Alpha-synuclein RT-QuIC assay in gastroduodenal and skin biopsies of Parkinson disease patients
Abstract
In this study, we compared the value of pathological alpha-synuclein (αSyn) seed amplification assay (SAA) in gastric and duodenal biopsies with skin biopsies in Parkinson disease (PD) patients with different disease duration. The accuracy of αSyn SAA was 87.7% in skin, 67.4% in duodenum, and 80.0% in gastric biopsies, with significantly higher sensitivity in advanced PD (skin: 81.8%; gastric: 88.9%; duodenal 58.8%). Misfolded αSyn was detected with higher sensitivity in advanced PD across all matrices, likely reflecting the progression of αSyn pathology. The seeding activity was lower in the duodenal than in the gastric wall, indicating differences in αSyn burden.
Introduction
In synucleinopathies, misfolding of alpha-synuclein (αSyn) triggers the formation of protein aggregates by seeding the conversion of monomeric αSyn. This process leads to the development of toxic intracellular aggregates, forming Lewy bodies and neurites, the histopathologic hallmarks of Parkinson's Disease (PD).1 While postmortem brain examination remains the gold standard for definitively diagnosing synucleinopathies, recent methods for accurately detecting αSyn aggregates in vivo have become available.2 These methodologies, named seed amplification assays (SAA), exploit the seeding potential of misfolded αSyn in biofluid or peripheral tissues, including cerebrospinal fluid (CSF), skin, olfactory and gastro-intestinal mucosa, and potentially serum.3-10
Robust SAA data have been obtained using both CSF and skin, demonstrating high specificity (>90%) and sensitivity of 85–100% in PD and Dementia with Lewy Bodies (DLB)3-7, 11 and 75–90% in isolated REM-Behavior Disorder (IRBD).12-14 However, our understanding of the accuracy of αSyn SAA when applied to other accessible tissues and biofluids is still limited.8-10 In this explorative study, we investigated the value of the αSyn SAA Real-Time Quaking-Induced Conversion (RT-QuIC) in detecting pathology in gastric and duodenal biopsies, two matrices not yet assessed deeply in PD, and compared results to skin biopsies.
Materials and Methods
Subjects
We included 22 PD patients who are part of the PADUA-CESNE cohort undergoing biological, genetic, and clinical characterization of Parkinson's at the Movement Disorders Unit of the University of Padova according to previously published protocols.15, 16 These 22 PD patients are all genetically negative.16 Twelve patients with motor fluctuations were considered as advanced PD17 and underwent percutaneous endoscopic gastrostomy at time of initiation of levodopa jejunal infusion. Ten had stable medication response and were considered “early PD” (median disease duration 5.5 years).18 They underwent an esophageal-gastro-duodenoscopy for research purposes. During these procedures, the operator sampled an average of four 3 mm3 duodenal-wall biopsies and four 3 mm3 gastric wall biopsies. For each site, two biopsies were snap-frozen in liquid nitrogen and stored at −80°C, and two were formalin-fixed.19, 20 Additionally, one skin punch-biopsy (ø = 3 mm; depth 3 ± 2 mm) of the cervical C7 paravertebral dermatomes was obtained under local anesthesia. After collection in phosphate-buffered saline (PBS) solution, the biopsy was cut in half using a microtome blade: one half was snap-frozen in liquid nitrogen and stored at −80°C, and the other half was fixed in Zamboni solution. One advanced PD refused skin biopsy while 3 advanced PD had only duodenal biopsies (no gastric samples).
Twenty-one healthy subjects undergoing routine screening diagnostic endoscopy were included as controls (HC) for the gastroduodenal biopsies. HC were evaluated clinically and interviewed to exclude neurological disorders. As a control group for the skin matrix, we used cervical (C7) 3 mm punch biopsies from a previously described independent cohort of 52 patients affected by various non-neurodegenerative neurological disorders (NeuC).7
The Padua Province ethical committee approved the study protocol for clinical experimentation (Prot. n. 0034435, 08/06/2020). Informed consent for the use of biological samples was obtained from all patients. All procedures on human tissue samples were carried out in accordance with the Declaration of Helsinki.
αSyn RT-QuIC SAA
Duodenal and gastric samples were weighed and washed twice in cold 1× PBS to remove blood residues. Next, tissue samples were homogenized at 2% (w/v) in cold 1× PBS supplemented with a protease inhibitor cocktail (Roche) using a pestle. Skin samples were homogenized as previously described.7
The αSyn RT-QuIC assay was performed as previously described with minor modifications according to the used matrix. Specifically, skin samples were run at 10–2 dilution, while gastric and duodenal samples were run at 10–3 dilution. Moreover, the established time cutoff to establish the positive outcome (when ≥50% of the four loaded replicates exceeded the threshold) was 30-h for skin samples and 40-h for duodenal and gastric biopsies. Based on previous experience, the threshold was set at 30% of the median of the maximum fluorescence intensity (Imax) reached by the positive control replicates to take into account the slight increase (below 20% of the mean Imax of positive controls in most cases) of the fluorescence signal sometimes occurring over the tested period in the negative controls.7 When only one of the four loaded replicates crossed the threshold, the analysis was considered “unclear” and repeated up to three times.
All RT-QuIC experiments were performed at the Laboratory of Neuropathology of ISNB by personnel blind to clinical data and diagnostic status.
Demographic and clinical variable differences between Parkinson's Disease (PD) and Healthy Controls (HC), as well as within early and late stages of PD, were compared using appropriate statistical tests. The normality of the data distribution was assessed using the Kolmogorov–Smirnov test. Based on the results of this normality test, either the Student's t-test (for normally distributed data) or the Mann–Whitney U test (for non-normally distributed data) was used for two-group comparisons. Fisher's exact test was employed for dichotomous variables. Statistical analysis and plotting of fluorescence values were performed using GraphPad Prism 9. The time required to reach the threshold (LAG) and the Imax were extracted for each positive sample replicate.
Results
Patient demographics and clinical information
Table 1 shows demographic and clinical information for study participants.
| PD | HC | NeuC | PD versus HC (vs. NeuCb) | PD Early/Middle versus Advanced stage | |||
|---|---|---|---|---|---|---|---|
| All n = 22 median (IQR) | Early/Middle n = 10 median (IQR) | Advanced n = 12 median (IQR) | n = 21 median (IQR) | n = 52 median (IQR) | Mann–Whitney/Fisher exact text | Mann–Whitney/Fisher exact text | |
| Age at biosample collection, yrs | 66.5 (61.3–72.3) | 69.0 (62.0–73.0) | 64.5 (59.8–71.3) | 69.0 (59.5–76.0) | 66.5 (60.5–77.3) | 0.34211 | 0.4274 |
| Males, n (%) | 12 (54.5) | 4 (40.0) | 8 (66.7) | 11 (52.4) | 31 (59.6) | 0.9999 | 0.2305 |
| Age at motor onset, yrs | 55.5 (46.0–62.0) | 57.5 (53.0–66.0) | 50.0 (43.5–58.5) | – | – | 0.092 | |
| Time since PD diagnosis, yrs | 9.5 (4.0–12.0) | 3.5 (2.0–7.0) | 11.5 (10–14.0) | – | – | 0.00007 | |
| MDS-UPDRS part III, score | 23 (16.5–31.5) | 23 (17.3–31.5) | 23.5 (15.0–31.0) | – | – | 0.99999 | |
| Hoehn e Yahr scale, score | 2 (1.8–2.1) | 2 (1.0–2.0) | 2 (2.0–2.5) | – | – | 0.058 | |
| αSyn RT-QuIC skin, pos/tota (%) | 14/22a (63.6%) | 5/10 (50.0%) | 9/12a (75.0%) | na | 2/52 (3.8%) | <0.0001b) | 0.6594 |
| αSyn RT-QuIC duodenum, pos/tota (%) | 9/22 (40.1%) | 2/10 (20.0%) | 7/12 (58.3%) | 1/21 (4.8%) | na | 0.0118 | 0.0115 |
| αSyn RT-QuIC stomach, pos/tota (%) | 13/22 (59.1%) | 5/10 (50.0%) | 8/12 (66.7%) | 2/21 (9.5%) | na | 0.0011 | 0.3498 |
- Continuous data are expressed as median (IQR). MDS-UPDRS part 3 and Hoehn e Yahr were assessed during ON state.
- CI, confidence interval; HC, healthy controls; MDS-UPDRS, Movement Disorder Society-unified Parkinson's disease rating scale; na, not available; NeuC, neurological controls; PD, Parkinson disease; pos/neg, positive/negative results; RT-QuIC, real-time quaking-induced conversion; yrs, years; αSyn, α-synuclein; −, not applicable.
- a One advanced PD refused skin biopsy while 3 advanced had no gastric biopsy.
- b NeuC constituted the control group for the skin matrix. In Table 1, as requested from the reviewers, we highlighted the percentage and frequency of positive cases over the total assessed cases, and we have provided statistical comparisons among the groups using Fisher's exact test to better clarify the accuracy.
Detailed clinical data for the PD group are provided in the Supporting Information Tables.
αSyn RT-QuIC assay
In skin biopsies, the αSyn RT-QuIC showed a positive result in 14/21 (66.7%) PD patients and 2/52 controls (3.8%).
Two advanced and 5 early PD participants showed a negative αSyn RT-QuIC result (Supporting Information Tables). There was a significant correlation between the weight of the biopsy material analyzed and the αSyn RT-QuIC outcome (20.0 ± 7.7 mg in the positive vs. 12.5 ± 9.8 mg in the negative group, p = 0.038). A trend toward the same association was also evident when the analysis was limited to the early-PD group (14.7 ± 4.1 vs. 9.4 ± 4.5, p = 0.088).
Overall, the skin αSyn RT-QuIC showed 66.7% sensitivity (95% CI, 43.0% to 85.4%), 96.2% specificity (95% CI, 86.8% to 99.5%), and 87.7% accuracy (95% CI, 77.9% to 94.2%).
The mean weight of duodenal biopsies was lower than that of skin biopsies (8.8 ± 3.9 mg, range 2.2–21.8 mg vs. 17.5 ± 9.0 mg, range 5.4–33.8 mg, p = 0.0010) with no significant difference between the two PD groups. The αSyn RT-QuIC showed a positive result in 9 of the 22 (40.9%) PD subjects and 1/21 (4.8%) controls. Seven of the 12 advanced-stage patients and only 2/10 in the early-PD group showed positive αSyn seeding activity.
Overall, the duodenal αSyn RT-QuIC showed 40.9% sensitivity (95% CI, 20.7% to 63.7%), 95.2% specificity (95% CI, 76.2% to 99.9%), and 67.4% accuracy (95% CI, 51.5% to 80.9%).
The mean weight of gastric biopsies was 6.8 ± 3.6 mg (range 1–12 mg) with no significant difference between the two PD groups. The αSyn RT-QuIC was positive in 13 of the 19 (68.4%) PD subjects analyzed and 2/21 (9.5%) control subjects. Eight of the 9 advanced and 5/10 early PD showed positive αSyn seeding activity.
Overall, the gastric αSyn RT-QuIC showed 68.4% sensitivity (95% CI, 43.4% to 87.4%), 90.5% specificity (95% CI, 69.6% to 98.8%), and an accuracy of 80.0% (95% CI, 64.4% to 90.9%).
The analysis of αSyn RT-QuIC kinetic parameters namely the LAG and the Imax did not differ between early and advanced PD in each of the three matrices (Fig. 1).
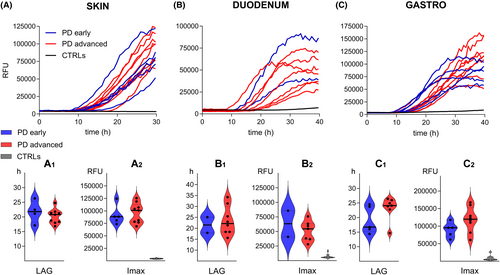
A comparison of clinical parameters between αSyn RT-QuIC positive and negative patients showed a significantly higher frequency of autonomic symptoms in patients with a positive αSyn RT-QuIC (Table S3).
Discussion
We assessed the performance of RT-QuIC SAA in detecting misfolded αSyn in gut and skin biopsies from both early and advanced-stage PD patients. The detection sensitivity was notably higher in advanced PD patients, likely reflecting differences in the burden and distribution of pathological αSyn within peripheral tissues in the early stages of the disease compared to advanced ones.21
Significant variations were also observed across tissues, particularly in samples of the gastro-intestinal wall. Advanced PD patients showed similar sensitivity of αSyn SAA in the skin (9/11) and gastric biopsies (8/9) but a lower sensitivity in the duodenal biopsies (7/12). Similarly, early PD manifested comparable sensitivity of αSyn SAA in the skin (5/10) and the gastric biopsies (5/10), but lower in the duodenal biopsies (2/10). These findings suggest a lower αSyn pathology burden in the duodenal than in the gastric wall, regardless of disease stage. Different pathological burdens might be related to the different anatomical distribution of vagal afferents (predominant in the initial gastro-intestinal tract) in the stomach and duodenum. These structures present a crucial role in gastro-intestinal motility and general function and are heterogeneous in terms of embryological origin, neurophysiological/morphological characteristics and response to stimuli. Moreover, it can be hypothesized that different regions across the gastro-intestinal system might present different susceptibility to pathological αSyn deposition, mirroring a similar process observed in the brain, where distinct regions and cell types might be affected.22, 23 Detecting αSyn in the gastro-intestinal system, especially in the vagus-innervated tract, is particularly intriguing due to its hypothesized role in “early” phases of pathogenetic processes.19, 20 These results encourage further research in the premotor stage of PD to explore the extent of the early peripheral Lewy body pathology and its relevance to disease progression. In this regard, we observed a higher frequency of vegetative symptoms (namely those related to the gastro-intestinal items) in patients with a positive αSyn RT-QuIC. Autonomic dysfunction is an early manifestation of PD, with a significant impact on quality of life and consistent αSyn pathology in related structures, including the peripheral sympathetic/parasympathetic nerves and the enteric nervous systems.
No other studies to date have assessed the sensitivity of αSyn SAA in the gastric wall while in duodenal biopsies we detected αSyn seeds with lower sensitivity compared to a recent study in which 22 out of 23 patients with advanced PD showed αSyn seeding activity.9 Similarly, the sensitivity of skin αSyn RT-QuIC in this study was slightly lower than in previously published studies.7, 11 These divergent results may be related to tissue availability for αSyn SAA, given that the amount of skin and duodenal tissue obtained per patient in our cohort was, on average, lower than in the aforementioned studies. Indeed, one or sometimes two whole skin biopsies were tested in our previous study on LBD patients.7 Similarly, the average tissue weight of the duodenal biopsy in the study by Vascellari et al was significantly higher than in this study (̴20 mg vs. ̴9 mg).9 On the same line, we recently showed that analyzing two skin biopsies instead of one increases SAA sensitivity.13 Therefore, at least for the skin, the analysis of two whole punches should be the recommended standard. However, the relationship between tissue availability, sample weight, and αSyn RT-QuIC accuracy should be further addressed, especially for gastro-intestinal biopsies.
Future studies should also consider assessing the performance of the αSyn SAA in different peripheral tissue matrices, particularly in populations at risk of developing PD, to define the heterogeneity of tissue distribution.24 Understanding this variability and the potential roles in different stages of the disease may significantly enhance the early detection of synucleinopathies and shed light on the pathophysiology of PD.
Funding Information
The project was supported by the grant Ricerca Finalizzata-2021-12374386, funded by the Ministry of Health, the #NextGenerationEU (NGEU) project MNESYS (PE0000006), funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), the Next Generation EU – National Center for Gene Therapy and Drugs based on RNA Technology and from the National Recovery and Resilience Plan, Investment PE8 – Project Age-It: “Ageing Well in an Ageing Society”.
Conflict of Interest
The authors declare no conflicts of interest related to the manuscript's content.
Disclosures
AA has received compensation for consultancy and speaker related activities from UCB, Bayer, Ever Pharma, Britannia, AbbVie, Zambon, Bial, Theravance Biopharma, Jazz Pharmaceuticals, Roche, and Medscape; he receives research support from Horizon 2020, Italian Ministry of University and Research (MUR), Italian Ministry of Health, Next Generation EU – National Center for Gene Therapy and Drugs based on RNA Technology and National Recovery and Resilience Plan, Investment PE8 – Project Age-It: “Ageing Well in an Ageing Society”. MC has received travel grants from Zambon and receives support from Progetto di ricerca di Rilevante Interesse Nazionale – PRIN 2022FJAXY8. All the other authors have no financial disclosure to declare.
Author Contributions
M.C. (Marta Campagnolo), A.A., A.E., and P.P. contributed to conception and design of the study. M.C. and M.C2. (Miryam Carecchio) performed the skin biopsies. M.S. and A.M. performed the RT-QuIC assays. M.R. and F.M. prepared the recombinant synuclein substrate. A.M., A.E., and M.R. analyzed the RT-QuIC data. A.E., M.C., M.C2., E.S., F.P.R., L.W., A.P., and A.A. contributed to acquisition and/or analysis of clinical and genetic data. A.E., S.B., and P.P. contributed to drafting the text and preparing the figure. P.P. and A.A. supervised the study. All authors reviewed the manuscript for intellectual content.
ACKNOWLEDGEMENTS
There are no acknowledgements to disclose.
Open Research
Data Availability Statement
All data collected or analyzed during this study are available upon reasonable request to the corresponding author.




