Expanding molecular and clinical spectrum of CPT1C-associated hereditary spastic paraplegia (SPG73)—a case series
Abstract
Autosomal-dominant variants in the CPT1C gene have been associated with hereditary spastic paraplegia type 73 (SPG73), which typically presents with slowly progressive lower limb weakness and spasticity and is therefore considered a pure form of hereditary spastic paraplegia. However, we report two unrelated males with novel CPT1C variants (NM_001199753.2: patient 1: c.2057_2061del (p.Ile686SerfsTer8) and patient 2: c.2020-1G>C (p.?)) who presented with lower limb spasticity at 4 and 3 years old, respectively. Both patients also experienced significant cognitive impairment, seizures, or neurobehavioral symptoms. These cases illustrate a broader and more complex clinical spectrum of SPG73, extending beyond the traditionally recognized pure motor symptoms.
Introduction
The hereditary spastic paraplegias (HSPs) are an expanding group of monogenic disorders characterized by progressive spasticity and weakness of the lower limbs.1 HSPs are traditionally categorized as either pure or complicated HSP based on the presence or absence of additional neurological manifestations, including cognitive dysfunction, peripheral neuropathy, or bulbar impairment.1, 2
Individuals presenting with symptoms of HSP in early childhood continue to be misdiagnosed with cerebral palsy due to overlapping clinical features and in many cases, slow initial disease progression.1 Advancements in the accessibility of genetic testing over the past decade have facilitated accurate diagnosis and differentiation between these conditions.1, 2
To date, there are over 80 different subtypes of HSP, with an increasing number of associated genetic loci. The most prevalent subtype is pure adult-onset autosomal dominant HSP, which stems from variants in various genes including SPAST (SPG4), ATL1 (SPG3A), and REEP1 (SPG31).
Over the past decade, a total of three studies have identified variants in the carnitine palmitoyl-transferase (CPT1C) gene as the cause of hereditary spastic paraplegia type 73 (SPG73),3 categorized as a pure autosomal dominant form.2, 4, 5 The first previously identified variant was found to impair regulatory/catalytic protein domains, indicating compromised CPT1C function.4 In contrast, other variants led to nonsense-mediated mRNA decay, with neither full-length nor truncated proteins detected.2, 5 The predominant clinical manifestations have been slowly progressive lower limb weakness and spasticity, indicative of a gradually evolving clinical course.4, 5 Reported patients exhibited normal cognitive function with no documented behavioral concerns, suggesting that CPT1C variants typically result in an adult-onset pure motor form of HSP.4, 5 Nevertheless, more recent reports describe two additional patients who presented with a complex form of HSP, accompanied by seizures or visual impairment.2 Notably, similar to previous cases, these patients also exhibited normal cognitive functioning and no behavioral issues.2 Despite only a few reported cases, these findings underscore the expanding genetic complexity of HSP and the need for further research into its underlying mechanisms.
In this study, we present two families with novel variants in CPT1C. Our patients are both male individuals who inherited a Pathogenic (Patient 1) or Likely Pathogenic (Patient 2) variant in CPT1C from their mothers, who also displayed lower leg cramps and gait impairment. Both patients presented with early-onset and progressive lower limb spasticity with mild–to-moderate impairment of gait. Remarkably, our patients also exhibited cognitive impairments and significant neurobehavioral concerns, which have not been described prior.
Case 1
The patient is a 10-year-old male with an unremarkable prenatal history. He reached appropriate developmental milestones of sitting unsupported at 6 months, standing unsupported at 9 months, and walking unsupported at 11 months. At the age of 2, his parents noticed his legs crossing. By ages 4–5, the family observed increased clumsiness and frequent falls, attributed to dragging or tripping over his feet. While he was able to run and play adequately, the family noted morning leg tightness and difficulties with fine motor skills. His initial evaluation revealed bilateral lower leg spasticity, ankle clonus, a positive Babinski sign, and an abnormal, unsteady gait (GMFCS 1). Notably, there was no muscle wasting, urinary or stool incontinence, or neuropathy. MR imaging of the brain showed a Chiari 1 malformation but was otherwise unremarkable.
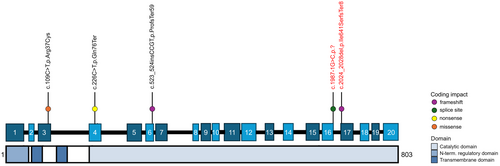
Variant c.2057_2061del (Ile686SerfsTer8) in CPT1C (NM_001199753.2) was identified through multi-gene panel testing and classified as Pathogenic (Fig. 1). This variant was found to be inherited from his mother who had also been experiencing bilateral leg cramps and impaired vibration sensation in both feet over the past year. There was no additional developmental history available for the mother, and the rest of the family history was otherwise unremarkable.
Approximately 2 years after the molecular diagnosis, the family noted worsening spasticity. On examination, his Spastic Paraplegia Rating Scale (SPRS) score was 8, indicating mild gait impairment and lower extremity muscle weakness and spasticity (Table 1). Spasticity is currently managed with baclofen and physical therapy.
| Patient | Family 1 (Rinaldi et al. 2016) | Family 2 (Hong et al. 2019) | Case 3 (Wang et al 2022) | Case 4 (Wang et al. 2022) | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Variant (NM_001199753.2) | c.109C>T, p.Arg37Cys | c.226C>T, p.Gln76Ter | c.524_527dup, p. Pro177ArgfsTer59 | c.226C>T, p.Gln76Ter | c.2057_2061del, p.Ile686SerfsTer8 | c.2020-1G>C, p.? |
| Inheritance | AD | AD—maternal | AD—maternal | AD—paternal | AD—maternal | AD—maternal |
| Affected family | Multi-generational family with 6 affected individuals | Mother asymptomatic | Mother asymptomatic | Father asymptomatic | Mother symptomatic with worsening bilateral leg cramps and impaired vibration sensation | Mother symptomatic from age 14, with progressively worsening cramping, pain, spasticity in lower extremities, leading to frequent falls |
| Sex | 3M, 3F | 2F, 1M | M | M | M | M |
| Age of presentation | Adult onset (19–48 years) | Infancy for both children | Infancy (age 2) | Infancy (from birth) | Infancy (age 4) | Infancy (age 3) |
| Progression | Slowly progressive—ranging from mild impairment to loss of ambulation 10–15 years after initial symptoms | Both children with reversible progression by age 10 | Independently walk with abnormal gait | 1 year old at follow up—cannot walk or hold head up currently | Progressive spasticity but walking without aid | Progressive weakness and spasticity requiring wheelchair for ambulation |
| Spasticity | Progressed to moderate/severe | + | + | + | Mild | Moderate |
| Weakness | Mild | + | − | + | Mild | Moderate |
| developmental delay | None | Delayed walking milestone (achieved independent walking at age 3 and 3.5 years) | Delay of gross motor and speech | Severely delayed milestones | Reached initial gross motor, then developed spasticity | Gross motor and speech delay |
| Cognition | Normal | Normal | Normal | Normal | Normal | Moderate intellectual disability with deficits in working memory, processing speed, and notable impairment in adaptive behaviors |
| MRI brain | Normal | Normal | Cortical dysplasia | Thinning of corpus callosum; abnormal signal of bilateral paraventricular; enlargement of bilateral ventricles | Mild Chiari 1 malformation, but otherwise unremarkable | Normal |
| Seizures | No | No | + | No | No | + |
| Behavior | − | − | − | − | ADHD, anxiety disorder | ADHD, anxiety disorder, autism spectrum disorder, depressive disorder, disruptive mood dysregulation disorder, conduct disorder |
Notably, the patient has exhibited separation anxiety from a young age, with continued significant anxiety as he aged leading to a formal diagnosis of anxiety disorder. Further evaluation led to a diagnosis of attention-deficit/hyperactivity disorder. Current medications include amphetamine/dextroamphetamine (Adderall) and sertraline.
Case 2
The patient is a 17-year-old male with an unremarkable prenatal history. He initially met developmental milestones but began experiencing muscle cramps in his legs around age 3, which progressed to lower limb spasticity by age 8. By age 14, progressive spasticity affected both upper and lower extremities, leading to contractures of the ankles, muscle wasting, and significant spontaneous ankle clonus (GMFCS 2–3).
Genetic testing identified a likely pathogenic variant in CPT1C (NM_001199753.2: c.2020-1G>C) (Fig. 1), inherited from his mother, who self-reportedly met all neurodevelopmental milestones as a child but then started to exhibit neurological symptoms beginning around age 14–15. These included progressive spasticity, bilateral lower extremity muscle cramps, nocturnal pain, and frequent falls. His maternal grandfather also had comparable walking difficulties (File S1).
On follow-up at age 18, the patient's SPRS score increased from 16 to 17, indicating moderate walking impairment and lower extremity spasticity. He now requires orthotics and wheelchair assistance for longer distances (GMFCS 3–4) (Table 1).
Interestingly, the patient also has a history of generalized tonic–clonic seizures, first occurring at age 5, with EEG findings revealing sharp waves in the temporal region. Antiseizure medications were initially prescribed but discontinued after 2 years of seizure freedom.
In addition to his neurological challenges, the patient faces significant cognitive difficulties, with a history of aggressive and impulsive behavior from a young age. His IQ testing suggests moderate cognitive impairment (full scale IQ: 57), with notable deficits in working memory and processing speed. An adaptive behavior score of 54 indicates severe difficulties in daily living and socialization. Diagnoses include ADHD, anxiety disorder, depressive disorder, disruptive mood dysregulation disorder, conduct disorder, and autism spectrum disorder.
Current pharmacotherapy includes sertraline, lurasidone, and olanzapine, which appear effective. However, the cognitive and behavioral issues continue to significantly impact his daily life.
Discussion
In this report, we describe two unrelated male patients with inherited, novel, pathogenic CPT1C variants. Consistent with previous observations of CPT1C variants, both patients showed progressive lower limb spasticity, with the second individual exhibiting moderate impairment in mobility that required the use of a wheelchair for longer distances. Notably, both patients also presented with a range of neurological symptoms beyond those typically associated with CPT1C variants, including cognitive impairment, seizures, neurobehavioral disorders, and psychiatric symptoms. Notably, case 2 displayed significant cognitive deficits, poor adaptive behaviors with significant difficulties in daily living skills, low IQ, and severe behavioral dysregulation. Both patients also had neurodevelopmental comorbidities such as ADHD and ASD, with Patient 2 additionally experiencing concurrent mood disorders including anxiety disorder, depressive disorder, disruptive mood dysregulation disorder, and conduct disorder. These cases highlight a more complex manifestation of HSP than the previously described benign clinical course typically associated with CPT1C variants, broadening the understanding of the clinical phenotype linked to SPG73 with its diverse manifestations.
The literature describes four distinct families with documented variants of CPT1C, with details listed in Table 1. The first family, detailed by Rinaldi et al. in 2016,4 is a multi-generational family comprising six individuals who experienced adult-onset lower limb spasticity and weakness, with notably slow disease progression. In contrast, three other families reported symptoms beginning in infancy.2, 5 Family 2, in particular, exhibited a reversible progression of symptoms, whereas Family 3 experienced only minimal gait impairment. The fourth case involved a patient who faced significant motor delays, unable to hold up their head at 1 year of age; however, there is limited information on subsequent progression. Of the 4 cases, cases 3 and 4 were diagnosed with complex HSP due to seizures and vision impairment, respectively. Overall, all affected individuals in these families exhibited normal cognitive function and no behavioral abnormalities, a significant difference from the two families in our study. In contrast, existing literature on other families with CPT1C variants demonstrates mild symptom severity, with many cases exhibiting mild motor issues with normal cognitive function and no neurobehavioral issues, highlighting the distinct clinical profile of our patients.
The two cases presented here feature novel, Pathogenic and Likely Pathogenic CPT1C variants located toward the end of the gene, a frameshift deletion and splice-site mutation, respectively. Of note, the observed phenotypic variability within families, especially among mothers who seem to exhibit mild symptoms, may be influenced by factors such as environmental stressors. Additionally, the presence of other genetic modifiers could contribute to incomplete penetrance and variable expressivity of the clinical phenotype within families. Previous research has linked loss-of-function (LoF) variants in CTP1C to a decrease in both the number and size of lipid droplets (LD).4 This is particularly significant given that corticospinal neurons, where CPT1C is expressed in the brain and spinal cord, are especially vulnerable to disruptions in LD metabolism.3 Moreover, the pathogenesis of HSP is associated with impairments in LD biogenesis. Our identified LoF variants, which result in notable frameshift and splice site changes, are likely to result in significant protein dysfunction, further reducing LDs. Due to the limitation of available tissue samples, functional analysis of the CPT1C variants in Patient 1 and Patient 2 was not possible, however, this remains a key focus for future research. Overall, these cases expand the understanding of the clinical phenotype of SPG73 and patients with CPT1C variants, underscoring the need for further investigation into their diverse manifestations.
Author Contributions
AKB, VQ, and DEF conceptualized the study. AM, VQ, LS, and DEF provided cohort data. JEA and LS created Fig. 1. AKB performed the literature review and drafted the original manuscript. All authors contributed to editing the final draft of the manuscript.
Acknowledgments
The authors thank the patients and their families for supporting research on movement disorders. Research in the Ebrahimi-Fakhari Laboratory is supported by the Spastic Paraplegia Foundation, the Boston Children's Hospital Translational Research Program, and the National Institutes of Health/National Institute of Neurological Disorders and Stroke (K08NS123552-01). Vicente Quiroz is supported by a fellowship from the International Parkinson and Movement Disorder Society. Luca Schierbaum is supported by a fellowship from the German Research Foundation (536105452).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data are available from the corresponding author upon reasonable request.




