Serotonergic and Dopaminergic Function in Neuropsychiatrically Asymptomatic People With HIV on Antiretroviral Therapy
Funding: This work was supported by Intramural Research Program at the National Institutes of Health: Clinical Center (CL010357; Center for Infectious Disease Imaging, Department of Radiology and Imaging Sciences), National Institute of Neurological Disorders and Stroke (Section of Infections of the Nervous System), and National Cancer Institute (Center for Cancer Research). The study was also funded through an office of AIDS Research (OAR) Innovation fund grant (PI: Dima A. Hammoud).
Chuen-Yen Lau and Anna Lyndaker contributed equally to this work.
ABSTRACT
Objective
People with HIV (PWH) on antiretroviral therapy (ART) still experience neurocognitive dysfunction and accelerated brain volume loss. To assess whether the serotonergic and dopaminergic systems are affected, we used [11C]DASB positron emission tomography (PET) to assess presynaptic serotonergic function and [18F]FDOPA PET to measure presynaptic dopaminergic reserve in neurocognitively and psychiatrically asymptomatic PWH on ART, compared to seronegative controls (SCs).
Methods
We compared [11C]DASB binding (BPND) (n = 17 PWH/19 SCs) and [18F]FDOPA influx constant (Ki) (n = 20 PWH/19 SCs) of PWH and SCs. We assessed correlations of Ki and BPND with CSF cytokines and imaging/clinical/neuropsychological outcomes using multivariable and univariable models.
Results
BPND, Ki, and neurocognitive and psychiatric scores did not differ between PWH and SCs. Higher BPND correlated with better neurocognitive scores in the whole group. Ki did not correlate with neurocognitive scores. Neither BPND nor Ki correlated with depression scores. Caudate and putamen MRI volumes trended smaller in PWH. While CSF inflammatory cytokines were higher in PWH, they did not correlate with either PET measure.
Interpretations
Presynaptic serotonin transporter density (measured by [11C]DASB) is a good correlate of the neurocognitive function in PWH and SCs. Despite long-term ART, PWH still showed trends for basal ganglia volume loss, consistent with known disproportionate global and regional volume loss in PWH compared to SCs. Aspects of serotonergic and dopaminergic function, namely, presynaptic SERT density and dopaminergic reserve, however, are not different in this PWH cohort on ART from SCs, despite persistent neuroinflammation and trends of volume loss. This could reflect a functional compensatory process versus minimal, below-detection-level dysfunction.
1 Introduction
Nearly half of people with HIV (PWH) experience some degree of HIV-associated neurocognitive disorder (HAND) despite effective combination antiretroviral therapy (cART) [1] and approximately one-third suffer from depression [2]. While HAND epidemiology has shifted to milder forms with modern cART [1], structural imaging with MRI has repeatedly shown evidence for neuronal injury in optimally treated PWH, depicted as general and regional volume loss [3, 4]. Fewer studies, however, have focused on corresponding cellular and molecular pathophysiology [5, 6] including individual neurotransmitter systems' function.
In the pre- and early-cART era, multiple studies had demonstrated dopaminergic and serotonergic dysfunction. One study evaluating serotonergic presynaptic activity with [11C]DASB PET in treated and untreated PWH found decreased serotonin reuptake transporter (SERT) binding in PWH compared to controls, suggesting serotonergic neuronal loss [7]. The dopaminergic system may also be dysregulated in chronic HIV. PET imaging of the dopamine reuptake transporter (DAT) in HIV-associated dementia showed decreased DAT activity in the basal ganglia relative to seronegative controls (SCs) [8]. Animal studies have shown a similar dysfunction: in acutely SIV-infected macaques, SERT density was elevated at multiple time points following infection [9], while evaluation of HIV-1 transgenic rats showed changes in pre- and post-synaptic dopaminergic transmission [10, 11]. Given the roles of dopamine and serotonin in mood and cognition [12, 13], we hypothesized that dysfunction of these neurotransmitter systems may correlate with neurocognitive changes in chronic HIV.
We evaluated presynaptic integrity of the dopaminergic and serotonergic systems in multiple brain regions of neurocognitively and psychiatrically asymptomatic, virologically suppressed PWH on ART using two PET radioligands: [11C]DASB which assesses presynaptic serotonin reuptake [14], and [18F]FDOPA, which measures presynaptic dopamine synthesis [15]. We compared PWH PET findings to SCs. The groups in this study were part of a prospectively followed, thoroughly characterized cohort of PWH on long-term ART and socioeconomically similar SCs (NIH-DOD NeuroHIV Consortium). In view of recently demonstrated significantly higher longitudinal gray matter volume loss in PWH compared to SCs within the same large cohort from which our participants were recruited [4], we hypothesized that PWH would show decreased [11C]DASB binding potential (BPND) and [18F]FDOPA influx constant (Ki) compared to SCs, reflecting presynaptic disruption of both neurotransmitter systems in the setting of HIV-associated neuronal injury, despite the lack of overt neurocognitive and psychiatric symptoms. To explore clinical implications, [18F]FDOPA influx and [11C]DASB binding were then correlated with MRI brain volumes, CSF inflammatory markers, clinical measures, and neuropsychological outcomes.
2 Materials and Methods
2.1 Standard Protocol Approvals, Registration, and Participant Consents
Participants were recruited and imaged through protocol #18-CC-0117 (NCT03581305: PET Imaging of the Dopaminergic and Serotonergic Systems in Treated HIV Positive Participants) and protocol #13-N-0149 (NCT01875588: An Evaluation of HIV-associated Neurocognitive Disorders in Virologically Controlled Patients), conducted at the National Institutes of Health (NIH). Protocols were approved by the NIH Institutional Review Board. All participants provided written informed consent.
2.2 Participants
We enrolled 20 PWH and 23 SCs from 2018 to 2022. The final populations after exclusions (detailed in the Results section) consisted of 17 PWH and 19 SCs in the serotonergic arm and 20 PWH and 19 SCs in the dopaminergic arm. In the final included populations, 17 PWH and 17 SCs enrolled in both arms. As a result, even though most participants in the serotonergic and dopaminergic arms overlapped, the populations were not exactly the same.
PWH participants had documented HIV-1 infection, plasma HIV-RNA below the limit of detection (< 40 copies/mm3) for at least 1 year, and at least 1 year of continuous ART prior to the last documented suppressed viral load on current ART. Socioeconomically similar HIV-seronegative participants were included in control groups.
Participants were excluded for conditions (e.g., intracranial tumors) that could interfere with study assessments, non-HAND conditions associated with cognitive impairment, psychiatric diseases known to affect dopaminergic or serotonergic systems, current substance use that would interfere with PET results per investigator judgment, use of any drug with dopaminergic or serotonergic activity within 6 months before imaging, pregnancy or lactation, exceeding the recommended annual limit of radiation, and any adverse reaction to carbidopa (dopaminergic arm).
2.3 Clinical and Laboratory Evaluation
All participants underwent medical history review, physical examination, and laboratory testing. Parameters associated with HIV infection were recorded for PWH. The laboratory measures we included in our analysis were C-reactive protein (CRP), D-dimer, CSF cytokines selected for assay detectability, and relevance to infection/inflammation (IL-8, IP-10, MCP-1, MIP-1β; Millipore Sigma HCYTOMAG-60 K) and hepatitis C virus (HCV) antibody with HCV RNA quantitative tests. One participant was positive for HCV antibodies, but not HCV RNA, and was not excluded. Participants underwent urine toxicology screening for amphetamines, benzodiazepines, cocaine, opiates, and cannabinoids on the day of scanning. Participants with positive cannabinoid results were initially excluded, but subsequently allowed after protocol amendment. Cardiovascular markers (blood pressure, cholesterol levels, diabetes, smoking, and use of anti-hypertensives, statins, or aspirin) were collected to calculate the atherosclerotic cardiovascular disease (ASCVD) risk scores [16].
2.4 Neuropsychiatric Evaluation
Neuropsychological data was available for all subjects except for two SCs in the [18F]FDOPA group whose evaluations were obtained more than 1 year from the PET scan.
Participants completed a detailed, thorough neuropsychological testing, covering 7 neurocognitive domains. These included (1) working memory, (2) information processing speed, (3) immediate recall, (4) delayed recall, (5) executive functioning, (6) verbal fluency, and (7) fine motor manual dexterity. Additional details about the specific tests are included in Supporting Information.
The outcome measures were overall T-score (overall neurocognitive functioning), global deficit score (GDS) (cognitive impairment), and the Beck Depression Inventory-II (BDI-II) (depression), as previously described [17]. Briefly, for each participant, the T-score was the average of demographically adjusted T-scores obtained from the battery tests (14 tests). The T-score range is 1–99, with a mean of 50 and a normal range of 40–60. Low T-scores reflect neurocognitive impairment [18]. Global Deficit Scores (GDS) were computed by first converting each individual T-score into a deficit score, ranging from 0 (T-score of 40 or greater) to 5 (T-score less than 20) and then averaging the deficit scores across the 14 tests. GDS measured the severity and frequency of performance deficits across these tests, with higher scores reflecting cognitive impairment [18, 19]. BDI-II scores were calculated using a 21-question test, with a score range from 0 to 63: scores of 14–19, 20–28, and 29–63 reflect mild, moderate, and severe depressive symptoms, respectively [20]. Finally, participants were diagnosed as having either “no HAND” or “HAND” based on the composite neuropsychological evaluation. All participants in this study with HAND or HAND-equivalent diagnosis (for SCs) had asymptomatic neurocognitive impairment (ANI), with no diagnoses of mild neurocognitive dysfunction (MND) or HIV-associated dementia (HAD) in any group [21].
2.5 Magnetic Resonance Imaging (MRI)
MRI imaging was performed as previously described [4]. MRI data was used if collected within a year from the PET. MRI scans closest to the PET date were selected if all necessary sequences for volumetric measurements were available.
Classification using DErivative-based Features (C-DEF) was used for automated segmentation of MRI to measure brain volumes (in mm3), as previously described [4]. Volumes for white matter, gray matter, and “white matter lesions” were quantified. The latter are foci of periventricular, deep and/or subcortical white matter high signal intensity on FLAIR images, generally seen with aging. When they are more than expected for age, and in the absence of other possible etiologies, they presumably reflect chronic small vessel ischemic changes due to atherosclerosis or other cardiovascular risk factors [22]. Gray matter to total parenchyma (white and gray matter) ratio was calculated.
For each MRI scan, thalamus, putamen, and caudate volumes were quantified using Freesurfer [23]. The Freesurfer volumes are expressed as a percentage of estimated total intracranial volume (% of eTIV) [24]. Additional details are included in the Supporting Information.
2.6 PET Imaging and Data Analysis
Details about the PET scanner used and [11C]DASB/[18F]FDOPA scan acquisitions are included in the Supporting Information.
PET images were analyzed using the PNEURO tool in PMOD version 4.105 (PMOD Technologies LC, Zurich, Switzerland). PET images were motion-corrected for head movement and co-registered to corresponding MR images. Volumes of interest (VOIs) in the caudate nuclei, putamina, thalami, amygdala, raphe nuclei, and anterior cingulate cortex (ACC) were identified using a custom atlas (modified from Hammers-N30R83 atlas) for the serotonergic arm. Additional regions with lower binding were compared between groups, including the frontal cortex (FC), occipital cortex, ACC, and hippocampi. For the dopaminergic arm, the caudate nuclei, putamina, thalami, and FC VOIs were selected. VOIs were mapped to each PET image using anatomical brain MR as a reference. Partial volume effect (PVE) correction was applied during analysis [25]. Both PVE-corrected and uncorrected output values were included in the statistical analysis. PET and MRI data were processed to generate VOI-specific time activity curves.
We calculated [11C]DASB binding potential normalized to non-displaceable tissue radioligand (BPND) and [18F]FDOPA influx rate constant (Ki). Analyses were performed using the batch analysis function in PNEURO, except for one [18F]FDOPA PET scan that was processed manually due to motion.
For [11C]DASB BPND calculations, we first used Ichise's Multilinear Reference Tissue Model-1 (MRTM1) to obtain region-specific fixed clearance rate (k2’), then averaged the k2’ values of the caudate, putamen, thalamus, and raphe nuclei for each participant. We then constrained this average k2’ to be identical across all brain regions. [11C]DASB BPND was then estimated using Ichise's Multilinear Reference Tissue Model-2 (MRTM2) with cerebellar gray matter (excluding the vermis) as the reference region (ideally free of specific binding due to low SERT) [26, 27].
[18F]FDOPA Ki, which reflects decarboxylation of [18F]FDOPA to and storage as [18F]fluorodopamine (estimate of the presynaptic dopaminergic function), was calculated using a linear Patlak Reference Tissue Model. As with previous studies, the occipital cortex was used as a reference region [28].
2.7 Statistics
Target sample size was determined based on anticipated differences between PWH and SCs based on Parkinson's Disease literature for FDOPA and prior experience with depressed PWH for DASB. For the FDOPA arm, the target sample size with alpha = 0.05 and 80% power was 23 participants per group. For the DASB arm, the target sample size with alpha = 0.05 and 90% power was 19 subjects per group.
Data was analyzed using SAS (version 9.4) and SAS/STAT (version 14.3) (SAS Institute Inc., Cary, NC, USA). Data availability for each parameter is specified in the tables.
Demographics between PWH and SCs in either arm were compared using the unpaired t-test (age) or χ2-test (gender and ethnicity distributions). Among PWH, the estimated duration of HIV positivity and estimated duration of treatment were compared between the dopaminergic and serotonergic arms using the unpaired t test, while the frequency of Nadir CD4 count < 200 versus Nadir CD4 > 200 (cells/μL) was compared using χ2-test. The unpaired t test was used to compare PVE-corrected and uncorrected BPND, Ki values, brain volumes, and neuropsychological measures between PWH and SCs. For clinical variables with non-parametric distribution, the Mann–Whitney test was used for comparison (ASCVD scores, CRP and D-dimer levels). For CSF cytokines, multiple unpaired t tests were used to compare CSF IL-8, IP-10, MCP-1, and MIP-1b levels between PWH and SCs separately in each arm, with p values adjusted by the Benjamini–Hochberg method for controlling for false discovery rate (FDR) [29]. For the comparison of proportions of HAND diagnoses in PWH and SC cohorts in the two arms, χ2-test with Yates' correction was used. To compare the age distributions of PWH and SCs cohorts in each arm, we used the Kolmogorov–Smirnov test.
In order to assess whether PET measures correlated with neuropsychiatric measures, we performed Pearson correlations within PWH only for BPND with GDS, T-scores, and BDI-II scores as well as Ki with GDS, T-scores, and BDI-II scores; the same analyses were then repeated in the entire group (PWH and SCs).
We also performed Pearson correlations of caudate, putamen, and thalamus BPND with corresponding caudate, putamen, and thalamus Ki values in patients who underwent both scans (n = 17 PWH and 17 SC). Then each Pearson correlation was tested against the null hypothesis that equals 0. An approximate t distribution was used to determine the p-value.
To explore the combination of clinical variables that best predict PVE-corrected and uncorrected BPND and Ki values in the entire group (PWH and SCs), we proceeded to perform multivariable analyses with the following explanatory variables: age, gender, 10-year ASCVD, CRP, D-dimer, CSF levels of IL-8, IP-10, MCP-1, and MIP-1β, HIV status, “white matter lesions” volume, gray matter volume, total parenchyma volume, gray matter/total parenchyma, total thalamus volume, total caudate volume, and total putamen volume. Age was included as a variable to account for its possible effect on the PET measures. Because the data on the candidate explanatory variables contained missing values, considering all the explanatory variables in one stepwise run would have resulted in the exclusion of many values, considerably weakening the analysis. Instead, the process of selecting the “best” (or most predictive) set of explanatory variables for the final model for BPND and Ki was achieved based on an automated stepwise approach using p-values and Schwarz Bayesian Criterion (using the SAS GLMSELECT procedure) combined with a manual iterative procedure that considered different subgroups of explanatory variables. As variables selected for the final multivariable model may be interrelated, univariable analyses was performed to interpret direction of individual slopes in final models. We elaborated on correlations with variables selected in the multivariable analysis, and on correlations that persisted after FDR adjustment in the univariable analysis.
All p-values were adjusted by FDR. Following the 2019 American Statistical Association recommendation [30], we did not label results “significant” or “non-significant”, but reported corresponding p-values, and when appropriate, point estimates with 95% confidence intervals. Although tables show results with various p-values, we elaborated only on results with p-values < 0.05.
3 Results
3.1 Study Population and Clinical and Laboratory Data
Among 18 PWH and 22 SCs originally included in the serotonergic arm, two SCs had neuropsychiatric findings meriting exclusion. One PWH and one SC had markedly decreased uptake of [11C]DASB in the brain, comparable to levels in people on SSRIs [31] despite no reported use of such medications by the subjects. When their [11C]DASB BPND values were compared to all participants, they were at least two standard deviations lower than mean values in high binding areas, and thus excluded as outliers (Figure S1). Our final population consisted of 17 PWH and 19 SCs (Table 1).
| Variable | Serotonergic arm | Dopaminergic arm | ||||
|---|---|---|---|---|---|---|
| PWH (n = 17) | SCs (n = 19) | p [FDR-adjusted p] | PWH (n = 20) | SCs (n = 19) | p [FDR-adjusted p] | |
| Age (years) | 59.31 ± 5.20; 59.50 (56.40–62.40) | 55.68 ± 7.37; 55.10 (52.60–58.80) | 0.100a | 59.07 ± 4.83; 59.15 (56.68–62.08) | 56.06 ± 7.40; 56.20 (52.60–59.60) | 0.139a |
| Sex, male: female | 13:4 | 10:9 | 0.137b | 14:6 | 10:9 | 0.421b |
| Ethnicity, white: black: other | 10:7:0 | 9:10:0 | 0.492b | 11:9:0 | 8:11:0 | 0.265b |
| Estimated duration of infection (years) | 21.72 ± 9.77; 24.07 (14.71–30.50) | N/A | N/A | 21.41 ± 9.21; 22.76 (14.11–29.29) | N/A | N/A |
| Estimated duration of antiretroviral treatment (years) | 17.62 ± 9.16; 19.02 (7.48–25.33) | N/A | N/A | 17.25 ± 8.78; 18.28 (9.55–24.10) | N/A | N/A |
| CD4 at time of scan (cells/μL) | 604 ± 218; 591 (463–708) | N/A | N/A | 613 ± 203; 589 (465–718) | N/A | N/A |
| Number of PWH with Nadir CD4 < 200 cells/μL, (%) | 7 (41.2%) | N/A | N/A | 8 (40%) | N/A | N/A |
| 10-Year ASCVD (%) | 9.79 ± 9.01; 6.40 (5.35–10.05) | 7.55 ± 6.73; 5.50 (2.40–9.90) | 0.216c | 9.75 ± 8.32; 6.90 (5.45–10.08) | 8.26 ± 6.67; 5.60 (2.60–10.30) | 0.399c |
| CRP (mg/L) | 5.58 ± 9.27; 1.70 (0.60–8.20) (n = 15) | 1.92 ± 1.73; 1.80 (0.45–3.45) (n = 17) | 0.427c | 6.66 ± 10.36; 1.90 (0.68–8.40) (n = 18) | 2.15 ± 1.69; 2.00 (0.60–3.65) (n = 17) | 0.530c |
| D-dimer (mcg/mL FEU) | 0.32 ± 0.29; 0.34 (0.00–0.55) (n = 14) | 0.46 ± 0.34; 0.37 (0.27–0.70) (n = 17) | 0.326c | 0.34 ± 0.26; 0.34 (0.00–0.53) (n = 17) | 0.46 ± 0.34; 0.37 (0.27–0.70) (n = 17) | 0.395c |
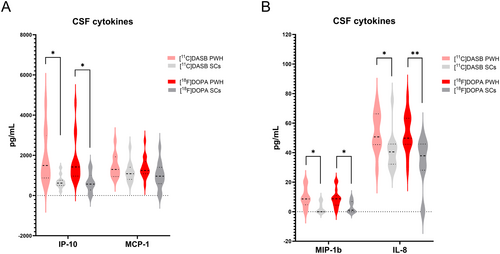
| IL-8 (pg/mL) | 54.46 ± 13.23; 55.43 (45.61–65.28) (n = 12) | 41.13 ± 12.75; 40.52 (32.20–45.81) (n = 12) | 0.020 [0.026] a | 53.36 ± 13.35; 49.81 (45.66–63.29) (n = 14) | 39.74 ± 8.34; 41.81 (31.27–46.18) (n = 10) | 0.002 [0.010] a |
| IP-10 (pg/mL) | 1791.76 ± 1222.28; 1443.64 (879.33–2667.34) (n = 12) | 671.02 ± 313.70; 618.62 (500.39–759.95) (n = 12) |
0.006 [0.011] a |
1726.91 ± 1138.16; 1422.85 (966.35–2081.50) (n = 14) | 775.95 ± 413.37; 630.39 (508.64–1163.18) (n = 10) |
0.005 [0.011] a |
| MCP-1 (pg/mL) | 1458.46 ± 539.60; 1359.69 (993.21–1849.03) (n = 12) | 1163.88 ± 490.84; 1088.02 (803.12–1406.73) (n = 12) | 0.176a | 1395.15 ± 539.99; 1247.94 (1087.52–1689.86) (n = 14) | 1184.55 ± 555.76; 1088.02 (764.25—1527.69) (n = 10) | 0.105a |
| MIP-1β (pg/mL) | 9.52 ± 6.33; 9.00 (5.15–13.50) (n = 12) | 2.50 ± 3.51; 0.00 (0.00–6.47) (n = 12) | 0.003 [0.011] a | 8.95 ± 6.15; 8.70 (4.46–11.03) (n = 14) | 2.27 ± 3.36; 0.00 (0.00–5.53) (n = 10) | 0.010 [0.014] a |
- Note: Data reported as mean ± standard deviation; median (IQR). Data was unavailable for some laboratory values. CD4 at the time of scan is reported for the closest sampling timepoint available. Significant p-values < 0.05 are shown in bold.
- Abbreviations: PWH, people with HIV; SCs, seronegative controls.
- a Unpaired t tests were used for comparing PWH and SCs.
- b Chi-squared tests were used for comparing PWH and SCs.
- c Mann–Whitney tests were used for comparing PWH and SCs.
We recruited 20 PWH and 22 SCs for the dopaminergic arm. The same two SCs who were excluded from the serotonergic arm for neuropsychiatric findings were excluded from the final [18F]FDOPA analysis. Additionally, one SC had markedly increased uptake of [18F]FDOPA across multiple brain regions, which was at least two standard deviations higher than the mean Ki of all participants; thus, it was excluded as an outlier (Figure S1). Our final population consisted of 20 PWH and 19 SCs (Table 1).
3.2 Demographics of Both Study Arms
The age ranges of our cohorts after the exclusions were 39.5–69.9 years for SCs and 49.3–68.2 years for PWH participants in both arms. The age ranges, mean, medians, interquartile ranges (IQRs), and distributions (histograms) of the cohorts in both arms are shown in Table 1 and/or in Figure S2.
In either the serotonergic or dopaminergic arms, there were no appreciable differences between PWH and SCs in age (Unpaired t test, p > 0.05), age distributions (Kolmogorov–Smirnov tests, p > 0.05), gender, or ethnicity distributions (χ2-tests, p > 0.05). Among PWH, both arms had similar estimated durations of HIV positivity, estimated durations of treatment (Unpaired t test, p > 0.05 for both), and frequencies of Nadir CD4 count < 200 (χ2-tests, p > 0.05) (Table 1) since the participants who underwent both scans did so within a short period of time.
3.3 Comparison of Laboratory and Clinical Measures Between PWH and SCs
There were no appreciable differences between D-Dimer and CRP levels or in ASCVD scores between PWH and SCs in either study arm (p > 0.05). When evaluating CSF cytokines, PWH participants had higher CSF IL-8, IP-10, MCP-1, and MIP-1b compared to SCs, with p-values < 0.05 for IL-8, IP-10, and MIP-1b in both groups (Table 1, Figure 1), before and after FDR correction.
3.4 Comparison of Neuropsychiatric Measures Between PWH and SCs
GDS, BDI-II, and overall T-scores did not differ between PWH and SCs in either the [11C]DASB or [18F]FDOPA groups (Table 2, Figure S3).
| Variable | PWH | SCs | p [FDR-adjusted] | |
|---|---|---|---|---|
| Serotonergic arm: Neuropsychological measures | GDS |
0.21 ± 0.25; 0.14 (0–0.36) |
0.19 ± 0.37; 0.07 (0–0.14) | 0.839 |
| BDI-II | 5.65 ± 5.06; 5.00 (1.00–9.50) | 3.68 ± 4.14; 1.00 (0.00–7.00) | 0.209 | |
| Overall T-score | 50.82 ± 6.49; 48.86 (45.79–53.90) | 54.44 ± 8.28; 53.64 (50.00–59.86) | 0.157 | |
| HAND (or HAND-equivalent diagnosis of asymptomatic neurocognitive dysfunction (ANI)a): no HAND (n) | 5:12 | 2:17 | 0.314b | |
| Serotonergic arm: MRI volumetric measures | Thalamic volume (% of eTIV) | 0.95 ± 0.11; 0.90 (0.88–1.00) | 0.95 ± 0.08; 0.93 (0.90–1.00) | 0.896 |
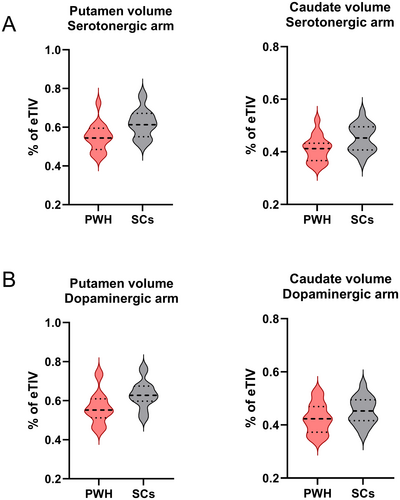
| Caudate volume (% of eTIV) | 0.41 ± 0.05; 0.41 (0.37–0.43) | 0.45 ± 0.05; 0.45 (0.41–0.50) | 0.023 [0.082] | |
| Putamen volume (% of eTIV) | 0.55 ± 0.07; 0.54 (0.49–0.59) | 0.62 ± 0.07; 0.61 (0.55–0.67) | 0.009 [0.061] | |
| White matter lesions volume (mm3) | 5564 ± 8081; 4355 (2197–5098) | 2893 ± 2509; 1781 (1181–3325) | 0.207 | |
| Gray matter volume (mm3) | 606,489 ± 75,302; 605,000 (538,106−682,851) | 622,719 ± 50,924; 622,762 (585,577–658,806) | 0.450 | |
| Total parenchyma volume (mm3) | 1,116,648 ± 151,091; 1,114,727 (990,870–1,250,848) | 1,130,300 ± 103,680; 1,097,591 (1,041,690–1,229,173) | 0.752 | |
| Gray matter/total parenchyma | 0.54 ± 0.02; 0.54 (0.53–0.55) | 0.55 ± 0.02; 0.55 (0.54–0.57) | 0.247 | |
| Dopaminergic arm: Neuropsychological measures | GDS | 0.21 ± 0.27; 0.07 (0.00–0.40) | 0.13 ± 0.17; 0.07 (0.00–0.18) | 0.294 |
| BDI-II | 6.20 ± 5.64; 5.50 (1.00–9.75) | 4.18 ± 6.83; 1.00 (0–6.50) | 0.330 | |
| Overall T-score | 51.92 ± 7.25; 51.43 (46.09–56.27) | 56.26 ± 6.97; 57.36 (50.64–60.04) | 0.073 | |
| HAND (or HAND-equivalent diagnosis of asymptomatic neurocognitive dysfunction (ANI)a): no HAND (n) | 6:14 | 1:17 | 0.111b | |
| Dopaminergic arm: MRI volumetric measures | Thalamus volume (% of eTIV) | 0.96 ± 0.11; 0.96 (0.88–1.00) | 0.98 ± 0.08; 0.98 (0.92–1.04) | 0.545 |
| Caudate volume (% of eTIV) | 0.42 ± 0.06; 0.42 (0.37–0.47) | 0.45 ± 0.05; 0.45 (0.42–0.49) | 0.126 | |
| Putamen volume (% of eTIV) | 0.56 ± 0.08; 0.55 (0.51–0.61) | 0.63 ± 0.07; 0.63 (0.60–0.68) | 0.015 [0.105] | |
| White matter lesions (mm3) | 5563 ± 7529; 4498 (2194–5417) | 2273 ± 2012; 1777 (1093–2485) | 0.074 | |
| Gray matter volume (mm3) |
603,738 ± 73,401; 606,445 (531,526–676,797) |
618,105 ± 41,975; 622,762 (588,709–651,500) | 0.463 | |
| Total parenchyma volume (mm3) | 1,113,469 ± 143,716; 1,117,554 (986,175–1,204,580) | 1,117,853 ± 96,547; 1,091,754 (1,040,254–1,201,553) | 0.916 | |
| Gray matter/Total parenchyma | 0.54 ± 0.02; 0.54 (0.53–0.55) | 0.55 ± 0.02; 0.55 (0.54–0.57) | 0.088 |
- Note: Data reported as mean ± standard deviation; median (IQR). Unpaired t-tests compared PWH and SC unless otherwise noted. Unadjusted p-values are reported. p-values < 0.05 are in bold but did not persist after false discovery rate adjustment. T-score range is 1–99, with a mean of 50. GDS range is 0 (no impairment) – 5 (severe impairment). BDI-II score range is 0–63.
- Abbreviations: BDI-II, Beck Depression Inventory-II; GDS, global deficit score; PWH, people with HIV; SCs, seronegative controls.
- a There were no participants diagnosed with either mild neurocognitive disorder or HIV-associated dementia.
- b Comparison of proportions done using the chi-squared test with Yates' correction.
3.5 Comparison of Brain Volumes on MRI Between PWH and SCs
Brain volumes were smaller in PWH compared to SCs in both arms prior to FDR correction. In [11C]DASB participants, putamen (difference = −0.07% of eTIV, 95% CI [−0.12, −0.02], p = 0.009) and caudate (difference = −0.04% of eTIV, 95% CI [−0.08, −0.01], p = 0.023) volumes were smaller in PWH compared to SCs. In the [18F]FDOPA participants, putamen (difference = −0.07% of eTIV, 95% CI [−0.12, −0.01], p = 0.015) volumes were also smaller in PWH compared to SCs (Table 2). After adjustment for FDR, however, all p-values were > 0.05 (Figure 2, Table 2).
3.6 Comparison of [11C]DASB BPND and [18F]FDOPA Ki Between PWH and SCs
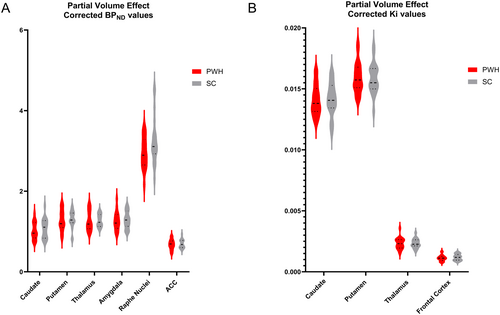
Though regional [11C]DASB BPND was mostly lower in PWH than SCs, there were no differences between the two groups, neither before nor after PVE correction (p > 0.05, unpaired t-test). There were no [18F]FDOPA Ki differences between PWH and SC in any assessed brain region, neither before nor after PVE correction (p > 0.05, unpaired t-test) (Figure 3).
3.7 Correlations of PET Measures With Neuropsychological Measures
All participants who underwent [11C]DASB scanning contributed neuropsychological data. In the [18F]FDOPA group, neuropsychological data for two SCs was not used because it was obtained more than 1 year from the scan (n = 17).
No correlations were found in the PWH group.
In the combined groups (PWH and SCs), there was a negative correlation between GDS and caudate BPND (PVE-corrected: slope = −0.39, p = 0.018, FDR-adjusted p = 0.034; PVE-uncorrected: slope = −0.36, p = 0.033, FDR-adjusted p = 0.053) and putamen BPND (PVE-corrected: slope = −0.42, p = 0.012, FDR-adjusted p = 0.035; PVE-uncorrected: slope = −0.37, p = 0.025, FDR-adjusted p = 0.074). In addition, caudate BPND correlated positively with overall T-score (PVE-corrected: slope = 0.38, p = 0.023, FDR-adjusted p = 0.034; PVE-uncorrected: slope = 0.35, p = 0.035, FDR-adjusted p = 0.053).
On the other hand, [18F]FDOPA Ki showed no correlations with GDS or overall T-scores.
Neither [11C]DASB BPND nor [18F]FDOPA Ki correlated with BDI-II scores.
3.8 Regional Correlations Between [11C]DASB BPND and [18F]FDOPA Ki
In participants who underwent both PET scans (n = 17 PWH and 17 SCs), [11C]DASB BPND correlated with [18F]FDOPA Ki in thalamus (PVE-corrected and uncorrected) among the full cohort of PWH and SCs (PVE-corrected: Pearson = 0.47, FDR-adjusted p = 0.017; Uncorrected: Pearson = 0.48, FDR-adjusted p = 0.017). Although the same correlations were identified among just PWH, FDR-adjusted p-values were > 0.05 (PVE-corrected: Pearson = 0.49, p = 0.045, FDR- adjusted p = 0.135; PVE-uncorrected: Pearson = 0.50, p = 0.041, FDR-adjusted p = 0.135).
3.9 Multivariable and Univariable Analyses for Exploration of the Combination of Variables That Best Predict BPND and Ki Values
Details of notable models are shown in Table 3. Multivariable analyses showed [11C]DASB BPND in the caudate correlated positively with gray matter/total parenchyma ratio. The gray matter/total parenchyma ratio was the only variable selected for both PVE-corrected (slope = 5.51, p = 0.004) and PVE-uncorrected (slope = 5.67, p = 0.004) BPND models.
| Regional BPND | Explanatory variables | Multivariable analysis | Univariable analysis | |||
|---|---|---|---|---|---|---|
| Slope [95% CI] | p | Slope [95% CI] | FDR-unadjusted p | FDR-adjusted p | ||
| Caudate PVE-corrected | GM/Parenchyma | 5.51 [1.87, 9.15] | 0.004 | N/A | ||
| Caudate PVE-uncorrected | GM/Parenchyma | 5.67 [1.98, 9.37] | 0.004 | N/A | ||
| Putamen PVE-corrected | GM/Parenchyma | 6.72 [3.57, 9.87] | 0.0001 | 6.90 [3.36, 10.44] | 0.0004 | 0.006 |
| WM lesions (mm3) | −1.36 × 10−5 [− 2.37 × 10−5, −3.64 × 10−6] | 0.009 | −1.66 × 10−5 [−2.93 × 10−5, −3.90 × 10−6] | 0.012 | 0.102 | |
| Caudate volume (% of eTIV) | −1.61 [−2.70, −0.51] | 0.005 | ||||
| Putamen PVE-uncorrected | GM/Parenchyma | 8.22 [4.36, 12.08] | 0.0001 | 7.59 [3.53, 11.65] | 0.001 | 0.010 |
| Caudate volume (% of eTIV) | −1.88 [−3.24, −0.52] | 0.008 | ||||
| Thalamus PVE-corrected | Total parenchymal volume (mm3) | −9.04 × 10−7 [−1.35 × 10−6, −4.55 × 10−7] | 0.0003 | −7.24 × 10−7 [−1.21 × 10−6, −2.37 × 10−7] | 0.005 | 0.053 |
| Age (years) | −0.01 [−0.02, 0.00] | 0.004 | ||||
| Thalamus PVE-uncorrected | Total parenchymal volume (mm3) | −1.02 × 10−6 [−1.51 × 10−6, −5.20 × 0−7] | 0.0002 | −8.20 × 10−7 [−1.36 × 10−6, −2.84 × 10−7] | 0.004 | 0.064 |
| Age (years) | −0.01 [−0.02, 0.00] | 0.004 | ||||
| Raphe nuclei PVE-uncorrected | IP-10 (pg/mL) | −4.35 × 10−4 [−6.44 × 10−4, −2.27 × 10−4] | 0.0003 | |||
| MCP-1 (pg/mL) | 7.88 × 10−4 [3.75 × 10−4, 1.20 × 10−3] | 0.001 | ||||
|
ACC PVE-uncorrected |
D-dimer (mcg/mL FEU) | −0.19 [−0.30, −0.08] | 0.001 | −0.16 [−0.29, −0.03] | 0.020 | 0.166 |
| WM lesions (mm3) | −8.09 × 10−6 [−1.34 × 10−5, −2.74 × 10−6] | 0.005 | −8.11 × 10−6 [−1.47 × 10−5, −1.50 × 10−6] | 0.018 | 0.166 | |
| Thalamus size (% of eTIV) | 0.48 [0.12, 0.84] | 0.011 | ||||
- Note: All multivariable models are shown. The explanatory variables in the multivariable models were selected from the following variables: age, gender, 10-year ASCVD, CRP, D-dimer, CSF levels of IL-8, IP-10, MCP-1 and MIP-1β, HIV status, “white matter lesions” volume, gray matter volume, total parenchyma volume, gray matter/total parenchyma, total thalamus volume, total caudate volume, and total putamen volume. Univariable correlations for the selected explanatory variables with unadjusted p < 0.05 are shown. Multivariable models with a single explanatory variable selected, or univariable results that persisted after FDR, are in bold.
- Abbreviations: % of eTIV, percentage of estimated total intracranial volume; ACC, anterior cingulate cortex; FEU, fibrinogen equivalent units; GM, gray matter; PVE, partial volume effect; WM, white matter.
For putamen, the gray matter/total parenchyma ratio was also selected in the multivariable model (PVE-corrected: slope = 6.72, p = 0.0001; PVE-uncorrected: slope = 8.22, p = 0.0001) and the univariable analysis confirmed the positive correlation persisting after FDR (PVE-corrected: slope = 6.90, FDR-adjusted p = 0.006; PVE-uncorrected: slope = 7.59, FDR-adjusted p = 0.010). The “White Matter Lesions” variable was selected with the corrected model (slope = −1.36 × 10−5, p = 0.009) and the univariable analysis confirmed the negative correlation (slope = −1.66 × 10−5, p = 0.012), but this did not persist after FDR. Caudate size was selected in the multivariable PVE-corrected (slope = −1.61, p = 0.005) and uncorrected (slope = −1.88, p = 0.008) putamen models; however, the correlation was not seen in the univariable analysis.
By multivariable analysis, both PVE-corrected and uncorrected [11C]DASB BPND values for the thalamus correlated with total parenchymal volume (PVE-corrected: slope = −9.04 × 10−7, p = 0.0003; PVE-uncorrected: slope = −1.02 × 10−6, p = 0.0002); age was also selected in both models (PVE-corrected: slope = −0.01, p = 0.004; PVE-uncorrected: slope = −0.01, p = 0.004). The relationship between thalamus BPND and total parenchymal volume, but not age, was found in the univariable analysis (PVE-corrected: slope = −7.24 × 10−7, p = 0.005; PVE-uncorrected: slope = −8.20 × 10−7, p = 0.004). These univariable correlations did not persist after FDR.
Uncorrected raphe [11C]DASB BPND did not correlate with brain volumes but did correlate with CSF IP-10 (slope = −4.35 × 10−4, p = 0.0003) and MCP-1 (slope = 7.88 × 10−4, p = 0.001). These correlations, however, were not detected in the univariable analysis.
Uncorrected ACC correlated with D-dimer (slope = −0.19, p = 0.001), thalamus size (slope = 0.48 BPND p = 0.011), and “white matter lesions” (slope = −8.09 × 10−6, p = 0.005). The univariable analysis mirrored the multivariable correlations between PVE-uncorrected ACC BPND and D-dimer (slope = −0.16, unadjusted p = 0.020) and white matter lesions volume (slope = −8.11 × 10−6, unadjusted p = 0.018), but not thalamus volume. However, adjusted p-values were > 0.05 for D-dimer and “white matter lesions” volume.
No other variables were selected in the multivariable or univariable models for any region. Specifically, HIV seropositivity was not selected as a predictive variable in any analysis.
Multivariable analysis showed no correlations between [18F]FDOPA Ki and clinical or laboratory variables.
4 Discussion
Looking at the combined groups of PWH and SCs, we found that presynaptic serotonin transporter (SERT) density as measured by [11C]DASB PET is a good correlate of neurocognitive function, as estimated by GDS (lower BPND with higher GDS) and overall T-score (lower BPND with lower T-scores), regardless of HIV status. We also found trends for smaller subcortical brain volumes in our cohort of virologically controlled neurocognitively asymptomatic PWH with no clinical depression, compared to socioeconomically similar SCs on MRI. This is consistent with previous findings that, despite advancements in HIV treatment and decreased severity of HAND, PWH on long-term ART still have regional (mainly basal ganglia) and global volume loss compared to SCs [3, 32-34], including in the larger cohort from which our participants were recruited [4]. Using PET, however, we found no concurrent differences in [11C]DASB binding (which reflects presynaptic SERT density [35]), or [18F]FDOPA PET influx constant (Ki) (which reflects DOPA neuronal transport/decarboxylation and dopamine storage capacity [36]) between PWH and SCs. This is different from prior studies from the pre- and early cART eras where dysfunctional dopaminergic and serotonergic systems have been described [7, 8, 37, 38]. This discrepancy between structural and functional abnormalities in the setting of otherwise effective ART could potentially reflect an efficient compensatory mechanism involving the remaining specialized neurons in the basal ganglia and brainstem, maintaining overall presynaptic function of both systems. Similar neurological compensation has been previously shown in HIV using other imaging techniques such as fMRI [39, 40]. An alternative explanation is that the level of dysfunction affecting the two neurotransmitter systems is below detection levels for our imaging method and sample sizes.
[11C]DASB is a SERT-specific ligand with high affinity and selectivity for SERT over DAT and norepinephrine transporter (NET) [35]. SERT has a key role in the fine-tuning of serotonin neurotransmission by controlling the magnitude and duration of serotonin transport into and release from the presynaptic neuron. [11C]DASB PET has demonstrated abnormalities in multiple neuropsychiatric conditions, including major depressive disorder [41], substance use [42], and Parkinson's disease [43]. Prior work revealed that sub-optimally controlled PWH showed lower [11C]DASB binding compared to SCs7. Depressed PWH had higher BPND values than non-depressed PWH, suggesting SERT upregulation above baseline, resulting in lower synaptic serotonin [7]. Furthermore, upregulation of SERT binding was demonstrated longitudinally in SIV-infected monkeys [9]. Even though [11C]DASB BPND was generally lower in the current non-depressed PWH and SC cohorts, we found no differences in SERT expression before or after PVE correction despite smaller caudate and putaminal volumes in PWH (Table 2). PVE-corrected and uncorrected BPND in caudate and putamen also positively correlated with the ratio of gray matter/total parenchyma; lower [11C]DASB binding was seen in participants with more cortical and subcortical neuronal loss. The physiologic consequences of decreased SERT expression are unclear. In our combined cohort of PWH and SCs, BPND of the caudate and putamen correlated negatively with GDS (lower BPND with higher GDS) and BPND of the caudate correlated positively with overall T-score (lower BPND with lower T-scores), both measures of decreasing cognitive capacity, regardless of HIV serostatus. Our findings are consistent with other studies which showed that lower SERT expression, mainly in the limbic regions, correlated with greater deficits in auditory-verbal and visual–spatial memory and semantic fluency in people with mild cognitive dysfunction [44].
Considering that serotonin plays an important role in mood regulation [45], we sought but found no correlation between [11C]DASB BPND and BDI-II in our PWH cohort or when PWH and SCs are combined. Our findings could be due to a lack of clinically depressed participants in our cohorts. Inclusion of unmedicated depressed PWH would have been valuable, but would have been logistically difficult and ethically challenging since such participants would need to be immediately started on antidepressants upon diagnosis and, as a result, cannot get [11C]DASB PET.
Involvement of the dopaminergic system in the pathophysiology of neuroHIV has long been suspected. Early in the epidemic, dopamine-rich areas were shown to be particularly affected, namely the striatum, substantia nigra, and pre-FC, with higher numbers of infected cells and HIV RNA detected in those regions [37, 46]. Also, SIV-infected animals with pharmacologically increased dopamine showed higher brain viral loads [47]. Subsequently, PET imaging of DAT in PWH showed decreased DAT availability in PWH with dementia versus those without dementia [8].
We measured [18F]FDOPA Ki, an influx constant representing [18F]FDOPA decarboxylation and storage as [18F]fluorodopamine, for quantification of presynaptic dopaminergic function [28]. [18F]FDOPA has mainly been used in Parkinson's disease (PD), which is caused by dopaminergic neuronal degeneration [48]. We found no Ki differences between our PWH on ART and SC cohorts, and no correlations between Ki and brain volumes or clinical parameters. In our PWH, however, striatal volumes trended smaller than those of SCs despite similar Ki values. This could reflect increased dopamine synthesis (preserved Ki) to compensate for neuronal loss (smaller volumes), sustaining dopaminergic reserve. As with other conditions such as early PD, this could potentially result in pseudo-normalization of Ki values [49]. It could have been useful to include PWH participants with parkinsonian symptomatology where dopaminergic neuronal loss is advanced with decreased Ki values seen [50]. However, considering the complicated pathophysiology of later stages of PD, with heterogeneous manifestations, it would have been difficult to extract the specific role of HIV in this process using PET imaging sample numbers.
Our cohort is different from previously evaluated cohorts. While a 2004 study showed decreased DAT levels in PWH8, that population consisted mostly of viremic participants, despite all, except one, being on ART. Since 2004, HIV treatment advances have resulted in more robust suppression of viral replication and concurrent decreases in neurocognitive and neuropsychiatric dysfunction. All our PWH had undetectable viral loads and showed no differences in neurocognitive function compared to SCs (Table 2). The same processes which preserve SERT density and dopaminergic reserve probably underlie the preservation of neurocognitive function in PWH, despite trends for more brain volume loss compared to SCs. Our results, however, should not be generalized to PWH with neurocognitive abnormalities or psychiatric manifestations since our cohort of PWH had no overt neuropsychiatric symptoms.
It is important to note that in our PWH cohort, measurable CSF cytokines, including IL-8, IP-10, and MIP-1b values, were appreciably higher in our PWH compared to SCs (Table 1), with p-values < 0.05 in both study arms (Figure 1). This suggests a persistent neuroinflammatory state in PWH, despite viral suppression both in the periphery and CNS. This, however, does not seem to affect the evaluated neurotransmitter systems as no strong correlations between those cytokines and [11C]DASB BPND or [18F]FDOPA Ki were found to suggest neuroinflammation as a factor.
Our study has limitations. While our PET measures reflect important aspects of serotonergic and dopaminergic function, they do not provide a complete picture of the full functionality and integrity of those neurotransmitter systems. Assessing other components such as pre- and postsynaptic receptors, vesicular transporters, and other transporters might provide different results. Also, findings from this study should not be generalized to PWH who have symptomatic HAND, are depressed, or whose disease is not well-controlled, in whom prior studies detected differences [7, 8]. Cross-sectional design precludes assessment of longitudinal changes of function between PWH and SCs, which would be more informative, as longitudinal intra-person differences could be assessed. Even though our sample numbers are appropriate for PET studies, it is possible that our study was underpowered and that differences between PWH and SCs are below detection levels for the sample sizes. The fact that CSF cytokines are significantly higher in PWH and brain volumes trended lower than SCs suggests that the lack of differences in PET measures is less likely to be solely due to small sample sizes and supports the alternative possibility of compensatory mechanisms. The small sample size, on the other hand, could be the reason that subcortical brain volume reductions in PWH compared to SCs lost significance after FDR adjustment, especially that similar results from the larger cohort (which includes our participants) showed significant volume loss in PWH compared to SCs [4]. Another limitation is that, even though we had some information regarding ART regimens, historical information regarding previous treatment was not completely available in this chronically treated population. As such, it was not feasible to stratify the various regimens in categories and assess for their potential effects on PET measures. Finally, the PWH and SC populations were not strictly age matched. There were, however, no differences in ages or age distributions between the groups in either arm, and age was included as a variable in the univariable and multivariable analyses.
In conclusion, despite subcortical brain volume loss trends and persistent neuroinflammation, we did not find differences in [11C]DASB BPND and [18F]FDOPA Ki to suggest disrupted presynaptic SERT density or dopaminergic reserve in our virologically controlled PWH on ART compared to socioeconomically similar SCs. This is different from older reports that described neurotransmitter dysfunction in PWH. Although our results could be partially due to sample size limitations and decreases below the level of detection, the known very high sensitivity of PET imaging and the concurrent identification of structural and inflammatory changes in the same cohorts argue for compensatory serotonergic and dopaminergic mechanisms in PWH.
Author Contributions
D.A.H., A.N., B.R.S. and C.-Y.L. contributed to the conception and design of the study. C.-Y.L., A.L., S.S., P.W., A.M., C.M., A.K., T.L.C., G.N., J.S., B.R.S. and D.A.H. contributed to the acquisition and analysis of data. C.-Y.L., A.L., S.S., B.R.S., A.N. and D.A.H. contributed to drafting the text or preparing the figures.
Acknowledgments
This work has been funded by the Intramural Research Program at the National Institutes of Health: Clinical Center (CL010357; Center for Infectious Disease Imaging, Department of Radiology and Imaging Sciences), National Institute of Neurological Disorders and Stroke (Section of Infections of the Nervous System), and National Cancer Institute (Center for Cancer Research). The study was also funded through an office of AIDS Research (OAR) Innovation fund grant (PI: Dima A. Hammoud). We are grateful for the contributions of the NIH Clinical Center PET Department, our protocol support staff, and our study volunteers.
Conflicts of Interest
The authors declare no conflicts of interest. The data will be made available upon request, subject to NIH's data sharing policies. The views, information or content, and conclusions presented do not necessarily represent the official position or policy of, nor should any official endorsement be inferred on the part of, the Clinical Center, the National Institutes of Health, or the Department of Health and Human Services.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




