Cerebral autoinflammatory disease treated with anakinra
Funding Information
This study was supported by National Research Foundation of Korea (NRF) grants funded by the Ministry of Science, ICT & Future Planning, and the Republic of Korea (2016R1C1B2011815 and 2016 M3C7A1914002). Y.J., K.A.W. and S-T.L. were supported by the Lee Sueng Moon Research Fund of Seoul National University Hospital (3020170130).
Abstract
Interest in autoimmune encephalitis has been growing since the discovery of various autoimmune antibodies, such as N-methyl D-aspartate receptors antibody and leucine-rich glioma-inactivated 1 antibody. However, in contrast to autoimmune encephalitis associated with dysregulated adaptive immunity in the brain, the question of whether innate immunity-mediated autoinflammatory diseases exist in the brain has drawn much attention. Herein, we report a patient with microglia-dominant acute autoinflammatory encephalitis successfully treated with anakinra, an including interleukin-1 receptor blocker. In comparison to systemic autoinflammatory disease, we term this encephalitis cerebral autoinflammatory disease. Cerebral autoinflammatory disease could suggest new conceptual approaches to patients previously diagnosed with an unspecified encephalitis.
Introduction
Autoimmune encephalitis is an emerging neurologic disease that is mainly mediated by autoantibodies reacting to neuronal synapses or intracellular antigens, such as N-methyl D-aspartate receptors (NMDAR) and leucine-rich glioma-inactivated 1 (LGI1).1 The pathogenesis of this antibody-mediated encephalopathy is being elucidated, and various modes of pathogenesis, such as antigen presentation, B-cell proliferation by germinal center reaction, and plasma cell infiltrations into the brain, are under investigation.1-3 Meanwhile, in some encephalitides, such as steroid-responsive encephalitis with autoimmune thyroiditis (SREAT), there are no detectable pathogenic antibodies, and T-cell–mediated pathogenesis is possible.4, 5 Nevertheless, antibody-mediated and effector T-cell–mediated encephalitis are associated with adaptive immune responses in the brain, and the idea that there exists innate immunity-mediated autoinflammatory diseases in the brain has not attracted significant attention thus far.
In contrast to autoimmune diseases, in which T and B lymphocytes are dysregulated, autoinflammatory diseases are differently defined by innate immunity disorders, mediated by monocytes, macrophages, and specific dominant cytokines, including interleukin (IL)-1α and IL-1β.6 Systemic autoinflammatory diseases (SAIDs) include hereditary febrile diseases (such as familial Mediterranean fever, a cryopyrin-associated periodic fever), Still's disease, Schnitzler syndrome, gout, and type 2 diabetes.6 In the central nervous system, microglia are tissue-resident macrophages that play a pivotal role in the innate immune system.7 Findings of the last decade have led to a growing understanding of the contribution of activated microglia to brain injury in neurodegenerative diseases, trauma, and stroke.8-11 However, it is not clear if the acute autoinflammatory encephalitis primarily mediated by excessive microglial activation, as a brain version of SAID, is possible in the brain.
Here, we show an evidence for microglia-dominant acute neuroinflammation in brain, and suggest that anakinra, the IL-1 receptor (IL-1R) blocker, can be a therapeutic option for the otherwise unexplained cerebral inflammatory syndromes.
Patient and Methods
Clinical presentation of the patient and initial treatment
A 51-year-old man was admitted due to progressive altered mental health. He was previously healthy and had no recent history of vaccination. At 12 days before admission, he developed fever, which persisted for 1 week and was treated with oseltamivir for possible influenza infection. However, 2 days before admission, he developed agitative and disorderly behaviors and became disoriented, which rapidly progressed to a semicoma state during his stay in another local hospital. On the day of admission (day 0), the patient was unresponsive to verbal or visual stimuli and showed minimal movements in response to pain with decorticated posture. Babinski signs were positive bilaterally. Brain magnetic resonance imaging (MRI #1, day 0) showed multifocal, patchy T2-hyperintensity in white matter, which showed a strong diffusion restriction in diffusion-weighted imaging (DWI) (Figure 1A). Cerebrospinal fluid (CSF) testing revealed elevated protein (82 mg/dL) without a cell, and normal glucose CSF/serum ratio (81 mg/dL/115 mg/dL). Cytology and oligoclonal band test were negative, with normal immunoglobulin G index (0.57). Viral polymerase chain reactions (PCR), serological tests of pathogens, and autoimmune autoantibodies, such as anti-ganglioside, myelin oligodendrocyte glycoprotein, aquaporin 4, myelin-associated glycoprotein, and autoimmune encephalitis antibodies were all negative in both serum and CSF (detailed profiles listed in Data S1). Malignancy screenings with chest computed tomography and stool hemoglobin were negative. The only positive test was Influenza B in the PCR analysis of sputum, suggesting a recent infection.
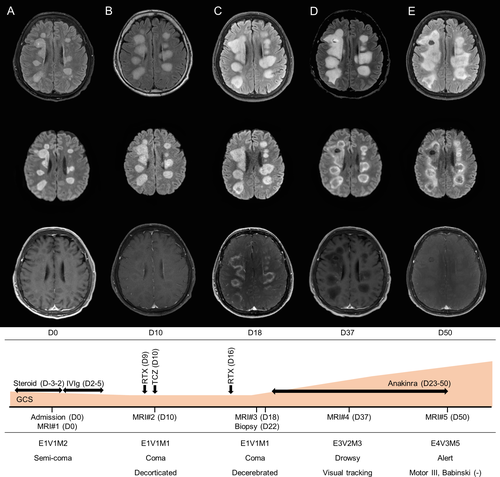
In suspicion of postviral acute demyelinating encephalomyelitis (ADEM), intravenous methylprednisolone (1000 mg per day, from day 3 to day 2) was administered for the 5 days, but he progressed into a coma. We then treated the patient with 5 days of intravenous immunoglobulin (IVIg) (400 mg/kg per day, from day 2 to 5), but the patient and MRI progressed further. MRI #2 at day 10 showed increased patchy T2-hyperintense lesions, newly involving the pons and bilateral cerebellar hemispheres. Each lesion still showed strong diffusion restriction without enhancement (Figure 1B). Given that some intractable ADEM and autoimmune encephalitides respond to rituximab or tocilizumab,12, 13 we tried rituximab (375 mg/m2 weekly, twice, at day 9 and 16) and tocilizumab (4 mg/kg, once, at day 10). Nevertheless, MRI #3 at 18 days revealed more aggravation of T2-hyperintense lesions with persistent strong diffusion restriction and newly exhibited rim enhancement on the lesion margins (Figure 1C).
Result
Pathologic analysis
The diffusion restriction in DWI indicates either cytotoxic edema or high cellularity, and the former cases usually show normalized diffusion within 2 weeks.14 Because the MRI showed strong prolonged diffusion restriction for 20 days, high cellularity pathology was suggested for this case (Figure 1D). Accordingly, a brain biopsy was performed to define the encephalitis pathology, with some suspicion of the involvement of lymphoproliferative diseases.
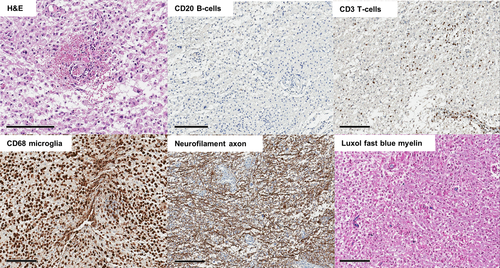
Brain biopsy on day 22 targeted a right subcortical area. The pathology report indicated massive CD68/CD163-positive microglial proliferation as the key feature of the lesion. There was also demyelination on Luxol fast blue stain and minimal CD3-positive T-cell infiltration, suggesting the disease can be put into the ADEM category. There was no vasculitic finding nor CD20-positive B-cell infiltration. Massive microglial proliferation caused high cellularity, showing the prolonged MRI diffusion restriction in the lesion (Figure 2).
Anakinra treatment and response
Based on the pathologic result, microglia, the innate immune cells, were targeted. Because microglia react to IL-1,15 the interleukin-1 receptor antagonist anakinra was administered daily (100 mg per day) from day 23 for 1 month. On day 28, the patient first recovered a blinking response to visual threats. On day 37, he became capable of mirroring a few tasks, including mouth opening. At MRI #4 on day 37, the diffusion restriction began to disappear, indicating the breakdown of high cellular microglia clouding. T2 lesions remained equivocal, with no T1 contrast enhancement. On day 50, the last day of anakinra administration, the patient was able to obey simple verbal commands and show spontaneous limb movements against gravity. MRI #5 on day 50 showed normalizing diffusion restriction (Figure 1E). Currently, the patient is alive and is undergoing standing and assisted walking exercises in a rehabilitation unit.
Inflammatory cytokines
We measured inflammatory cytokines in the initial serum and CSF. Even though we used high-sensitive ELISA measurement of IL-1β, it was not detected in CSF, as in another case.16 IL-1β might be locally active, and even if released into CSF, it would not be easily detectable. Accordingly, measuring IL-1β might not be a biomarker for cerebral autoinflammatory diseases. Other cytokine results are listed in the Table S1. The analysis of autoantibodies and cytokines was approved by Seoul National University Hospital Institutional Review Board.
Discussion
Herein, we demonstrate a case of ADEM-like syndrome with pathologic evidence of microglia-dominant neuroinflammation in brain which was successfully treated with anakinra targeting IL-1β. Our case implies that an uncontrolled innate immune reaction can be a possible pathophysiology for encephalitis otherwise unexplained previously.
The clinical and microscopic manifestation of autoinflammation in the central nervous system (CNS) is worth further investigation since as it could introduce newer therapeutic options in refractory encephalitis, as in our case. In parallel with the conventional definition of autoinflammatory diseases,6, 17 the following criteria would be helpful in establishing the concept of cerebral autoinflammatory disease (CAID) for further discussion: (1) increased cerebral inflammation mediated by the cells and molecules of the innate immune system, (2) minimal engagement of autoantibodies or autoreactive T cells, and (3) exclusion of alternative causes. As noted in the case description, clinical response to immunomodulating agents targeting an adaptive immune system can be poor, including corticosteroid, IVIg, and rituximab. Since the term focuses on the pathogenesis of inflammation, the status of CAID as a disease entity remains to be clarified among the broad spectrum of encephalitis.
In the brain, microglia are the resident innate immune cells and are responsible for the early control of infection and damaged brain tissues. Similar to other innate immune cells, microglia generate NLR family pyrin domain containing 3 (NLRP3) autoinflammasomes by using NOD-like receptor (NLR) and damage-associated molecular pattern (DAMP), and finally produce IL-1 for their proliferation and the activation of antiviral reactions and acute inflammatory immune cascades.15 In our case, instead of switching to the adaptive immune system, the immune process seems to have halted at the stage of microglial activation by an unknown cause (possibly the previous viral infection), promoting a vicious cycle of autoinflammation. Thus, regarding the pathogenesis, our case can be categorized as CAID. This is further supported by the patient's favorable response to anakinra, an IL-1R antagonist, which blocks the initiation of the microglial activation.6 However, there is a possibility that the previous immune therapies could have influenced the clinical and microscopic manifestation of the patient by removing lymphocytes (or other pathologies). Moreover, the possibility cannot be excluded that the natural history of the encephalitis coincidentally affected the patient's prognosis regardless of the anakinra response. Our case has a limitation that serial biopsy, which could have supported the hypothesis by showing the proliferation and the removal of microglia, was impossible because of the ethical issue.
The presence of CAID could suggest new conceptual approaches to patients previously diagnosed with an unspecified encephalitis. First, the molecular pathways related to the inflammasome may be promising candidates to be investigated in addition to seeking novel antibodies, which is the current mainstream research. Given that few neutrophils were shown in the patient's microscopic examination and that tissue-resident macrophages are derived from embryonic development, not from bone marrow,18 the patient's autoinflammation seems to be an innate reaction of the brain. It is a limitation that we could not perform the genetic test for known causes of autoinflammation such as NLRP3/CIAS1, MEFV, MVK, IL1RA, and TNFRSF1A.17 Verification of the genetic mutations on the innate immune system would be an important next step in researching CAIDs. Second, immune therapies targeting the molecules of the innate immune system can be alternative therapeutic options for patients diagnosed with CAID, as our case responded to anakinra. Lastly, further studies should be performed to find a biomarker differentiating CAIDs from autoimmune encephalitis because, for now, analyzing the profile of dysregulated immune cells via brain biopsy is the only way to make a decision.
In conclusion, our case suggests that anakinra, the IL-1R blocker, can be utilized as a therapeutic option for clinically refractory encephalitis with microglia-dominant neuroinflammation in the CNS. We propose the concept of CAID to elucidate the clinicopathological spectrum of encephalitis attributable to the predominance of innate immune response. Further studies are required with larger numbers of patients previously diagnosed with unspecified and otherwise refractory encephalitis to verify the extent of cerebral autoinflammation.
Acknowledgments
This study was supported by National Research Foundation of Korea (NRF) grants funded by the Ministry of Science, ICT & Future Planning, and the Republic of Korea (2016R1C1B2011815 and 2016 M3C7A1914002). Y.J., K.A.W. and S-T.L. were supported by the Lee Sueng Moon Research Fund of Seoul National University Hospital (3020170130).
Author Contributions
Y.J, K.A.W, and S.T.L. wrote and revised the manuscript. S.T.L analyzed the autoantibody tests. K.A.W, Y.J., and S.T.L. collected clinical data. S.H.P. analyzed the pathologic results. S.T.L. provided study concepts. K.C. and S.K.L. provided materials and funding. S.T.L. supervised the study. All authors reviewed the manuscript.
Conflict of Interests
The authors declare no conflict of interests.




