Spatiotemporal spinal cord stimulation with real-time triggering exoskeleton restores walking capability: a case report
Abstract
Objective
Motor recovery is challenging for spinal cord injury (SCI), especially in low-level SCI.
Methods
A 16-year-old patient with complete SCI at T12 presented flaccid paralysis and inability to control defecation and was scored as ASIA A at admission. The patient underwent spinal cord stimulation (SCS) implantation at the T11-L1, followed by an innovative algorithm combining spatiotemporal SCS with real-time triggered exoskeleton training (EXS-SCS).
Results
After 1 month of treatment, she gained substantial improvement in her iliopsoas and quadriceps femoris muscle strength to grade 3–4 as well as percutaneous EMG, allowing for assisted standing and walking, and was reassessed as ASIA C.
Interpretation
This case reveals the potential of SCS-EXS regimen in restoring walking capability of SCI patients.
Introduction
Spinal cord injury (SCI) is a severe systemic condition that directly results in locomotor dysfunction and significantly increases the risk of bed-related complications. Spinal cord stimulation (SCS) has emerged as one of the most promising neuromodulation therapies for treating SCI, modulating neural activity based on the polarization principles of neurons and the intrinsic electrical activity of spinal cord conduction pathways.1-3 Notably, research led by Courtine G. et al. revealed that epidural SCS could activate spinal cord neurons, enabling three patients with lower limb paralysis to regain the ability to perform everyday activities such as walking, cycling, and swimming.1, 4
SCS can deliver precise spatiotemporal activation of motor neurons in specific spinal cord segments through accurate electrode placement and pre-established stimulation protocols. This spatiotemporal activation pattern is essential for the restoration of a natural gait cycle.5 Nevertheless, SCS by itself has been found insufficient to attain voluntary control of the lower limbs, notably in instances of severe low-level SCI. The absence of afferent feedback signals in these cases hinders the formation of a complete motor-sensory circuit.6, 7
Exoskeletons are critical rehabilitation devices that aid patients in adapting to weight-bearing positions and support both passive and active lower limb movement feedback training.8 Gorgey AS et al. reported successful cases of restoring motor function following the use of spinal cord stimulation in conjunction with exoskeleton-assisted rehabilitation therapy.9, 10 This integration is able to augment the muscular activity and motor control through central-peripheral functional mapping.9 SCS facilitates muscle activation by delivering targeted electrical impulses to the spinal cord, thereby modulating the neural circuits responsible for movement. Concurrently, the exoskeleton provides physical support and kinesthetic feedback, which is essential for gait training and weight-bearing practices.9 In this report, we presented a case in which spatiotemporal SCS combined with real-time exoskeleton-triggering (EXS-SCS) successfully restored lower limb motor function in a patient with complete SCI.
Case Report
The participant was a 16-year-old female with a complete T12 SCI due to a car crash a year ago, who presented sensory disturbances, urinary and fecal incontinence, and paralysis of both lower limbs, and was classified as ASIA A. She underwent thoracolumbar fracture reduction and internal fixation via a posterior approach (T10-L2) and maintained consistent rehabilitation treatments, but there was no significant recovery in lower limb movement or bowel control.
Upon admission, physical examination showed that the muscle strength of the bilateral iliopsoas and quadriceps femoris was grade 1, while the remaining muscles of both lower extremities were graded at 0. Sensation was absent below the L1 level. The anal sphincter showed no voluntary contraction, and there was no pressure sensation. Both physiological and pathological reflexes were absent, and muscle tone was markedly reduced. Imaging confirmed stable internal fixation, no space-occupying compression in the spinal canal, and clear adhesions between the T12 spinal cord segment and the dorsal dura (see Fig. 1).

SCS electrodes (5–6-5 paddle, RISHENA Medical Device Co., Ltd.) were implanted at the T11-L1 spinal segment. Intra-operatively, electrical stimulation was applied with the following parameters: a frequency of 5 Hz, a pulse width of 200 microseconds, and an amplitude ranging from 6 to 20 mA. These parameters were used to test the activation thresholds of different muscle groups, allowing for the identification of the optimal stimulation configuration for each patient. Multiple configurations were tested based on intraoperative electrophysiological results, and electrodes 14 and 15 were optimal for stimulation, which provided the most effective activation of the patient's right adductor muscles, quadriceps femoris, and gastrocnemius, with minimal activation of nontarget muscles (see Fig. 2).
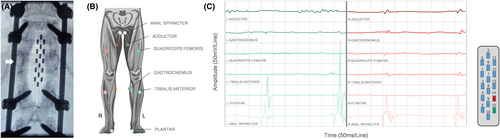
During the 2-week postoperative testing period, the patient was fitted with an external temporary SCS stimulator. On the first day, there was a slight improvement in quadriceps femoris strength, a sensory level descent to L2, and an initial ASIA score of grade A. Baseline percutaneous electromyography (EMG) revealed an extended distal motor latency of the left common peroneal nerve, a 95% reduction in amplitude with mid-ankle stimulation, a 25% decrease in conduction velocity of the left tibial nerve, and an 83% reduction in amplitude upon stimulation at the medial malleolus, with no other responses detected.
A long-duration electrical stimulation protocol was employed alongside passive lower limb exoskeleton training. Based on surface EMG data and the patient's subjective sensations, the SCS activation patterns were categorized into three groups: motor stimulation, sensory stimulation, and stimulation for defecation and urination functions (see Table 1). The motor stimulation group was activated for 4 h daily, during which the patient participated in physical rehabilitation, including exoskeleton-assisted lower limb training, therapist-assisted muscle exercises, cycling, and standing exercises. The sensory stimulation group was activated for 10.5 h daily, inducing a tingling sensation in the bilateral iliacus and quadriceps femoris muscles, aimed at enhancing sensory function. The defecation and urination function group was activated for 9.5 h during sleep, slightly stimulating anal sphincter activity. The choice of biphasic pulses for SCS was made to ensure charge balance within the tissue, minimizing the risk of electrolysis and tissue damage, which is particularly important for long-term stimulation protocols.
| Group | Activated Electrodea | Frequency/Hz | Pulse width/μs | Amplitude/mA | Cycle | Activation time | Effect |
|---|---|---|---|---|---|---|---|
| A1 | 9, 10 | 5 | 200 | 12 | 600 s on, 300 s off | 09:30–11:30 | To facilitate the recovery of muscle strength, surface electromyography verifies the effective activation of the right quadriceps, bilateral iliopsoas, tibialis anterior, plantar muscles, and gastrocnemius. |
| A2 | 14, 15 | 5 | 200 | 9 | NA | 15:00–17:00 | |
| B1 | 7, 3 | 60 | 500 | 6 | NA | 07:30–9:30; 17:00–22:00 | To promote sensory recovery, the patient reported subjective sensations of mild tingling bilaterally, encompassing the surface from the iliopsoas to the quadriceps muscles. |
| B2 | 7, 14 | 60 | 300 | 4 | NA | 11:30–15:00 | |
| C | 5, 6 | 14 | 210 | 5 | NA | 22:00–07:30 (+1) | To facilitate the recovery of bowel and bladder functions, a mild promotion of anal sphincter contraction is initiated during sleep time. |
- a The signals SCS applied were biphasic.
After 2 weeks, the patient's bilateral iliacus muscle strength improved to grade 3, the quadriceps femoris to grade 2, the sensory level descended to L2, deep pressure sensation in the anal region was restored, and her ASIA score improved to B. Remarkably, the patient was also able to independently move her legs while lying in bed.
The patient subsequently underwent permanent SCS implantation, with a rechargeable pulse generator placed in the lower back. One week postoperatively, the patient started EXS-SCS therapy. We developed an algorithm to synchronize the exoskeleton (RoboCT Technology Development Co., Ltd.) with the SCS device (RISHENA Medical Device Co., Ltd.), enabling simultaneous stimulation and active muscle training within a 2-sec cycle (see Fig. 3). The exoskeleton system was equipped with a drive that detected the signals from SCS through wireless transmission. When the patient moved either leg, the corresponding muscles were activated, accompanied by a tingling sensation. The exoskeleton allowed for resistance to the movements derived by active assistance facilitation, which involves the participant actively contracting their muscles against a resistance while the exoskeleton passively facilitates the overall movement (see Video S1). This approach combines the benefits of active muscle engagement with the support of passive movement assistance, allowing for controlled resistance training within the constraints of the participant's current motor abilities.
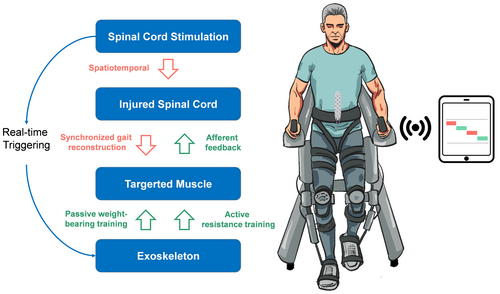
The patient engaged in the EXS-SCS training regimen lasting 30 min to 1 h daily, contingent upon their physical condition. The SCS parameters were set at a frequency of 5 Hz, a pulse width of 200 microseconds, and an amplitude of 9 mA, while the long-duration stimulation parameters outlined in Table 1 were maintained during other periods. After 1 month, the patient's bilateral iliacus muscle strength improved to grade 4, and the quadriceps femoris to grade 3, enabling her to stand and walk with assistance. The ASIA score was reevaluated as grade C. Two months postoperatively, percutaneous EMG results showed varying degrees of improvement in the motor conduction velocity of the left common peroneal and tibial nerves (see Table 2).
| Location | Pre-operation | Two months postoperation | ||||
|---|---|---|---|---|---|---|
| Latency/ms | Amplification/mV | Velocity/m·s−1 | Latency/ms | Amplification/mV | Velocity/m·s−1 | |
| L-Peroneal n.(middle ankle) | 8.2 | 0.2 | NA | 7.9 | 0.44 | NA |
| L-Tibial n.(medial ankle) | 4.8 | 1.0 | NA | 4.15 | 1.13 | NA |
| L-Tibial n.(popliteal fossa) | 14.6 | 0.9 | 37.6 | 13.9 | 0.51 | 38.8 |
Discussion
The primary objective of this study was to report the potential of spatiotemporal SCS synchronized with exoskeleton-assisted training to restore walking capability in an individual with complete T12 SCI. This combinatory regimen was grounded in the recognition that traditional rehabilitation often reaches plateaus in restoring functional mobility, particularly in patients with complete SCI, due to the lack of afferent signals from muscles. In a study by Takeoka A. et al., mice with Egr3 mutations were constructed as a model for impaired muscle spindle afferentation, and it was found that activity-dependent muscle spindle feedback played a crucial role in neural circuit reconstruction and motor recovery following SCI.6 The integration of SCS provides a novel avenue for enhancing neuromuscular activation through the recruitment of myelinated sensory feedback circuits at the dorsal region of spinal cord.1 Therefore, SCS might further enhance muscle sensory signals through central activation, thereby reconstructing the neural functional circuitry following SCI9.
Current researches on SCI are increasingly shifting toward understanding the adaptive changes in neural circuits and the role of feedback regulation, including upstream brain networks and downstream nerves and muscles, with the goal of promoting function-oriented retraining locomotor recovery.6, 11-13 Empirically, weight-supported locomotor rehabilitation is one of the most common and effective methods for promoting motor recovery.14 Based on Hebbian plasticity, which follows the principle that “neurons that fire together, wire together,” researchers have begun exploring methods to synchronize neural circuit activation. This includes co-stimulation of spinal cord-muscle circuits,12, 15 and brain-spinal cord circuits,16 both of which have shown unexpectedly promising results.
SCS, which involves placing electrodes in the epidural space, can alleviate pain based on the Gate Control Theory and has seen extensive clinical use and research in this area.17 In the context of SCI, electrical stimulation not only promotes neural growth in a targeted manner by recruiting large-diameter afferent fibers at the dorsal root ganglia entry zone but also enhances the motor drive capabilities of the corticospinal tracts, thereby improving fine muscle control postinjury.2-4 However, clinical practice has shown that the long-term effects of SCS on motor function improvement are suboptimal, primarily due to the interference of proprioceptive input caused by electrical stimulation.18
To summary, this case report described the use of spatiotemporal SCS that mimics the natural gait cycle, with targeted application of electrical stimulation to specific spinal cord segments in a temporally coordinated manner corresponding to the sequence of muscle activations during walking. Simultaneously, an exoskeleton, triggered by the SCS, was employed to engage the corresponding muscles, thereby enhancing the afferent nerve feedback. The exoskeleton also enabled the patient to perform weight-bearing active resistance training, allowing for conscious active control during the rehabilitation process. This approach facilitated coordinated brain-spinal cord-muscle feedback training, significantly improving motor recovery. Within just 1 month, the patient progressed from a preoperative bedridden state (ASIA A) to assisted standing and walking with assistance (ASIA C). This case highlights an innovative rehabilitation strategy for SCI, involving coordinated peripheral and central stimulation, which merits further prospective clinical research to explore its efficacy and identify the appropriate patient population.
Nevertheless, the study is constrained by several limitations, including a diminutive sample size, absence of a control cohort, and an indistinct prognosis for long-term efficacy. Moreover, it is imperative that future studies should explore the ramifications of SCS on autonomic dysregulation, gastrointestinal and urinary function, and analgesic management, as well as SCS mapping patterns.19, 20 Concurrently, elucidating the neural plasticity underpinnings of functional amelioration is essential.4 Accounting for patient heterogeneity, which encompasses the level of SCI and concomitant morbidities, is pivotal for delineating the subset of patients optimally positioned to derive maximal benefit from this EXS-SCS training regimen.
Author Contributions
Penghao Liu and Yuanchen Cheng contributed equally to this work. Penghao Liu led the study design and manuscript drafting, while Yuanchen Cheng managed data analysis. Zhuofan Xu was responsible for patient preparation before surgery. Xiaoyu Li conducted intraoperative electrophysiological recordings and analysis. Zan Chen participated in patient management. Wanru Duan supervised the project, provided critical revisions, and served as the corresponding author. All authors approved the final manuscript.
Acknowledgments
None.
Funding Information
This research was supported by National Key R&D Program of China, “Research on Prevention and Control of Common Diseases,” Supported by Ministry of Science & Technology of PRC (no. 2023YFC2509700), Beijing Natural Science Foundation-Haidian Original Innovation Joint Fund, Supported by Beijing Municipal Science & Technology Commission (L232141), and Research and application of clinical characteristic diagnosis and treatment Program, Supported by Beijing Municipal Science & Technology Commission (Z221100007422019).
Conflicts of Interest
The authors have no relevant financial or nonfinancial interests to disclose.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




