An Analysis for IDH-Mutant Grade 4 Astrocytoma Based on WHO CNS 5: Implication of Clinical Practice
Funding: This work was supported by Beijing Science and Technology Innovation Medical Development Foundation (No. KC2023-JX-0288-BM150), the National Natural Science Foundation of China (8240072705) and Science and Technology Development Fund of Shanghai Pudong New Area (PKJ2021-Y48).
ABSTRACT
Purpose
There is ongoing debate regarding the therapeutic approach and prognosis for IDH-mutant grade 4 astrocytoma, a newly defined subtype of diffuse glioma in the 2021 WHO classification system for central nervous system tumors (WHO CNS 5). The aim of this study was to explore the clinical outcome and prognosticators for newly diagnosed IDH-mutant grade 4 astrocytoma based on our single institutional data.
Methods
This retrospective analysis included 53 consecutive patients with newly diagnosed IDH-mutant grade 4 astrocytoma, who underwent radiotherapy between September 2021 and December 2023. All patients were administered concurrent and adjuvant temozolomide. Eleven patients received adjuvant tumor-treating fields (TTFields).
Results
The median follow-up was 15.7 months. Twenty patients had tumor relapse; three patients died, all of whom were without TTFields therapy. The median PFS for the entire cohort was 19.3 months, and the median OS was not reached. Univariate analysis indicated patients younger than 40 years (p = 0.11) or without homozygous deletion of CDKN2A/B (p = 0.11) tended to have better PFS. In addition, the TTFields group tended to have longer median PFS than the non-TTFields group in both analyses before and after propensity score matching (PSM) (24.4 vs. 18.5 months, p = 0.097, before PSM; 24.4 vs. 15.9 months, p = 0.080, after PSM). No significant independent prognostic factor was found in the multivariate analysis.
Conclusions
The study reveals important insights into clinical practice for IDH-mutant grade 4 astrocytoma. Younger age and tumor without deleted CDKN2A/B might be predictive of better outcomes. The addition of TTFields trended towards improved PFS, necessitating prospective clinical trials for further investigation.
1 Introduction
Adult-type diffuse gliomas represent the most common entities of primary infiltrative brain tumors existing on a spectrum of aggressiveness [1, 2]. The 2021 vision of WHO classification of central nervous system tumors (WHO CNS5) subdivided adult-type diffuse gliomas into three divergent categories: isocitrate dehydrogenase (IDH)-mutant astrocytoma, IDH-mutant oligodendroglioma, and IDH-wildtype glioblastoma [1]. The transition to a novel molecular-based diagnostic algorithm of adult-type diffuse gliomas has generated challenges regarding prognosticators and standards of care in the newly defined tumor types.
Noticeably, IDH-mutant grade 4 astrocytoma is a newly proposed diffuse glioma subtype. According to WHO CNS 5, the grading of IDH-mutant astrocytoma is no longer entirely based on histological features [1, 2]. The presence of cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) homozygous deletion, even in the absence of microvascular proliferation or necrosis, can designate IDH-mutant astrocytoma as grade 4 [1]. Traditionally, the strategies used to treat diffuse gliomas, which are divided into low- and high-grade gliomas, have been mostly based on landmark clinical trials conducted while prior tumor classification systems were in place [3-8]. However, the results might stem from a wide range of heterogeneous glioma entities [9, 10].
After the release of WHO CNS 5, various international institutions have updated their treatment guidelines for diffuse gliomas [11-14]. Nevertheless, there is ongoing debate regarding the therapeutic approach for IDH-mutant grade 4 astrocytoma, with a main issue revolving around whether it should be managed similarly to IDH-mutant grade 3 astrocytoma or to IDH-wild glioblastoma. In the particular term, the efficacy of tumor-treating fields (TTFields) for IDH-mutant grade 4 astrocytoma, though recommended at a 2A level in the updated NCCN recommendations [14], necessitates deeper comprehension. The primary objective of this study is to conduct an initial investigation of the clinical outcomes and prognostic factors of newly diagnosed IDH-mutant grade 4 astrocytoma, treated by adjuvant therapy regimens similar to IDH-wild glioblastoma, based on the database in our center following the release of the WHO CNS5.
2 Methods
2.1 Patients
This retrospective research has been approved by the Institutional Review Board (IRB) of Huashan Hospital, Fudan University (Approval No. 2024–1128). Following the approved protocol by the Institutional Review Board, records of patients with newly diagnosed IDH-mutant grade 4 astrocytoma, who received the treatment of radiotherapy between September 2021 and December 2023, were retrospectively evaluated. All patients were diagnosed based on the criteria of WHO CNS 5, as they were consecutively collected after the release of WHO CNS 5. Medical records were reviewed for patient, tumor, and treatment features, as well as clinical outcome. In the context of molecular diagnostics, IDH mutations were determined using immunohistochemistry and sequencing methods. The entire cohort of this study was verified as IDH1-mutant type. CDKN2A/B homozygous deletions were assessed via fluorescence in situ hybridization (FISH), and MGMT promoter methylation was analyzed using methylation-specific polymerase chain reaction (PCR). Testing for 1p19q co-deletion via FISH was performed to exclude oligodendroglioma.
2.2 Treatment
All patients underwent tumor resection via craniotomy. Gross total resection was defined as no visible tumor residual tumor on postoperative conventional MRI with T1-weighted, T2-weighted, FLAIR, and T1-contrast sequences. Treatment planning for radiotherapy was based on CT simulation with a thermoplastic mask. A customized 5 mm thickness latex-free open-cell styrene butadiene rubber foam was fitted under the mask to accommodate the TTFields arrays for patients receiving TTFields during the radiotherapy period. In order to accurately delineate the target for radiotherapy, contrast-enhanced MRI, as well as 18Fluoro-O-(2) fluoroethyl-l-tyrosine ([18F] FET) or [11C] Methionine (MET) -PET-CT if available, were matched to simulation CT images. The gross tumor volume (GTV) was determined as any remaining tumor observed on T1 postcontrast, FLAIR, and MET- or FET-PET scans combined with the surgical cavity. The high-risk clinical target volume (CTV) (CTV-hr) was defined as GTV with a margin of 5-10 mm. The low-risk CTV (CTV-lr) was defined as GTV plus a 15–20 mm margin. The prescribed doses were 54–60 Gray (Gy) using standard fraction (1.8–2.0 Gy per fraction).
The recommended regimens for chemotherapy involved oral temozolomide at a dose of 75 mg/m2/day from the beginning to the end of radiotherapy, followed by at least 6 cycles of adjuvant oral temozolomide (at 150–200 mg/m2) for 5 days every 28 days.
For patients with supratentorial lesions, recommendations were provided to initiate TTFields therapy at the commencement of radiotherapy, within 1 week, as long as the condition of the scalp permitted. Among the patients, six initiated TTFields during radiotherapy. The transducer arrays were retained during radiotherapy sessions and replaced every 2–3 days in accordance with standard practice. The array location for TTFields administration was alternated between two different sites with each update of the array. The condition of the scalp was inspected by the physician and/or patient/care giver during transducer array changes.
2.3 Statistical Analyses
The evaluation of tumor response was performed using version 2.0 of RANO Criteria [15]. Adverse effects caused by radiation that occurred within 3 months of starting radiotherapy are classed as acute toxicities, whereas those that occurred afterwards are classified as late toxicities. The toxicities were scored using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Both the progression-free survival (PFS) and overall survival (OS) were assessed for every individual patient. PFS was defined as the duration of time between the surgery and the occurrence of disease progression. OS was calculated as the time from the date of operation to the date of death from any cause. The Kaplan–Meier method was used to estimate the rates of PFS and overall survival OS. Differences in survival were evaluated using the log-rank test. The Cox proportional hazards regression models were conducted to evaluate prognostic factors in both univariate and multivariate analyses. To reduce the risk of model overfitting, only variables with a p < 0.15 in univariate analysis were included in the multivariate Cox regression model. Propensity score matching (PSM) was performed between the TTFields group and the non-TTFields group to reduce potential bias in this retrospective study. The match tolerance (caliper) was set at 0.02, and 1:1 nearest neighbor matching was performed. As this was a retrospective exploratory data study, the p-values have a descriptive quality. The R software was utilized to conduct statistical analysis.
3 Results
3.1 Patients and Treatment
Between Sep 2021 and Dec 2023, a total of 53 consecutive patients diagnosed with newly diagnosed IDH-mutant grade 4 astrocytoma, following the release of the WHO CNS 5 classification system, were administered radiotherapy at our center. There were 30 males and 23 females, respectively. The median age of this entire cohort was 40 years, with a range of 28–67 years. Tumor invasion into the corpus callosum or ventricle, signifying a propensity for aggressive behavioral characteristics [16-18], was documented in 56.6% (30/53) of patients. An overview of KPS, MGMT promoter status, and CDKN2A/B status was provided in Table 1.
| Characteristics | N = 53 |
|---|---|
| Age | |
| Median (range), years | 40 (28–67) |
| Gender | |
| Male | 30 |
| Female | 23 |
| KPS | |
| > 80 | 36 |
| ≤ 80 | 17 |
| Tumor invasion into corpus callosum and/or ventricle | |
| Yes | 30 |
| No | 23 |
| CDKN2A/B status | |
| Non-homozygous deletion | 26 |
| Homozygous deletion | 16 |
| Unknown | 11 |
| MGMT promoter status | |
| Methylated | 39 |
| Un-methylated | 8 |
| Unknown | 6 |
| Tumor resection | |
| Gross-total resection (GTR) | 21 |
| Non-GTR | 32 |
| Radiotherapya | |
| Total dose of 60Gy | 39 |
| Total dose < 60Gy | 14 |
| TTFields Therapy | |
| Yes | 11 |
| No | 42 |
- a All patients received concurrent and adjuvant TMZ, based on Stupp protocol.
Regarding therapy, 39.6% (23 out of 53) of patients underwent gross-total resection of the tumor. Thirty-nine patients received irradiation with a dose of 60Gy, whereas 14 patients received a lesser irradiation dose, but not less than 54Gy. Each patient received concurrent and adjuvant TMZ (a minimum of 6 cycles) on the basis of the Stupp protocol for IDH-wild glioblastoma. Thirty patients completed over 6 cycles of adjuvant TMZ. No instances of early discontinuation or dose reduction of TMZ were observed. Eleven patients were administered adjuvant TTFields therapy; all of them had TTFields usage over 2 months. Among patients administered TTFields, five exceeded the recommended 75% adherence threshold. The median compliance rate across all patients was 73.5%. One typical case of IDH-mutant grade 4 astrocytoma, treated with radiotherapy, TMZ plus TTFields therapy, was illustrated in Figure 1.
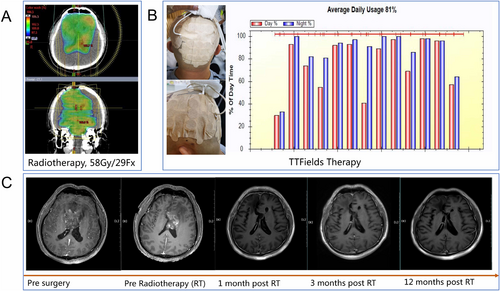
3.2 Survival Outcomes and Toxicities
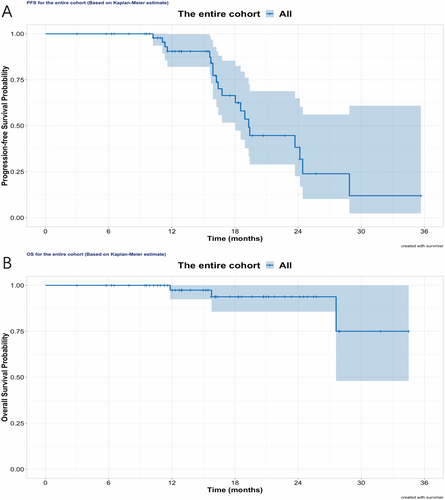
This analysis had a median follow-up period of 15.7 months. Twenty patients had tumor progression, translated into the median PFS of 19.3 months for the entire cohort, as shown in Figure 2. For tumor failure pattern at first progression among these cases, 16 (80%) were classified as local failure, two were distant failures out of the radiotherapy field, and two presented with both local and distant tumor relapse. Three patients had deceased, and none of them had received TTFields therapy. The median OS was not reached.
In all patients, radiotherapy was well tolerated and completed without interruption due to treatment-related toxicity. Fourteen patients developed pseudoprogression, which subsequently subsided either shortly or < 6 months after the completion of radiotherapy. No severe acute or late toxicities (grade 3 or above) related to radiotherapy were observed. Skin adverse events were specially assessed for patients administered with TTFields therapy. Most TTFields-related skin toxicities were mild (grade 1, n = 8) or moderate (grade 2, n = 1). No severe skin toxicity (grade 3 or above) was observed during the administration of TTFields.
3.3 Prognostic Factors
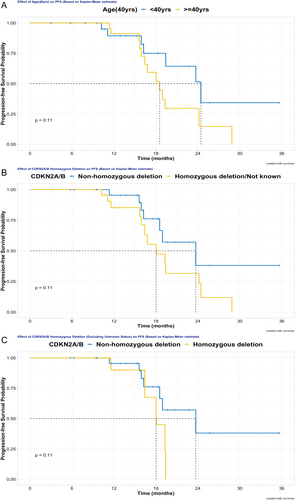
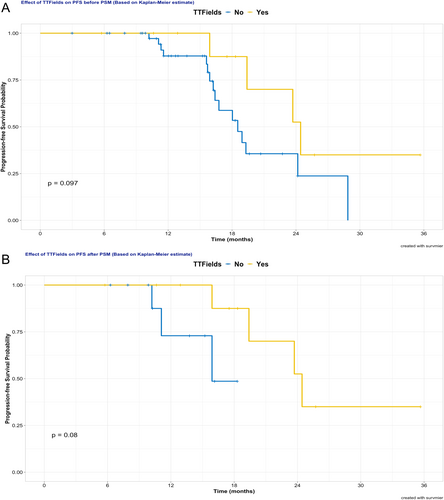
Numerous factors, including gender, age, KPS, extent of tumor invasion, MGMT promoter methylation status, CDKN2A/B homozygous deletion status, surgical resection degree, irradiation dose, and TTFields therapy usage, were examined for the prognostic value of PFS in both univariate and multivariate analyses, as these factors were considered clinically relevant. Due to limited events, prognostic factor analysis for OS was not performed in this analysis. Univariate analysis revealed that age, CDKN2A/B status, as well as TTFields usage, showed a trend to impact PFS. As detailed in Figure 3A, patients younger than 40 years (24.4 vs. 18.5 months, p = 0.107) exhibited a trend of prolonged PFS. Concerning the impact of CDKN2A/B, non-homozygous deletion, in contrast to homozygous deletion or unknown status, signified a tendency for extended PFS (23.7 vs. 18.0 months, p = 0.108). In the analysis of excluding unknown CDKN2A/B status (n = 11), patients without CDKN2A/B homozygous deletion continued to show a trend towards better PFS than those with deletion (p = 0.113, Figure 3C). Additionally, patients who received TTFields therapy tended to have longer median PFS than that of patients without TTFields therapy (24.4 months vs. 18.5 months, p = 0.097), as shown in Figure 4A. Other factors were statistically non-significant for PFS in univariate analysis. Moreover, no significant predictive factors for PFS were found in multivariate analysis, as illustrated in Table 2.
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR, 95% CI | p | HR, 95% CI | p | |
| Gender (male/female) | 1.50 (0.60–3.76) | 0.383 | n.a. | n.a. |
| Age (≥ 40 years/< 40 years) | 2.12 (0.83–5.41) | 0.107 | 1.36 (0.43–4.24) | 0.600 |
| KPS (> 80/≤ 80) | 0.88 (0.35–2.25) | 0.793 | n.a. | n.a. |
| Tumor invasion into corpus callosum or ventricle (No/Yes) | 0.89 (0.44–2.94) | 0.791 | n.a. | n.a. |
| MGMT promoter (Methy/Un-methy, not known) | 0.75 (0.29–1.98) | 0.563 | n.a. | n.a. |
| CDKN2A/B (Hom-deletion, not known/Non-hom-deletion) | 2.10 (0.83–5.29) | 0.108 | 1.74 (0.66–4.57) | 0.265 |
| Surgical resection (GTR/non-GTR) | 0.68 (0.26–1.79) | 0.433 | n.a. | n.a. |
| Radiotherapy dose (60Gy/< 60Gy) | 0.77 (0.31–1.91) | 0.568 | n.a. | n.a. |
| TTFields usage (Yes/No) | 0.40 (0.13–1.23) | 0.097 | 0.57 (0.15–2.20) | 0.412 |
3.4 Efficacy Analysis of TTFields by PSM
For further analysis of the efficacy of TTFields in PFS, PSM was performed between the TTFields group and the non-TTFields group in this retrospective study. Predictor covariates used in PSM included age and status of CDKN2A/B, both of which trended towards significance influencing PFS as indicated above. After 1:1 PSM, 22 patients were selected for further analysis. No statistically significant difference was found between the two groups in predictor covariates or other covariates after PSM (Table 3). After PSM, the TTFields group, compared to the non-TTFields group, tended to have improved median PFS (24.4 vs. 15.9 months, p = 0.080), as illustrated in Figure 4B.
| Characteristics | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| TTFields | Non-TTFields | p | TTFields | Non-TTFields | p | |
| Age (years) | 0.015 | 1.000 | ||||
| ≥ 40 | 2 | 27 | 2 | 2 | ||
| < 40 | 9 | 15 | 9 | 9 | ||
| Gender | 0.501 | 1.000 | ||||
| Male | 5 | 25 | 5 | 6 | ||
| Female | 6 | 17 | 6 | 5 | ||
| KPS | 1.000 | 0.586 | ||||
| > 80 | 8 | 30 | 8 | 10 | ||
| ≤ 80 | 3 | 12 | 3 | 1 | ||
| Tumor invasiona | 0.738 | 0.395 | ||||
| Yes | 4 | 19 | 4 | 7 | ||
| No | 7 | 23 | 7 | 4 | ||
| CDKN2A/B status | 0.745 | 1.000 | ||||
| Non-hom-deletion | 6 | 20 | 6 | 6 | ||
| Hom-deletion/not known | 5 | 22 | 5 | 5 | ||
| MGMTp status | 0.706 | 0.635 | ||||
| Methy | 9 | 30 | 9 | 7 | ||
| Unmethy/not known | 2 | 12 | 2 | 4 | ||
| Tumor resection | 1.000 | 0.670 | ||||
| Gross total resection (GTR) | 4 | 17 | 4 | 6 | ||
| Non-GTR | 7 | 25 | 7 | 5 | ||
| Radiotherapy | 0.494 | 1.000 | ||||
| Total dose of 60 Gy | 8 | 24 | 8 | 9 | ||
| Total dose < 60 Gy | 3 | 18 | 3 | 2 | ||
- a Tumor invasion into corpus callosum and/or ventricle.
4 Discussion
The results of this study are among the first real-world data, with the sample size of 53 cases, on IDH-mutant grade 4 astrocytoma following the release of the WHO CNS 5 classification system. With a median follow-up of 15.7 months, the median PFS reached 19.3 months for the entire cohort, over double that of historical data in IDH-wild glioblastoma. The most common tumor failure pattern, 80% of the first-observed relapse, was local recurrence. Patients with younger age or no CDKN2A/B homozygous deletion might have better prognosis as indicated by univariate analysis for PFS. The addition of TTFields therapy in the adjuvant phase was found to enable a trend improvement of median PFS both in the analysis before and after PSM.
Until the 2021 revision of the WHO classification, IDH-mutant grade 4 astrocytoma has been commonly referred to as IDH-mutant glioblastoma [1, 19, 20]. Various lines of evidence suggest that IDH mutations in adult-type diffuse gliomas are linked to a less aggressive phenotype and a more favorable prognosis, in comparison to their IDH-wild type counterparts [10, 21-24]. Therefore, the WHO CNS 5 classification clearly distinguished between the IDH-mutant and IDH-wildtype subtypes of diffuse gliomas in adult [1]. Furthermore, the grading of diffuse gliomas is no longer entirely based on histological features, as well as incorporating molecular markers. Noticeably, CDKN2A/B homozygous deletion can directly designate IDH-mutant astrocytoma as grade 4 in the novel classification. This recommendation was based on multiple researches indicating that the homozygous deletion of CDKN2A/B genes, which naturally encode for three proteins (p14, p16, and p15) acting as suppressors of the oncogenic CDK pathway, is a strong independent indicator of the poor prognosis within IDH-mutant astrocytomas [25-29].
As of now, there is no available randomized evidence specifically in IDH-mutant grade 4 astrocytoma. Most of the data supporting radiotherapy plus TMZ in the management of IDH-mutant grade 4 astrocytoma is extrapolated from the results of the CATNON trial [30, 31], in which 751 patients with 1p/19q-non-codeleted anaplastic gliomas were randomly divided into four different treatment groups. These groups included: radiotherapy alone, radiotherapy combined with concurrent TMZ, radiotherapy plus concurrent and adjuvant TMZ, and radiotherapy plus adjuvant TMZ. Based on a second interim analysis [30], adjuvant TMZ post radiotherapy, compared to radiotherapy alone, resulted in an improved median survival in the entire population (82 months versus 47 months). However, when IDH mutation status was taken into account, the survival benefit of TMZ was only seen in IDH-mutant tumors (117 months versus 78 months). In contrast, concomitant TMZ did not enhance overall survival, despite a nonsignificant tendency towards improvement in IDH-mutant tumors (117 versus 92 months, p = 0.17). Thus, regarding radiochemotherapy approaches, IDH-mutant grade 4 astrocytoma may be treated with radiotherapy followed by adjuvant TMZ based on CATNON results of IDH-mutant grade 3 gliomas, or radiotherapy with concurrent and adjuvant TMZ based on trial data in glioblastoma completed prior to WHO CNS 5 [13, 30, 32]. Both regimens are considered appropriate and rational by different expert groups.
Regarding TTFields therapy, the most recent version of NCCN guidelines recommended it at a 2A level in treating IDH-mutant grade 4 astrocytoma, largely based on the results of EF-14 trial [14, 33]. TTFields therapy received FDA approval in 2015 for the treatment of newly diagnosed glioblastoma, following the positive outcomes of phase 3 trial EF-14. The EF-14 trial, to describe it briefly, enrolled 695 supratentorial GBM patients who were at least 18 years old, with the primary endpoint of median PFS. The final analysis, via the use of statistical methods sufficient to power both endpoints of PFS and OS, demonstrated the addition of TTFields significantly improves both PFS (6.7 vs. 4.0 months, p < 0.001) and OS (20.9 vs. 16.0 months, p < 0.001). Therefore, the EF-14 trial provides indirect evidence for the addition of TTFields at face value. Nonetheless, several limitations of the EF-14 trial must be considered. First, the EF-14 trial was conducted between July 2009 and November 2014, when prior tumor classification systems had not yet incorporated the IDH gene into the diagnostic algorithm of gliomas. The 2017 EF-14 trial results by Stupp et al. [33] may have stemmed from a wide variety of heterogeneous glioma entities; meanwhile, subanalysis based on IDH gene status was not done due to insufficient data. Second, the EF-14 study was concluded early as a result of a planned interim analysis. There is evidence indicating that trials that have been prematurely terminated before reaching 500 events are highly susceptible to bias, leading to an overestimation of the extent of the effect, although not its direction. The predetermined cessation criteria do not alleviate this risk [34, 35].
Thus, the optimal treatment strategy for IDH-mutant astrocytoma grade 4 is still not well established, with two key areas of contention being the regimen of adjuvant radio-chemotherapy and the effectiveness of adding TTFields therapy. At our center, we prefer RT combined with concurrent and adjuvant TMZ to treat IDH-mutant grade 4 astrocytoma, since trials supporting this regimen would have included a small percentage of IDH-mutant grade 4 astrocytoma. Meanwhile, patients with IDH-mutant grade 4 astrocytoma of supratentorial lesions are recommended to be offered TTFields therapy.
The data presented in our study supports the addition of TTFields therapy to RT plus concomitant and adjuvant TMZ for the treatment of IDH-mutant grade 4 astrocytoma, offering an optimistic treatment efficacy. The main justifications supporting TTFields' utility in treating IDH-mutant astrocytoma are its distinct anti-tumor properties and tolerable toxicity profiles. Initially discovered for their antimitotic effects [36], TTFields are now understood to exert a variety of direct anti-tumor biological effects, acting synergistically with existing therapy techniques in several types of malignancies [37-42]. Noticeably, recent data indicated multiple immune modulatory roles of TTFields in heating up the “cold” tumor immune microenvironment [43-46]. Given that gliomas are extremely aggressive and immunosuppressive, any treatment that shows efficacy in halting the growth of the tumor will probably need to work in concert with mechanisms that combat the disease at the cellular, tumor microenvironment, and systemic levels simultaneously. Generally, TTFields therapy has a good safety profile as a localized and noninvasive treatment modality. The most common side effects are mild-to-moderate skin-related side effects, which are mostly manageable and resoluble. The addition of TTFields therapy was not associated with an increase in systemic toxicity.
During the pre-IDH era, diffuse astrocytomas in adults were a diverse group of tumors with varying prognoses that were somewhat difficult to predict. The molecular categorization of these tumors according to IDH mutation status has greatly improved prognostic accuracy compared with histologic categorization alone. Previous research revealed that survival in the IDH-mutant glioblastoma population is almost twice that of IDH-wildtype glioblastoma [47]. Once compatible with a glioblastoma diagnosis, identification of an IDH mutation in a diffuse astrocytic glioma with microvascular growth, necrosis, or homozygous deletion of CDKN2A/B is now described as IDH-mutant grade 4 astrocytoma. Nevertheless, prognostic factors for this newly defined tumor type require further examination. Factors that have traditionally been used to predict the outcome of IDH-mutant astrocytomas need to be reevaluated in light of the new information available as of WHO CNS 5 classification.
The CDKN2A/B status was of trend significant value when examining the prognostic factors statistically related with PFS in our univariate analysis. These findings are in line with the results of a recent prognostic stratification study of Weller et al. [48], which supported the strong unfavorable prognostic value of CDKN2A/B homozygous deletion in IDH-mutant astrocytoma. As previously reported, even among patients with WHO grade 4 tumors, the presence of homozygous deletion of CDKN2A/B is linked to worse prognosis for patients with IDH-mutant astrocytomas [25-27]. Diagnostic testing for CDKN2A/B homozygous deletion in IDH-mutant astrocytomas exhibiting histological symptoms of anaplasia comparable to grade 3 and 4 is advised by the WHO CNS 5 classification [1]. Hence, we advise that, in clinical practice, the status of CDKN2A/B homozygous deletion should be carefully evaluated when diagnosing IDH-mutant astrocytomas, as its presence directly defines grade 4 astrocytoma, per the 2021 WHO classification, despite not being ubiquitous in all grade 4 tumors.
With a 40-year cut-off in our univariate analysis, older age was indicative of a negative prognostic factor of trend, matching the findings of Weller et al. [48]. Age is one of numerous clinical prognostic markers that can impact the outcome for histologically low-grade gliomas. Early studies showed that younger patients typically had better outcomes than older ones; patients under 40 are classified as low risk, while those over 40 are classified as high risk. A meta-analysis of four sizable phase III studies involving patients with histologically proven low-grade gliomas (astrocytic and oligodendroglial) was conducted by Gorlia et al. [6], and the adjusted analysis revealed that age, with a cut-off of 40 years, was not a significant predictor of outcome. Another two studies on IDH-mutant astrocytomas indicated that the prognostic cut-off point might be higher than 40 years, as was previously thought [49, 50]. More research is needed to determine whether age affects prognosis in IDH-mutant grade 4 astrocytomas. Other potential confounding factors, such as extent of resection, KPS, and MGMT promoter methylation status, were not fully explored due to the retrospective nature and small sample size of our study. These variables should be considered in future research.
There are several limitations in our study that require discussion. First, this study in nature was obtained retrospectively from a single institution experience. Despite the fact that all patients in this analysis were consecutively collected after the release of WHO CNS 5, it is still possible that selection bias was present and cannot be disregarded. Second, due to the small sample size of 53 cases, though among the largest samples specific to IDH-mutant grade 4 astrocytoma to date, it is not possible to conduct a rigorous analysis to investigate the potential confounding relationships between survival and the variables under examination. Third, our detailed outcome analysis was primarily restricted to PFS due to the short median follow-up duration of 15.7 months, coupled with the limited number of death events. At present, it is challenging for us to provide long-term clinical outcomes. Fourth, the low number of events (n = 20 for progression) limits the statistical power of our findings and warrants caution in interpretation. Additionally, the high costs of TTFields therapy and its long duration of application present a challenge for healthcare systems. This is particularly relevant for patients with IDH-mutant grade 4 astrocytoma, who generally have better prognoses compared to IDH-wild glioblastoma. While TTFields therapy has shown promise in improving progression-free survival, the socio-economic burden and implications for healthcare resource allocation must be carefully considered in treatment planning. Balancing the costs with the potential benefits for patients is critical to optimize outcomes. Future studies should focus on larger multicenter cohorts to validate our findings and explore the molecular mechanisms underlying the observed prognostic factors. Additionally, prospective trials evaluating TTFields therapy in conjunction with emerging targeted therapies are warranted.
Author Contributions
Conception and design: Yang Wang and Zhiyong Qin. Acquisition of data: Xianxin Qiu, Liping Liang, Lingchao Chen, Jiabing Liu, Tianqi Wu, Mingyuan Pan, and Yihua Zhong. Analysis and interpretation of data: Xianxin Qiu, Pengjie Hong, Wanzun Lin, Zhirui Zhou, and Jing Gao. Drafting or revising the article: Xianxin Qiu and Yang Wang.
Acknowledgments
This study was supported by The National Natural Science Foundation of China (8240072705), Science and Technology Development Fund of Shanghai Pudong New Area (PKJ2021-Y48), and Beijing Science and Technology Innovation Medical Development Foundation (KC2023-JX-0288-BM150).
Ethics Statement
This retrospective research has been approved by the Institutional Review Board (IRB) of Huashan Hospital, Fudan University (Approval No. 2024–1128).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.




