelination with glycolipid metabolic processes and cholesterol metabolic processes) were distinct from the larger network. Two isolated “islands” of amino acid metabolic processes and mitochondrial protein degradation were also observed, consistent with the more heterogeneous ontologies.
| Description | Log10(p)a | Log10(q)b |
|---|---|---|
| AP4 adaptor complex | −10.42 | −6.07 |
| Endoplasmic reticulum tubular network organization | −9.11 | −5.23 |
| Axodendritic transport | −9.09 | −5.23 |
| SPG3A-SPG4-SPG31 complex | −7.80 | −4.16 |
| AP5-SPG11-SPG15 complex | −6.50 | −3.28 |
| Axon development | −5.91 | −2.85 |
| Endocytosis | −5.43 | −2.45 |
| Central nervous system myelination | −4.41 | −1.63 |
| Endosome organization | −4.03 | −1.31 |
| Regulation of extent of cell growth | −3.99 | −1.28 |
| Vacuole organization | −3.83 | −1.15 |
| Glycolipid metabolic process | −3.81 | −1.14 |
| Cytoskeleton-dependent cytokinesis | −3.71 | −1.09 |
| Cholesterol metabolic process | −3.61 | −1.00 |
| Golgi vesicle transport | −3.07 | −0.59 |
| Protein transmembrane transport | −3.05 | −0.58 |
| Nucleotide metabolism | −2.87 | −0.45 |
| Regulation of transmembrane transport | −2.80 | −0.41 |
| Mitochondrial protein degradation | −2.70 | −0.32 |
| Amino acid metabolic process | −2.20 | 0.00 |
- Note: Bolded entries shared with combined phenotype inherited neuropathy analysis.
- a p-value in log10.
- b Multi-test adjusted p-value in log10.
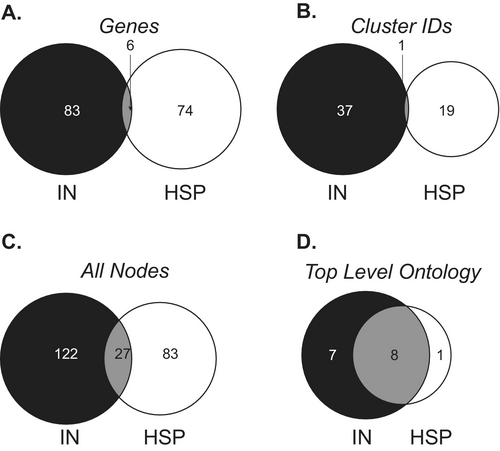
While initial comparison of HSP and the combined IN genes suggests markedly different pathophysiologies, an ontologic analysis demonstrates some common processes in these two length-dependent axonopathies. In a direct comparison of the gene lists between HSP and IN (Tables 1 and 6), only 6 genes were in common (Figure 3A). Similarly, when comparing the top statistically significant ontologic cluster identities (Tables S1 and S2), only one cluster, axodendritic transport, was shared between the two disorders (Figure 3B). However, when the associated ontologic term/nodes within a cluster were compared, there was considerably more overlap (Figure 3C), with nearly one quarter (27/110) of the HSP ontologic terms shared with IN. Furthermore, a more global view of the ontologic networks can be appreciated by review of the “top level” biologic processes ontologic terms. These top-level terms are the overarching, broad themes for branches of clusters. The top-level ontologic biological processes were also identified in the network analysis (Figure S3) and demonstrated considerable overlap between HSP and IN, with nearly 90% (8/9) of the HSP and over 50% (8/15) of the IN top level ontologies shared (Figure 3D). The shared top-level biological process ontologies include general pathways including “metabolic process,” “developmental process,” “cellular process,” positive and negative “regulation of biological processes,” and “biological regulation” as well as more specific terms including “response to stimulus” and “localization.”
4 Discussion
In this study, gene lists from a variety of IN with axonal, demyelinating, and intermediate phenotypes were analyzed for PPIs and ontological network associations. The goal of such an analysis is to gain insights into common versus disparate pathophysiologic mechanisms in a clinically and genetically heterogeneous group of disorders, as well as possible therapeutic targets. Insights into the ontologic pathways associated with IN were also extended into HSP, another family of inherited, length-dependent axonopathy, albeit in the central nervous system. The goal of this comparison was to identify pathophysiologic processes across the clinical (central to peripheral nervous system) spectrum.
Protein–protein interaction analyses describe shared versus distinct pathways as well as central points of integration. These “hubs” of convergent pathways suggest that these proteins may be a critical link in the pathophysiology of a given phenotype and, hence, reasonable targets for therapeutic development. When examined separately, the axonal, demyelinating, and intermediate phenotype analyses did not share any hubs. However, when combined into a single gene list, multiple hubs were identified, and the highly interconnected subnetworks of phosphoinositide metabolism/intracellular trafficking and tRNA aminoacylation were central and are attractive as more specific targets for therapeutic modulation. The integration of the protein interaction networks suggests that the demyelinating vs. axonal vs. intermediate clinical phenotypes may not necessarily be predictive of distinct pathophysiologic mechanisms: a perturbation of one hub may lead to mixed phenotypes (a phenomenon routinely appreciated in the clinic). Furthermore, the highly interconnected subnetworks may suggest that organelle function and dysfunction, especially the mitochondrion with its own tRNAs, may be especially involved in the development of IN. Similarly, when there is more than one distinct PPI network, as is most evident in the separation of SCN9A and SCN11A from the larger network of the combined phenotype study, then the pathophysiology of the disorders associated with these genetic mutations may be fundamentally different than all other disorders. As for therapeutic development, the central location of VCP and NTRK1 in the inherited neuropathy combined data set PPI network supports the continued development of VCP or MAPK pathway modulating therapeutics.
Insights from the PPI interaction networks can be extended by examination of gene ontology families. The ontologic analysis was completed at several levels of detail. Firstly, individual genes were associated with certain ontologic terms. These terms were then visualized as a network between terms as well as synthesized into larger ontologic clusters. Finally, as these clusters are themselves branches of larger ontologic trees, the “top level” biological process ontologies could be compared. The ontologic term and cluster network visualization suggested that not only was there more variety in the ontologic pathways associated with axonal phenotype IN as compared to the demyelinating and intermediate phenotypes (which may be due to the larger gene list), but that there was a general division between stress responses, ATP metabolism, and tRNA processing, suggesting multiple possible pathways to axonal degeneration. It is intriguing to consider mitochondria as being an organelle that shares these ontologic pathways, especially as related to possible alternation of intra-axonal metabolic health and distal axon degeneration through the SARM pathway [17]. The ontologic pathways of intracellular trafficking and mitochondrial organization, common to both demyelinating and axonal phenotype IN, further support this putative association, although the mechanisms by which these ontologies may lead to mitochondrial injury and axonal degeneration cannot be determined through this analysis. Interestingly, a separate study [18] of gene ontology from axonal compartment transcripts from healthy induced pluripotent stem cell-derived motor neurons also identified mitochondrial function and transport as a major network, suggesting the disease-focused analysis herein can identify fundamental biologic processes in healthy samples. However, the overlap of some of these ontologic terms, and especially the top-level biologic process ontologies between IN and HSPs, supports these insights as more generalizable to inherited distal axonopathies of both the central and peripheral nervous systems. The identification of cellular transport, axon development, and nucleotide synthesis pathways in the HSP ontologic clusters corroborates a 2014 study using whole exome sequencing from patients with HSP to identify novel mutations and common pathways [19]. In this pathway analysis, there are more clusters describing organelle dynamics and fewer within the synaptogenesis cluster previously [19] identified. Nonetheless, the current analysis supports the fundamental finding of genetic convergence onto several pathways involved in neuronal axon development and health in HSP specifically and inherited axonopathies, in general.
An additional insight from the ontologic network analysis is the marked integration of the demyelinating phenotype network with the axonal phenotype network. This integration prefigures a shared ontology and, perhaps, pathophysiology. The identification of a prominent demyelinating ontologic cluster was also seen in the analysis of the HSP gene list, despite it being considered a primary disorder of the axons rather than glia. Interestingly, a bovine model of HSP with Spastin mutation [20] was first identified [21] as a primary disorder of central nervous system myelin dysfunction, rather than axonal degeneration, supporting a fundamental overlap between axonal and myelin pathology in the development of length-dependent hereditary axonopathies.
Beyond insights into pathophysiologic mechanisms, an ontologic network analysis predicts other possible genetic etiologies underlying IN that have not yet been characterized. More specifically, when considering both the ontologic term identities, none of the terms were fully represented. That is, for each term identified in the inherited neuropathy gene lists, there were other genes associated with the ontologic term that were not included in the list. While there is an intrinsic limitation to a pathway analysis using a list of previously identified genes and potential unknown protein associations with a given ontologic term, the ability to expand a given term or association into other associated genes may identify new genetic candidates. Indeed, given their ontologic association, one would predict that perturbations of these non-listed genes could also cause an inherited neuropathy. These yet unassociated genes would serve as reasonable targets for novel gene identification in undiagnosed IN.
Finally, an ontologic network analysis expands the PPI characterization of possible therapeutic targets. Indeed, the identification of enriched, downstream transcription factor targets from the gene list provides direct targets for therapies as well as insights into other therapeutic strategies for IN. The prominent enrichment of MEF2D target genes in the axonal phenotype, and its modulation by HDAC, as well as the identified HDAC4 target gene transcription factor enrichment, support the study of HDAC inhibitors to attenuate the development of some [22, 23], but not all [24], IN. As genetic diseases provide basic science insights that can be applied to acquired disorders, these ontologic findings also predict the clinical effect of HDAC inhibitors in neuropathic pain [25]. In addition to direct therapeutic targets, the identification of transcription factor enrichment may also predict therapeutic targets outside of the axon. For example, MEF2D is most often associated with myocyte development and may, therefore, predict some of the postsynaptic changes seen in axonal phenotype IN [26-28], opening a potential new target for therapies.
The major limitation of these analyses is the inability to delineate between different mutations within a different gene and the phenotype, as there is a tremendous degree of phenotypic variability for certain genes and mutations. The combined gene data set analysis hoped to overcome this limitation, but it does so at the expense of a nuanced understanding of the changes in PPI and ontologic associations for a certain mutation. The differential effect of gain or loss of function mutations is not delineated in this analysis, for example. Indeed, the phenotypic variability is suggestive of complex, multigenic disorders with modifying genes or factors that may be more evident with ontologic pathways, but less so if these modifying factors are unknown. Put another way, a pathway analysis from genes associated with inherited axonopathies may not effectively delineate between risk versus causative genes. Nonetheless, this study's ontologic insights are supported by overlaps with other inherited axonopathies as well as therapeutic observations.
A pathway analysis of the genes associated with axonal, demyelinating, and intermediate phenotype IN, as well as those associated with HSP, identifies tRNA metabolism, axonal transport, and endomembrane/organelle trafficking as common ontologies across all phenotypes. PPI analysis found robust connectivity between the demyelinating and axonal phenotypes, suggestive of overlapping pathophysiology, while the sodium voltage-gated channel associated neuropathies were distinct from the rest of the PPI networks. Therapeutic targets include over 20 hubs by PPI networks, including two highly interconnected networks of tRNA aminoacylation and intracellular trafficking, and support the use of HDAC inhibitors for inhibition of downstream transcription factors enriched in the various pathways. This study provides a framework to expand our understanding of the current and help predict future genetic causes of inherited axonopathies as seen in neuropathies and HSP. Recent advances in artificial intelligence allow the analysis of large data sets, which is ideal for studies of genetic interactions and pathways. These larger datasets may permit ascertainment of risk versus causative genes, as well as integrate mutation-specific functional changes.
Author Contributions
Christopher R. Cashman: conceptualization (lead), Investigation (lead), methodology (lead), visualization (lead), writing-original draft preparation. Reza Sadjadi: conceptualization (supporting), methodology (supporting), visualization (supporting), supervision (lead), writing-review and editing. Craig Blackstone: conceptualization (supporting), methodology (supporting), visualization (supporting), supervision (supporting), writing-review and editing.
Acknowledgments
The authors would like to thank the Department of Neurology at the Massachusetts General Hospital and Neuromuscular Medicine Fellowship program for support of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the raw data for this study are available in the literature. The specific analyses of this study are available from the corresponding author upon reasonable request.




