Clinical and infarction patterns of PFO-related cryptogenic strokes and a prediction model
Abstract
Objectives
The higher than expected PFO rate in CS patients has raised concerns that paradoxical embolism maybe the pathophysiologic mechanism for strokes. However, only a small proportion of pathogenic PFOs cause CS. Therefore, accurate recognition of patients with pathogenic PFOs among all CS patients could guide clinical decision making in selecting the most appropriate treatment. The aim of this study was to devise a new algorithm to stratify cryptogenic stroke (CS) patients into pathogenic patent foramen ovale (p-PFO)- and non-p-PFO-related patients.
Methods
A total of 1201 patients with acute ischemic stroke were recruited from two different medical centers, and 253 CS patients were identified. Of the 253 patients, 111 were diagnosed with PFO using contrast transcranial Doppler. Data on medical histories, neuroimaging and laboratory tests were compared in CS patients with or without PFO.
Results
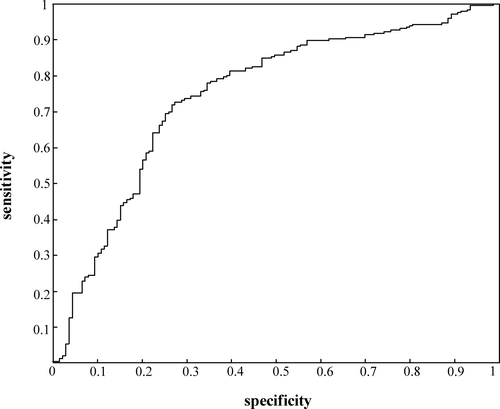
Compared with PFO-negative CS patients, PFO-positive CS patients showed younger onset age, lower incidence of hypertension and dyslipidemia, characteristic infarction pattern in magnetic resonance imaging and specifically altered platelet activity and coagulation function. Based on the above information, we constructed a PFO judgment formula (Hr-PFOJ) by means of feature weight estimation and predictive performance evaluation to predict pathogenic PFO in CS patients with a sensitivity of 76.3% and a specificity of 66.5%.
Interpretations
Hr-PFOJ judgment formula is a useful screening tool for identification of patients with pathogenic PFO who may benefit from PFO-related treatment.
Introduction
Cryptogenic stroke (CS) is a type of stroke without a well-defined etiology.1-3 Epidemiological studies demonstrated that the prevalence of patent foramen ovale (PFO) in CS patients (40–56%) is much higher than that in normal healthy population (4–18%), suggesting a potential association between PFO and CS.4-7 PFO may act as the pathway of thrombi from the venous circulation to cerebral circulation2 and could induce transient arrhythmia, both of which are recognized as potential underlying mechanisms for PFO-induced CS.8 However, some researchers considered PFO as an anatomic variant,9 which could be just an incidental event for CS, rather than a cause.8, 10 A few randomized clinical trials even showed that closure of PFO does not significantly reduce cerebral ischemic events when compared with controls.7, 11 One possible reason for the inconsistent conclusions in these studies is the nonstratification of patients with PFO into pathogenic and incidental groups, and many incidental patients with PFO who may not benefit from PFO closure were included. This speculation was supported by recent studies that enrolled patients with a potential high-risk PFO, such as younger patients,12 patients with an atrial septal aneurysm, hypermobility or large interatrial shunt,12-14 have demonstrated reduced recurrence rate by PFO closure. In addition, in line with the observation, another study enrolled 664 patients, of whom 81% had moderate or large PFO, also showed positive clinical outcome by PFO closure.15
Therefore, identifying featured data of pathogenic PFO for further stratification of CS patients is important. Previous studies described a few clinical features that may indicate pathogenic PFO, such as younger age, coexisting septal aneurysm, positive venous hypercoagulability tests and absence of vascular risk factors.3, 16, 17 However, instead of considering only partial aspect of the disease, studies that incorporate comprehensive data of clinical manifestations, neuro-imaging tests and laboratory results to recognize PFO-induced stroke remain lacking. In this study, we aimed to devise a new algorithm to stratify CS patients into pathogenic patent foramen ovale (p-PFO)- and non-p-PFO-related patients. We initially compared the onset age, stroke risk factors, infarction patterns in neuroimaging, platelet function, coagulation function and hemorheology in PFO+ and PFO- CS patients. We then evaluated the different clinical characteristics between the two groups and further constructed a pathogenic PFO prediction model termed PFO judgment formula (Hr-PFOJ) by feature weight estimation and predictive performance evaluation. Our Hr-PFOJ could serve as a valuable tool for identification of pathogenic PFO and the subsequent clinical treatment decision in CS patients.
Methods
Study population
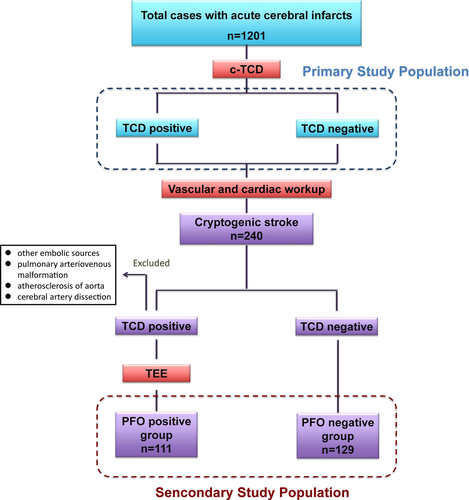
This study consecutively recruited 1417 patients with an acute ischemic stroke attending the stroke unit at Tongji Hospital and Wuhan Dongxihu People's Hospital between 1 October 2010 and 31 April 2017. Contrast-transcranial Doppler (c-TCD) was feasible in 1201 patients (81.64%). Of the 216 patients who did not receive c-TCD, 36 patients refused this examinations, 59 patients were unsuitable or could not tolerant the testing (such as pregnancy, aphasia and so on), 121 patients had poor temporal window or poor cooperation. Consequently, the study evaluated data from 1201 patients for the primary analysis. All the 1201 patients underwent clinical and neuroimaging assessment. The classification of stroke was based on comprehensive evaluations of clinical status and stroke etiology according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classifications.1 All the patients were classified into one of the following subtype: Large artery atherosclerosis (LAA), small vessel occlusion (SVO), cardioembolism (CE), stroke of undetermined etiology (SUE), and stroke of other determined etiology (SOE). For LAA, the patients should have clinical or neuroimaging evidence of more than 50% stenosis of major cerebral arteries or branch cortical arteries. For SAA, patients usually had lacunar infarction without the evidence of large artery atherosclerosis and cardiac embolisms. For CE, at least one source of cardiac embolisms should be found. For SOE, the causes of stroke were very rare, including artery dissection (11 cases in this study), hypercoagulation (five cases, including pregnancy, oral contraceptives use and thrombophilia), moyamoya (three cases), vasculitis (three cases), taking plane (one case), cancer (two cases) or hematologic disorders (three cases). For SUE, we could not confirm the reason of stroke after thorough work-up (which also be termed as CS in this study). Then, patients who were considered to have CS (patients with undetermined etiology) were further included in the secondary analysis. To elucidate the neurological characteristics of stroke-associated PFO, the following patients were excluded in the PFO group after the though clinical assessment: (1) patients with other potential paradoxical embolic sources, such as atrial septal aneurysm, persistent Eustachian valve, Chiari network, atrial septal defect, and cyanotic congenital heart defects; (2) patients with pulmonary arteriovenous malformation; and (3) patients with atherosclerosis of aorta.
This study was approved by the ethics boards of each hospital, and informed consent for participation was obtained from all participants or their relatives.
Clinical assessment
In both analyses, the clinical histories, including age, sex, history of hypertension, diabetes mellitus, cigarette smoking, alcohol intake, atrial fibrillation, dyslipidemia, migraine and ischemic events, among others, were collected for each patient. Physical and neurological examinations were performed by two different neurologists. All patients underwent routine blood and coagulation tests, computed tomography and/or cranial magnetic resonance imaging, carotid duplex, imaging of cerebral arteries (At least one of the following examinations, but not necessary all three: magnetic resonance angiography, computed tomography angiography or digital subtraction angiography), c-TCD, transesophageal echocardiogram (TEE) and transthoracic echocardiography (TTE).
Neuroimaging assessment
All patients underwent brain MRI, including the diffusion-weighted image (DWI), apparent diffusion-weighted image (ADC) and T2-weighted image (T2WI) within seven days of the stroke onset. The infarct pattern was assessed by two experienced neuro-radiologists who were blinded to the study groups. The agreement of different researches were assessed with kappa statistics, and listed in Table 1. According to the number, ischemic lesions were classified into single lesions and multiple lesions (the number of lesions are more than one).18 Further, they were classified as small lesions (<1 cm), large lesions (≧1 cm) and large hemisphere infarctions (>2/3 of MCA territory) according to the size of lesions.18 On the basis of vascular territory involved, infarctions were divided into anterior circulation, posterior circulation, both anterior and posterior circulation and bilateral anterior circulation.19 Finally, the distribution of the lesions was divided into: cortical (the gray matter of frontal, temporal, parietal, occipital, limbic lobe and cerebellum), subcortical (the whit matter of frontal, temporal, parietal, occipital, limbic lobe and cerebellum, corona radiate, centrum semiovale), cortical-subcortical (the lesion located across cortical and subcortical area), and deep areas (basal ganglia region, brainstem, vermis cerebelli).18, 20
| Lesion numbers | Distribution of single lesions | Distribution of multiple lesions | Lesion size | Vascular territory involved | |
|---|---|---|---|---|---|
| T2 phase | |||||
| PFO+ | 0.905 | 0.734 | 0.784 | 0.97 | 0.912 |
| PFO- | 0.929 | 0.656 | 0.796 | 0.975 | 0.949 |
| DWI phase | |||||
| PFO+ | 0.925 | 0.68 | 0.717 | 0.929 | 0.844 |
| PFO- | 0.968 | 0.743 | 0.637 | 0.911 | 0.936 |
- PFO, patent foramen ovale.
PFO assessment
As a noninvasive, cheap and repeatable technique with a sensitivity of 96.1% (95% confidence interval: 93.0–97.8%) and a specificity of 92.4% (95% confidence interval: 85.5–96.1%),21 c-TCD has been used to screen the PFO through recognizing right-to-left shunt (RLS). As described previously,8, 22 contrast agent was generated by agitating a mixture of 9 mL of isotonic saline solution and 1 mL of air between two 10 mL syringes connected by a three-way stopcock. A drop of the patient's blood was allowed to mix with the contrast agent, which can slightly increase the detection sensitivity. The contrast agent was injected as a rapid bolus during normal respiration and 5 sec prior to the start of a 10 sec Valsalva maneuver. RLS was quantified by counting the number of air embolic signals within 40 sec.
Although PFO is the major cause of RLS,23 we also performed examinations to rule out other potential reasons. First, we differentiate the cardiac and pulmonary shunt through controlling the time of appearance of microbubbles in the middle cerebral artery, and the signal change after the Valsalva maneuver.22 Second, according to c-TCD, patients were first divided into five subgroups based on the number of microbubbles (MBs) observed: normal, no MBs; small, 1–9 MBs; moderate, 10–25 MBs; shower, >25 MBs; Curtain: uncountable.24 For 58 patients with MBs (>=small MB), we carried out TEE to exclude the intrapulmonary RLS (such as pulmonary arteriovenous fistula) and other reasons of intracardiac RLS (such as atrial septal defect, atrial septal aneurysm), and to confirm the existence of PFO.21, 25 Third, TTE were also carried out for all the patients, which allows exclusion of aortic arch disease and other source of cardioembolism.3 Finally, the image of cerebral arteries helped us to exclude some rare reasons of RLS, such as subclavian arterial-venous fistula.26 The details of standardized protocol to assess PFO are listed in Figure 1.
Neighborhood component analysis based on feature selection and predictive performance evaluation
We estimated the likelihood of stroke-related PFO through feature weight estimation by neighborhood component analysis (NCA).27 In the data set of CS, the clinical features, stroke patterns (diffusion-weighted imaging [DWI] characteristics and T2 characteristics), serological indicators and other characteristics were included. To further quantitatively analyze the close relationship between various features and CS, considering the small sample size in the data set, this study used NCA to calculate the importance of the feature. The specifics of this feature subset were evaluated based on the sensitivity and specificity of the classification results based on support vector machine (SVM). First, each feature of the data set was normalized into standard data with zero mean and unit standard deviation. Second, the optimal weight matrix was obtained by minimizing a loss function using optimizing algorithm. Third, according to weight, the feature was sorted in descending order, and the bigger the feature weight, the closer the relationship with the CS. Finally, the former K (K = 1,2,… 55) features were used to train SVM classifier, which was tested by 10-fold cross-validation, and the classifier performance was evaluated by sensitivity and specificity.
Statistical analysis
Statistical analyses were performed using the statistical program for social sciences version 13.0 (SPSS; IBM, West Grove, PA, USA). Differences between groups were analyzed with t-test or Wilcoxon rank test for continuous variables and chi-squared test for nominal variables. The agreement of different researches were assessed with kappa statistics. The level of significance was set at P < 0.05.
Results
Clinical feature and ischemia type for all stroke patients
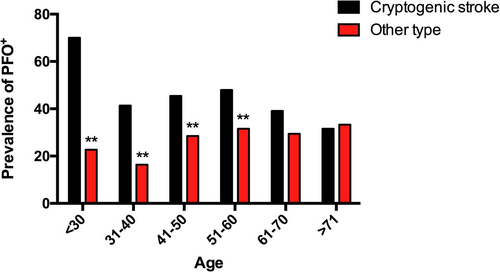
In the primary analysis, the clinical characteristics of the patients are shown in Table 2. According to the TOAST classification, 379 cases (31.56%) were caused by atherosclerosis of large vessels; 333 (27.73%), cardiac embolism; 208 (17.32%), small vessel diseases; 28 (2.33%), other defined causes; and 253 (21.07%), undetermined cause (cryptogenic stoke). In all the 1201 patients, RLS (mainly caused by PFO) was demonstrated with TCD in 378 patients (positivity: 31.47%, mean age: 51.34 ± 12.53). We found that the prevalence of RLS in the CS group was higher than that in the other TOAST subclass at <30 (+47.27%, P = 0.000), 31–40 (+25.02%, P = 0.004), 41–50 (+16.98, P = 0.007) and 51-60 (+16.39, P = 0.009) age groups (Fig. 2). Cryptogenic stoke in the RLS+ group (30.42%) was significantly higher than that in the RLS- group (16.77%, P < 0.001), whereas gender and age showed no difference between the two groups (Table 2). Furthermore, by taking medical history (Table 2), we analyzed the vascular risk factors in these patients. The RLS+ group had relatively lower incidence of hypertension (26.46% vs. 43.74%, P < 0.001) and dyslipidemia (43.39% vs. 67.68%, P < 0.001) and higher incidence of history of migraine (19.05% vs. 10.10%, P < 0.001) and recurrent stroke/transient ischemic attack (14.55% vs. 10.10%, P = 0.009) than the RLS- group. These results indicate that RLS+ patients showed less atherosclerosis-related factors, suggesting that other mechanisms of stroke, such as paradoxical embolism, may exist in these patients.
| Full cohort | RLS+ | RLS- | P value | |
|---|---|---|---|---|
| Number | 1201 | 378 (31.47) | 823 (68.53) | |
| Age, years | 50.01 ± 13.12 | 51.34 ± 12.53 | 49.97 ± 13.46 | 0.163 |
| Female:male | 356:845 | 127:251 | 241:582 | 0.132 |
| TOAST | ||||
| L | 379 (31.56) | 68 (17.99) | 311 (37.79) | P < 0.001 |
| C | 333 (27.73) | 130 (34.39) | 203 (24.67) | |
| S | 208 (17.32) | 57 (5.08) | 151 (18.35) | |
| O | 28 (2.33) | 8 (2.12) | 20 (2.43) | |
| U | 253 (21.07) | 115 (30.42) | 138 (16.77) | |
| Hypertension, % | 460 (38.30) | 100 (26.46) | 360 (43.74) | P < 0.001 |
| Diabetes mellitus, % | 127 (10.57) | 40 (10.58) | 87 (10.57) | 0.995 |
| Coronary heart disease, % | 111 (9.24) | 33 (8.73) | 78 (9.48) | 0.678 |
| Atrial fibrillation | 335 (27.89) | 112 (29.63) | 223 (27.10) | 0.363 |
| Dyslipidemia, % | 721 (60.03) | 164 (43.39) | 557 (67.68) | P < 0.001 |
| Smoking, % | 489 (40.72) | 156 (41.27) | 333 (40.46) | 0.791 |
| Alcohol intake, % | 362 (30.14) | 121 (32.01) | 241 (29.28) | 0.339 |
| History of migraine, % | 155 (12.91) | 72 (19.05) | 83 (10.10) | P < 0.001 |
| History of stroke/TIA | 133 (11.07) | 55 (14.55) | 78 (9.48) | 0.009 |
- Age is mean ± SD. RLS, right-to-left shunt; TOAST, Trial of Org 10172 in Acute Stroke Treatment; TIA, transient ischemic attack; c-TCD, contrast-transcranial Doppler.
Medical history and risk of paradoxical embolism score for CS patients with or without PFO
Considering the potential relationship between PFO and CS, we subsequently analyzed the clinical manifestation in CS patients with or without PFO to illustrate the distinct characteristics and better identify stroke-related PFO (Table 3). We screened 240 CS patients according to the process illustrated in Figure 1, with 111 PFO+ patients and 129 PFO- patients. Previous studies of PFO-related strokes to predict the probability of paradoxical embolism, such as a risk of paradoxical embolism (RoPE) score, are mainly based on the information from the medical history of the patients.28 Therefore, we initially studied the medical history of both PFO+ and PFO-CS patients. In consistent with the results from a RoPE score study, the patients with PFO were younger (the average age was 49.38 ± 1.22 for patients with PFO, while 53.46 ± 1.10 for patients without PFO, P = 0.015) compared with the patients without PFO, but no significant difference was found in the gender ratio between the two groups. From the perspective of risk factors for stroke, PFO+ patients were less likely to have a history of hypertension (36.05% vs. 53.49%, P = 0.007) compared with PFO- patients, but both groups showed no difference in the history of stroke, history of transient ischemic attack, history of diabetes, and history of smoking. Correspondingly, the RoPE score for patients with PFO+ patients showed no difference compared with that of PFO-patients. Together, these results suggest that more clinical features, in addition to medical history of the patients, are needed to be incorporated to accurately identify high-risk PFO.
| Full cohort | PFO+ | PFO- | P value | |
|---|---|---|---|---|
| Age (mean±SEM) | 51.74 ± 0.83 | 49.38 ± 1.22 | 53.46 ± 1.10 | 0.015 |
| Female:male | 60: 180 | 23: 88 | 37: 92 | 0.156 |
| Hypertension (n, %) | 109 (45.42) | 40 (36.04) | 69 (53.49) | 0.007 |
| Hyperlipidemia | 115 (47.92) | 55 (49.55) | 60 (46.51) | 0.639 |
| Diabetes | 48 (20.00) | 27 (24.32) | 21 (16.28) | 0.120 |
| Smoking | 132 (55.00) | 68 (61.26) | 64 (49.61) | 0.071 |
| Alcohol intake | 87 (36.25) | 41 (36.94) | 46 (35.66) | 0.837 |
| History of migraine | 21 (8.75) | 10 (9.01) | 11 (8.53) | 0.895 |
| History of stroke/TIA | 30 (12.50) | 17 (15.32) | 13 (10.08) | 0.221 |
| Recurrent stroke | 27 (11.25) | 15 (13.51) | 12 (9.30) | 0.303 |
| Onset age of stroke<55 years old | 127 (52.92) | 55 (49.55) | 72 (55.81) | 0.332 |
| RoPE score | 5.24 ± 0.12 | 5.11 ± 0.18 | 5.35 ± 0.15 | 0.310 |
- CS, cryptogenic stroke; PFO, patent foramen ovale; RoPE score, risk of paradoxical embolism score; c-TCD, contrast-transcranial Doppler.
Stroke pattern of CS patients with or without PFO
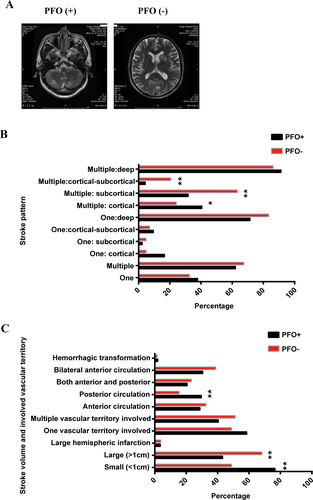
We investigated the stroke pattern between PFO+ patients and PFO- patients, as previous studies found that the infarction pattern is closely related to the mechanism of stroke. In our research, we found that stroke pattern differed based on the presence of PFO. We first analyzed the T2 lesion (Fig. 3). In this research, PFO+ patients and PFO- patients had no statistically difference in the number of lesions. However, if the location of the lesion was specifically analyzed, we found that the multiple lesions in PFO+ patients were more likely to be distributed in the cortex (40.58% vs. 24.14%, P = 0.028), and less likely to be in cortical-subcortical (4.35% vs. 20.69%, P = 0.000) or subcortical (31.88% vs. 63.22%, P = 0.003) when compared to those in PFO- patients. With regard to lesion size, the proportion of patients with small size (<1 cm) was much higher in the PFO+ group (76.57%) than the in the PFO- group (48.84%, P = 0.000). Compared with the PFO- group, the PFO+ group was more likely to influence the posterior circulation (P = 0.008).
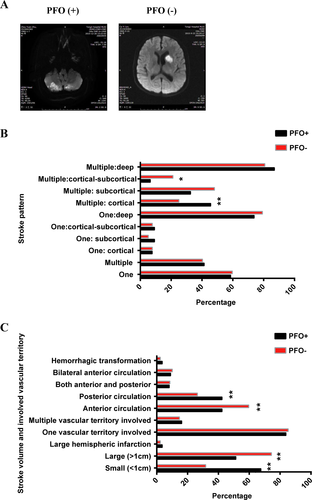
We then analyzed the DWI lesion which represented the stroke pattern of the latest attack (Fig. 4). The number of the lesions did not differ between the PFO+ and PFO- groups. For patients who had single lesions, the distribution of the lesion showed no difference between the two groups, while for patients who had multiple lesions, the percentage of lesions located in the cortical area was higher in the PFO+ group (45.65%) than in the PFO- group (25.00%, P = 0.000), while the percentage of lesions in the cortical-subcortical area was less in the PFO+ group (6.52% vs. 21.15%, P = 0.039). Similar to T2 flair, the percentage of small lesions in the PFO+ group (67.57%) was higher than in the PFO- group (31.78%, P = 0.000). While the percentage of large lesions in the PFO- group was higher than that in the PFO+ group (P = 0.000). most lesions in the PFO+ group were located in the posterior circulation (42.34%), whereas most lesions in the PFO- group were located in the anterior circulation (59.69%, P < 0.01). Together, these detailed unique characteristics of stroke pattern may provide a basis to identify PFO-related strokes and lay a foundation to construct a statistical model for prediction of PFO-related strokes.
Laboratory finding of CS patients with or without PFO
To further explore the clinical feature and possible mechanism for patients with pathological PFO, laboratory findings were summarized (Table 4). The mean platelet volume (MPV) (11.28 ± 0.29 vs. 10.42 ± 0.18, P = 0.031), platelet aggregation rate (63.96 ± 2.02 vs. 58.70 ± 1.71, P = 0.005), international normalized ratio (INR, 1.08 ± 0.02 vs. 1.04 ± 0.01; P = 0.009) and thrombin time (17.63 ± 0.93 vs. 17.08 ± 0.49) were significantly higher in patients with PFO compared with the control group. Meanwhile, total cholesterol (3.70 ± 0.10 vs. 3.94 ± 0.09) and homocysteine was relatively lower in patients with PFO (12.90 ± 0.73) than in the control group (15.93 ± 1.20, P = 0.039). Except for these factors, no statistically significant differences were found between the two groups with regard to the triglyceride, high-density lipoprotein, low-density lipoprotein, blood sugar, glycated hemoglobin, platelet count and platelet distribution, among others. Therefore, increased MPV by right-to-left shunt (PFO) increases the risk of stroke29 because large platelets aggregate easily and more rapidly compared with small platelets. Our results showed that platelet activity may be increased in CS patients with PFO compared with those without PFO. In summary, differences in platelet function and coagulation functions may also be possible risk factors for predicting pathologic PFO.
| All | Patients with PFO | Patients without PFO | P value | |
|---|---|---|---|---|
| Total cholesterol | 3.83 ± 0.07 | 3.70 ± 0.10 | 3.94 ± 0.09 | 0.024 |
| Triglyceride | 1.53 ± 0.08 | 1.52 ± 0.09 | 1.53 ± 0.13 | 0.397 |
| High-density lipoprotein | 1.03 ± 0.02 | 1.03 ± 0.03 | 1.02 ± 0.03 | 0.752 |
| Low-density lipoprotein | 2.26 ± 0.06 | 2.17 ± 0.09 | 2.33 ± 0.08 | 0.121 |
| Homocysteine | 14.53 ± 0.73 | 12.90 ± 0.73 | 15.93 ± 1.20 | 0.046 |
| Blood sugar | 5.85 ± 0.13 | 5.73 ± 0.19 | 5.94 ± 0.18 | 0.156 |
| Glycated hemoglobin | 6.12 ± 0.10 | 6.01 ± 0.13 | 6.21 ± 0.14 | 0.406 |
| Platelet count | 214.33 ± 5.12 | 214.13 ± 8.95 | 214.51 ± 5.61 | 0.273 |
| Platelet distribution width | 13.84 ± 0.19 | 13.57 ± 0.29 | 14.06 ± 0.25 | 0.369 |
| Mean platelet volume | 10.81 ± 0.17 | 11.28 ± 0.29 | 10.42 ± 0.18 | 0.031 |
| Platelet-large cell ratio | 33.00 ± 0.64 | 32.93 ± 0.94 | 33.06 ± 0.89 | 0.920 |
| Thrombocytocrit | 0.23 ± 0.005 | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.903 |
| D-Dimer | 0.63 ± 0.10 | 0.83 ± 0.10 | 0.48 ± 0.05 | 0.160 |
| Prothrombin time | 13.48 ± 0.08 | 13.58 ± 0.13 | 13.39 ± 0.09 | 0.073 |
| International normalized ratio | 1.06 ± 0.01 | 1.08 ± 0.02 | 1.04 ± 0.01 | 0.009 |
| Activated partial thromboplastin time | 36.82 ± 0.27 | 36.84 ± 0.35 | 36.81 ± 0.40 | 0.589 |
| Thrombin time | 17.33 ± 0.50 | 17.63 ± 0.93 | 17.08 ± 0.49 | 0.045 |
| Prothrombin activity | 95.99 ± 0.93 | 96.18 ± 1.27 | 95.82 ± 1.34 | 0.336 |
| Fibrinogen | 3.49 ± 0.05 | 3.53 ± 0.06 | 3.46 ± 0.07 | 0.306 |
| Platelet aggregation rate | 61.17 ± 1.33 | 63.96 ± 2.02 | 58.70 ± 1.71 | 0.005 |
| Plasma osmotic pressure | 291.33 ± 0.14 | 291.42 ± 0.21 | 291.25 ± 0.19 | 0.505 |
- CS, cryptogenic stroke; c-TCD, contrast-transcranial Doppler.
Feature weight estimation and predictive performance evaluation based on NCA
Considering the imperfect function of RoPE score, we attempted to incorporate the above unique clinical features of PFO identified in this study to develop a more comprehensive model for risk stratification of PFO-related CS. We initially used the NCA method to sort out clinical features for their contribution to predict PFO-related stroke. We included a total of 55 features, including history, stroke distribution and partial blood parameters, and sorted them by their relevance. We found that the maximal sensitivity and specificity of the model reached 74.98% and 72.87%, respectively, when modeling with the most relevant 25 features. These 25 selected features were listed in Table 5 with the order of importance, including triglyceride, thrombin time, sexual, smoking, hypertension, lesion size>1 cm in DWI image, posterior circulation involvement in T2 image and so on. As a result of feature selection, these characteristics were used to form a training data set to obtain the final SVM classifier. To predict whether a patient has CS, we used the following formula, which were named as PFO judgment formula (Hr-PFOJ):
| Feature name | Importance index | Classification accuracy | Specificity | Sensitivity | Positive prediction | AUC | |
|---|---|---|---|---|---|---|---|
| 1 | Triglyceride | 1 | 64.50% | 4.82% | 98.83% | 100.00% | 0.650 |
| 2 | Thrombin time | 0.963102 | 72.53% | 26.96% | 98.83% | 100.00% | 0.650 |
| 3 | Sexual | 0.908599 | 74.35% | 32.05% | 99.23% | 100.00% | 0.667 |
| 4 | Smoking | 0.899988 | 73.79% | 31.13% | 98.49% | 94.17% | 0.667 |
| 5 | Hypertension | 0.855357 | 73.29% | 33.21% | 96.88% | 84.86% | 0.708 |
| 6 | DWI Lesion size>1 cm | 0.813133 | 73.00% | 36.23% | 95.02% | 70.71% | 0.688 |
| 7 | T2: posterior circulation | 0.806407 | 76.36% | 49.63% | 92.72% | 78.42% | 0.738 |
| 8 | Diabetes mellitus | 0.770322 | 76.88% | 60.86% | 86.83% | 75.87% | 0.733 |
| 9 | Activated partial Thromboplastin time | 0.580412 | 75.28% | 58.85% | 85.73% | 74.75% | 0.736 |
| 10 | T2: large cerebral infarction | 0.511132 | 74.31% | 61.54% | 82.92% | 77.14% | 0.746 |
| 11 | DWI: posterior circulation | 0.510429 | 73.23% | 61.76% | 81.04% | 69.33% | 0.726 |
| 12 | DWI: anterior and posterior circulation | 0.478369 | 73.23% | 62.67% | 80.76% | 67.49% | 0.737 |
| 13 | Migraine | 0.423642 | 71.92% | 59.97% | 79.55% | 65.30% | 0.740 |
| 14 | T2 Lesion size>1 cm | 0.41433 | 71.92% | 63.68% | 77.44% | 65.12% | 0.753 |
| 15 | DWI: bilateral anterior circulation | 0.378646 | 72.18% | 67.87% | 75.68% | 63.21% | 0.734 |
| 16 | DWI: large cerebral infarct | 0.377091 | 72.18% | 67.87% | 75.68% | 62.43% | 0.732 |
| 17 | Single T2 lesion: cortical-subcortical infarct | 0.370463 | 71.88% | 67.58% | 75.76% | 62.62% | 0.741 |
| 18 | Single T2 lesion: subcortical infarct | 0.323721 | 72.67% | 69.68% | 75.79% | 62.51% | 0.742 |
| 19 | Thrombocytocrit | 0.313552 | 72.61% | 69.15% | 75.69% | 60.87% | 0.744 |
| 20 | Single DWI lesion: subcortical infarct | 0.208199 | 71.53% | 69.32% | 73.62% | 60.36% | 0.730 |
| 21 | Mean platelet volume | 0.201526 | 70.80% | 64.87% | 75.24% | 59.50% | 0.728 |
| 22 | Low-density lipoprotein | 0.179373 | 71.30% | 69.41% | 73.52% | 59.76% | 0.733 |
| 23 | Lesion size<1 cm | 0.178027 | 71.79% | 68.21% | 74.87% | 59.62% | 0.734 |
| 24 | Total cholesterol | 0.172122 | 72.55% | 69.03% | 74.44% | 59.63% | 0.743 |
| 25 | Multiple T2 lesions: cortical | 0.171787 | 74.98% | 72.87% | 76.02% | 60.44% | 0.747 |
| For the RoPE score | |||||||
| – | 50.83% | 76.74% | 20.72% | 43.40% | – | ||
- DWI, diffusion-weighted imaging; NCA, neighborhood component analysis.

Discussion
PFO is relatively common in one-fourth of healthy adults.30 Treating every patient with PFO causes overtreatment, and clinical decisions in patients with PFO depend on whether an observed PFO is pathogenically related to a CS event.31 Thus, prescreening stroke patients who require c-TCD or TEE for PFO detection and need subsequent therapy including PFO closure or secondary preventive medication, as well as elucidating the characteristics of PFO-related stroke and construction of prediction model are necessary.
To identify stroke patients who need PFO screening and treatment, we compared the clinical, neuroimaging and laboratory tests in CS patients with or without PFO in this study. Compared with PFO- CS patients, the infarction pattern of PFO+ CS patients in T2/DWI phase was more likely to be multiple cortical lesions and was less frequent in subcortical/cortical-subcortical area. Furthermore, the infarction size of PFO-related CS patients was generally smaller than that of non-PFO-related patients and was more likely to influence the posterior circulation. In contrast, the MPV, platelet aggregation rate and INR were significantly higher in patients with PFO than in the control group. Homocysteine and total cholesterol were relatively lower in patients with PFO. Finally, we constructed an Hr-PFOJ by means of NCA, which can effectively distinguish between high-risk PFO and incidental PFO (specificity: 66.5%, sensitivity: 76.3%).
Several studies explored the association of neuroradiological findings and PFO. Kim B. J. and his colleague found that large and superficial located lesions are more likely to be PFO-associated lesion.18 However, in their research, he only divided the lesions as superficial/deep and small/large lesion, which could not differentiate the PFO-related lesions to other embolic infarction. Therefore, to better differentiate PFO-related lesions, we described the stroke pattern at several more detailed levels to identify the neuroimaging characteristics of PFO-related CS. First, by evaluating both DWI and T2 phase of lesion, we analyzed newly developed stroke lesions and recurrent stroke lesions in both PFO+ CS patients and PFO- CS patients, respectively. No significant difference was found with regard to the number of lesions between the PFO+ CS and PFO- CS groups. However, when we further divided the superficial lesions into cortical, cortical-subcortical, subcortical and deep area, multiple lesions in PFO+ CS patients were likely to be located in the cortex compared with PFO- CS patients in both of the new stroke and recurrent stroke (less distributed in the subcortical or cortical-subcortical area of the brain), whereas the distribution of the single lesion showed no significant difference between the two group. These clinical features will help us to more accurately identify PFO-related stroke in general CS patients. Furthermore, whether the imaging features in patients with PFO+ CS can indicate a high risk of recurrent stroke requires further follow-up studies for clarification. Second, in terms of size, we observed that small lesions were more common in CS patients with PFO compared with CS patients without PFO, regardless of acute stroke or recurrent stroke. Jin Wo Kin et al. reported the likelihood of patients with small lesion increasing with the increase in RLS.32 A possible reason is that the emboli that can go through the PFO are often small.32 Moreover, previous studies based on DWI demonstrate a positive relationship between PFO size and infarct volume,33, 34 suggesting the possible cause-and-effect relationship between PFO and CS. Third, we also analyzed the vascular involvement in the two groups and found that PFO-related stroke mostly influenced posterior circulation. Consistent with our results, change in hemodynamics would also influence the lesion pattern. Hayashida et al. used 99mTc-MAA to monitor the passage of blood flow through PFO. They found that during physiological condition, the difference in blood flow between anterior and posterior circulation is nearly equal. However, with PFO, the blood flow in posterior circulation exceeds the anterior circulation by 16.1% under Valsalva maneuver.35 This may explain the increased frequency of PFO-related stroke in posterior circulation. Together, the above three features suggest the possibility of PFO-related stroke in CS patients and indicate that further stratification of the general CS patients is warranted.
Due to the existence of PFO, harmful substances in blood or vasoactive substance could go directly into the cerebral artery system, escaping filtration of the normal necessary pulmonary circulation and increasing the chance of stroke.36 Thus, we attempted to seek for some probable vasoactive substances related to high-risk PFO. To investigate the biological characteristics of PFO and the potential biomarker-related to pathogenic PFO, we compared the difference in laboratory tests between CS patients with or without PFO. In this study, we first found the difference in platelet reactivity between the two groups. Numerous studies have indicated that the MPV is a measure of platelet size, which is often used as a simple index to assess platelet function.37 In accordance with our results, Varol et al. demonstrated that the MPV is higher in patients with PFO compared with healthy control.37 Another study conducted in Turkey reported the decrease in MPV after percutaneous closure of PFO.38 Taken together, patients with PFO have elevated MPV and platelet aggregation time, particularly in CS patients. Our finding supported the pathological association between PFO and CS. Except for paradoxical embolisms, increased platelet activity may be the pathogenic reasons of stroke, which may increase thrombogenic mechanisms, such as venous thrombosis.39 Thus, patients with PFO having higher MPV and increased coagulative function may be more prone to stroke. Thus, MPV and coagulation function test may be considered as significant biomarkers to screen high-risk patients with PFO. Our results indicated the necessity of using antiplatelet aggregation drug in pathological PFO in the secondary prevention of cerebral infarction.
Making treatment decisions for patients with PFO depend on the likelihood that PFO is responsible for the stroke and risk of recurrence. RoPE score can be used to predict the possibility of paradoxical embolism. Therefore, it is often used to predict the presence of PFO-related high-risk stroke in clinics. Nevertheless, the sensitivity of RoPE score needs further improvement to be used as the basis for screening patients with PFO who require intervention.40 In our study, no significant difference in RoPE scores was found between the PFO+ and PFO- groups. Further analysis indicated that RoPE score mainly focused on onset age and traditional stroke risk factors (i.e., hypertension, diabetes, smoking, alcohol consumption). However, traditional stroke risk factors may co-exist with PFO-related CS, which may limit the application of RoPE score. To improve the prediction model, we present a novel index based on the clinical, neuroimaging and laboratory characteristics that may be useful to predict the probability of discovering a PFO in a patient with CS. This model considers patient history, stroke patterns, and possible physical and chemical mechanisms, which are relatively more comprehensive than the RoPE score system. With the incorporation of more samples from other medical centers and databases, the accuracy of the trainer gradually increases. Notably, a user-friendly interface is developed based on our model. Clinicians can simply enter the data of the CS patients to obtain concise prediction, without the need to understand the complex mathematical calculation process. Specifically, we found that when the index result was calculated as 1, it could be strongly associated with the increasing prevalence of PFO. Therefore, we believe that this model might be helpful in predicting patients who need PFO screening. More importantly, the model can help us distinguish high risk and incidental PFO. While still inaccurate, the new index could serve as a useful tool to guide patient inclusion for future clinical trials. Addition, our new prediction model is beneficial in selecting patients with PFO who need mechanical closure treatment.
Our research also has some limitations. First, we identified the presence of PFO by c-TCD in this study. Because elderly patients are difficult for TCD, there seemed to be selection bias of patients in this study. However, as a method which could quantify right-to-left shunt (RLS) directly, c-TCD has a class IIa recommendation for RLS detection as well as TEE,41 and have been recommended as the first choice for screening PFO.22 When compared with TEE,42 it has been demonstrated that contrast-TCD is more feasible in elderly patients. Further, we believe this limitation will have small effect on the Hr-PFOJ judgment formula to identify pathogenic PFO, which is mainly established in young adults but not in elderly patients.3, 13And these younger pathogenic PFO patients will likely benefit from PFO closure, as demonstrated in the resent large-scale RCT studies..12, 13, 15 Second, our study was performed in two hospitals of the same area, further multicenter study is necessary to improve the precision of the model.
Conclusions
We summarized the demographic characteristics, stroke patterns and blood tests of PFO+ CS patients to elucidate their clinical characteristics and developed a corresponding predictive model to predict the presence of PFO-related stroke in stroke patients. These data would serve as guide to doctors and patients for further clinical decisions on treating the disease.
Acknowledgments
This work was supported by the Natural Science Foundation of China (81301126 to Dan He and 81471200, 81000521 and 81771341 to Xiang Luo), Science and technology program of Guangzhou(201803010067 to Dan He),the Fundamental Research Funds for the Central Universities to Xiang Luo (2017KFYXJJ111),Clinical Research Physician Program of Tongji Medical College, HUST, Natural Science foundation of Hubei Province (2015CFB572), Project of Health and Family Planning Commission of Hubei Province (WJ2015 MB056) to Shabei Xu, Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Major Neurological Diseases (2014B030301035); The Southern China International Cooperation Base for Early Intervention and Functional Rehabilitation of Neurological Diseases(2015B050501003); Guangzhou Clinical Research and Translational Center for Major Neurological Diseases (201604020010); Guangdong Provincial Engineering Center for Major Neurological Disease Treatment. We thank the patients who participated in the study, Dr. Shujin Tang (Sun Yat-sen University) for advice regarding the diagnosis of PFO; Dr. Zifeng Cui (Sun Yat-sen University) for the statistical analysis.
Author Contributions
D.H., Q.S., G.J.X, W.W., and X.L. were responsible for designing the experiments; Q.S. was responsible for constructing the prediction model; H.Z., X.L, Q.L, Y.G., S.X., and Y.L. were responsible for acquiring and analyzing the data; D.H., Z.Y., W.W., and X.L. were responsible for drafting the manuscript figures. All the authors have reviewed, revised, and approved the final manuscript.
Conflicts of Interest
The authors have declared that no conflict of interest exists.




