Argatroban plus alteplase in posterior versus anterior circulation stroke
Abstract
Objective
ARAIS trial failed to demonstrate benefit of argatroban as an adjunct to alteplase for stroke. Given differences between anterior circulation stroke (ACS) and posterior circulation stroke (PCS), we performed prespecified secondary analysis to investigate whether benefit of argatroban was different between ACS and PCS.
Methods
In secondary analysis of ARAIS, patients with definite stroke territories based on responsible vessel examination were classified into ACS and PCS. The primary outcome was a 90-day excellent functional outcome (modified Rankin Scale score of 0 to 1). The efficacy was compared between argatroban plus alteplase and alteplase alone.
Results
This study included 356 patients from the full analysis set of ARAIS trial: 283 in the ACS group and 73 in the PCS group. Compared with alteplase alone, a higher likelihood of 90-day excellent functional outcome was associated with argatroban plus alteplase in PCS group (78.1% versus 61.0%; adjusted RD, 14.4%; 95% CI, 1.6% to 27.2%; p = 0.03), but similar in ACS group (61.7% versus 62.7%; adjusted RD, −2.4%; 95% CI, −10.1% to 5.2%; p = 0.54). After controlling unbalanced sample size bias by propensity score matching, significant interaction between efficacy and stroke territories was found (p = 0.01). The risk of symptomatic intracranial hemorrhage was higher following argatroban plus alteplase than alteplase alone in ACS group (p = 0.02).
Interpretation
Argatroban plus alteplase, compared with alteplase alone, was associated with improved functional outcomes in PCS. This study first demonstrated better benefits of argatroban plus alteplase in PCS, which deserves to be confirmed.
Introduction
Intravenous thrombolysis is recommended as one of effective therapies for acute ischemic stroke by current guideline.1 Achieving recanalization of responsible vascular following treatment was critical for improving functional outcome,2 but post-thrombolytic successful vascular recanalization was poor with the rate of about 30%.3 Furthermore, re-occlusion following vascular recanalization resulted in early neurological deterioration and poor long-term prognosis.4 Thus, it is important for a suitable therapy to restore recanalization and prevent neurological deterioration, thereby reducing disability and death.
Previous clinical studies suggested that the potential benefit of argatroban combined with intravenous alteplase for acute ischemic stroke based on its potential effect on promoting complete recanalization of large vessel occlusion and preventing the re-occlusion.5, 6 A recent study also found that argatroban could improve 3-month outcome in patients experiencing early neurological deterioration.7 However, the ARAIS (argatroban plus recombinant tissue-type plasminogen activator for AIS) trial did not prove that argatroban combined with intravenous alteplase significantly affected three-month functional outcomes compared with intravenous alteplase alone.8 The neural result is unexpected, which pushes us to explore the target population benefited from argatroban as an adjunct to alteplase.
Stroke can be classified into different subtypes such as anterior circulation stroke (ACS) and posterior circulation stroke (PCS) based on vascular territories experiencing injury. Although previous studies indicated that similar function outcome existed between two subtypes of stroke,9, 10 different clinical characteristics may exist, such as some risk factors of early neurological deterioration that was associated with long-term outcome.11 For example, presumed stroke cause including large artery atherosclerosis and embolization were more frequent in the PCS.12 Given that less large artery occlusion may lead to the negative results of the ARAIS trial8 and cardioembolic stroke may benefit from anticoagulation treatment,13, 14 we hypothesized that argatroban plus alteplase may be potentially effective in the population diagnosed with PCS. Therefore, we performed this prespecified secondary analysis to respectively compare the efficacy of argatroban plus alteplase with alteplase alone between patients with ACS and PCS.
Methods
Study design and population
This secondary analysis was reported by the Strengthening the Reporting of Observational Studies in Epidemiology guideline. Details of the ARAIS trial have been published,15 which was a randomized clinical trial to evaluate the efficacy of treatment with argatroban plus alteplase for ischemic stroke within 4.5 h of symptom onset. Participants were included if they were 18 to 80 years old, acute ischemic stroke with ≥6-point National Institutes of Health Stroke Scale (NIHSS) score at admission, and screened within 4.5 h after the stroke onset, and they were excluded if they were pre-stroke disability with ≥2-point modified Rankin Scale [mRS] score, experienced bleeding events, and needed other than anticoagulants treatments. The ARAIS trial was approved by Ethics Committees of General Hospital of Northern Theater Command (approval number: k [2018]45), registered with ClinicalTrials.gov (NCT03740958), abided by the Declaration of Helsinki, and obtained written informed consents.
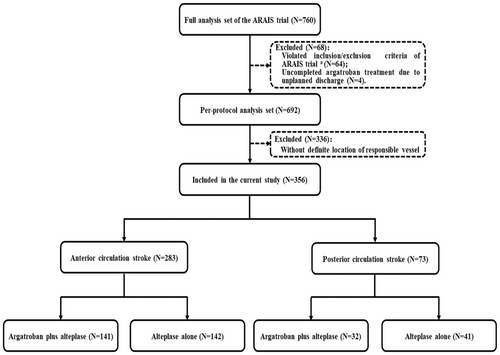
In the current analysis, participants were further screened from the full analysis set of the ARAIS trial. Patients were further excluded if they violated inclusion/exclusion criteria or received uncompleted argatroban treatment due to unplanned discharge. Additionally, considering symptoms may be confusing to localize the stroke territories, patients were excluded who did not justify the stroke territories by responsible vessel examination adjunct to patients' clinical presentation and neuroimaging at admission.
Procedures
Included participants were classified into two subtypes based on stroke territories: ACS and PCS groups. According to the randomization at admission, patients in each stroke subtype were further classified into argatroban plus alteplase treatment group and alteplase alone treatment group. Details of administration of alteplase and argatroban have been stated in the ARAIS trial.8 In addition to study agents, participants also received guideline-standard treatments.1
Baseline data were obtained at randomization to show the demographic and clinical characteristics of participants. Follow-up data included NIHSS score at 48 h and 14 days after randomization which were assessed to evaluate the neurological status, and mRS score and vascular events at 90 days after randomization. All the data were recorded in an electronic data capture system based on a website.
Outcomes
The primary outcome was a 90-day excellent functional outcome, which was defined as mRS scoring 0 to 1. The secondary outcomes included 90-day favorable functional outcome (mRS scoring 0 to 2), shift distribution of 90-day mRS score, early neurological improvement at 48 h (≥2-point decrease in NIHSS score compared with baseline),16 early neurological deterioration at 48 h (≥4-point increase in NIHSS score compared with baseline),17 change in NIHSS score at 14 days compared with baseline, and stroke18 or other vascular events within 90 days. The prespecified safety outcomes included symptomatic intracranial hemorrhage (bleeding on the head CT scan followed with ≥4-point increase in NIHSS score),19 parenchymal hematoma type 2 (bleeding occupying >30% of the infarct volume with mass effect),20 and major systemic bleeding (≥2 g/dL decrease in the hemoglobin level or ≥2-unit transfusion blood) that occurred during the trial. Baseline and follow-up data such as NIHSS score and bleeding events were assessed by the same investigator in each participating site, who was unblinded to treatment allocation. Follow-up data such as 90-day mRS score and vascular events were assessed by the trained investigators in participating sites, who were blinded to treatment allocation.
Statistical analysis
This analyses included part of participants from full analysis set of the ARAIS trial. Baseline characteristics were described by median (interquartile range) or number (percentage), which were compared between the included and excluded participants to explore potential bias from population selection bias and compared between treatment groups to show the balance. For the outcomes, we respectively compare the efficacy between treatments in each stroke subtype. For the primary outcome and secondary outcomes such as 90-day favorable functional outcome, 48-hour early neurological function, change in NIHSS score, and safety outcomes, we investigated the association by generalized linear models with risk difference (RD) or geometric mean ratio (GMR) and their 95% confidence intervals (CIs). For the 90-day mRS score distribution, we investigated it by ordinal logistic regression with odds ratio (OR) and 95% CI. For the stroke or other vascular events within 90 days, we investigated by Cox regression model with hazard ratio (HR) and 95% CI.
The primary analyses were adjusted due to imbalance between treatment groups, which included the covariates with p value <0.1. Interactions between treatment effect and stroke territories were assessed, which were conducted by including the treatment groups, stroke territories subgroups, and their interaction term as independent variables in the generalized linear model, ordinal logistic regression model, and Cox regression model, and calculated the pint values for the interaction term. As a sensitivity analysis of primary outcome, propensity score matching was performed to generate a new cohort with balanced sample size to address unbalanced sample size between stroke subtypes. Baseline characteristics including age, gender, time from symptom onset to thrombolysis, premorbid function, history of stroke or transient ischemic attack, and confounders with p value <0.1 compared between stroke subtypes were matched with 1:1 of ratio, 0.05 of settled caliper, and strategy of nearest-neighbor matching.
We conducted the analyses as exploratory and Two-sided p values with less than 0.05 were statistically significant. The SPSS software (Version 26.0, IBM) and R software (version 4.1.0, R Foundation for Statistical Computing) were used for all the analyses.
Results
After excluding 404 patients (64 violating inclusion or meeting exclusion criteria, 4 uncompleted argatroban treatment, and 336 without determined stroke territories due to lack of vessel examination or partial ACS) from full analysis set of the ARAIS trial, 356 patients were totally included in the current analysis: 283 in ACS (141 assigned into argatroban plus alteplase and 142 assigned into alteplase alone) and 73 in PCS (32 assigned into argatroban plus alteplase and 41 assigned into alteplase alone) (Fig. 1). There were some imbalances of baseline characteristics between included and excluded patients, including current smoking and drinking, blood pressure at randomization, history of hypertension, and presumed stroke cause (Table 1).
| Full analysis set (N = 760) | Included patients (N = 356) | Excluded patients (N = 404) | p value | |
|---|---|---|---|---|
| Age, y | 64 (57–71) | 64 (57–70) | 65 (56–72) | 0.61 |
| Sex (F) | 222 (29.2) | 93 (26.1) | 129 (31.9) | 0.08 |
| Current smoker | 272 (35.8) | 152 (42.7) | 120 (29.7) | 0.002* |
| Current drinkera | 138/743 (18.6) | 80/352 (22.7) | 58/391 (14.8) | 0.01* |
| Comorbiditiesb | ||||
| Hypertension | 426 (56.1) | 219 (61.5) | 207 (51.2) | 0.004* |
| Diabetes | 172/760 (22.6) | 83 (23.3) | 89/404 (22.1) | 0.69 |
| Previous strokec | 142 (18.7) | 56 (15.7) | 86 (21.3) | 0.05 |
| Previous TIA | 7 (0.9) | 1 (0.3) | 6 (1.5) | 0.08 |
| Blood pressure at randomization, mmHg | ||||
| Systolic | 150 (138–168) | 160 (143–175) | 145 (135–160) | <0.001* |
| Diastolic | 89 (80–98) | 90 (82–100) | 86 (80–94) | <0.001* |
| FBG at randomization, mmol/L | 6.70 (5.71–8.99) | 6.71 (5.70–9.10) | 7.57 (6.00–13.18) | 0.83 |
| NIHSS score at randomizationd | 9 (7–12) | 8 (6–12) | 9 (7–12) | 0.09 |
| Estimated premorbid function (mRS score)e | ||||
| Score, 0 | 609 (80.1) | 277 (77.8) | 332 (82.2) | 0.08 |
| Score, 1 | 141 (18.6) | 79 (22.2) | 62 (15.3) | |
| Score, 2 | 10 (1.3) | N/A | 10 (2.5) | |
| OTT, min | 160 (117–205) | 155 (115–202) | 162 (118–210) | 0.30 |
| Endovascular therapy | 23 (3.0) | 8 (2.2) | 15 (3.7) | 0.75 |
| Duration of hospitalization, d | 10 (7–13) | 9 (7–12) | 11 (8–14) | 0.08 |
| Presumed stroke causef | ||||
| Undetermined | 503/745 (67.5) | 139 (39.0) | 364/388 (93.6) | <0.001* |
| Large artery atherosclerosis | 141/745 (19.0) | 131 (36.8) | 10/388 (2.6) | |
| Small artery occlusion | 62/745 (8.3) | 53 (14.9) | 9/388 (2.3) | |
| Cardioembolic | 35/745 (4.7) | 31 (8.7) | 4/388 (1.0) | |
| Other | 4/745 (0.5) | 2 (0.6) | 2/388 (0.5) | |
- The data was shown with median (interquartile range) or number (percentage). p value represents the comparison between included and excluded patients.
- FBG, fasting blood glucose; NIHSS, National Institute of Health Stroke Scale; mRS, modified Rankin Scale; OTT, time from onset of symptom to intravenous thrombolysis; TIA, transient ischemic attack.
- a Defined as consuming alcohol at least once a week within 1 year prior to the onset of the disease.
- b The comorbidities were based on the patient or family report.
- c Previous stroke included ischemic and hemorrhagic stroke. Previous referred only to the patients with pre-stroke mRS ≤1.
- d Patients with NIHSS scores of more than or equal to 6 were eligible for this study; NIHSS scores range from 0 to 42, with higher scores indicating more severe neurological deficit.
- e Scores on the mRS of functional disability range from 0 (no symptoms) to 6 (death).
- f The presumed stroke cause was classified according to the TOAST (Trial of Org 10,172 in Acute Stroke Treatment) using clinical findings, brain imaging, and laboratory test results. Other causes included nonatherosclerotic vasculopathies, hypercoagulable states, and hematologic disorder.
- * p value <0.05.
As shown in Table 2, there were well balanced among the baseline characteristics between treatment groups across ACS and PCS, except higher diastolic blood pressure at randomization in the argatroban plus alteplase treatment group of ACS, longer hospitalization duration, and some imbalance in presumed stroke cause in the argatroban plus alteplase treatment group of PCS. In addition, compared with the PCS group, higher baseline NIHSS score and less defined stroke cause were found in ACS.
| ACS (N = 283) | PCS (N = 73) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Argatroban plus alteplase (N = 141) | Alteplase alone (N = 142) | p Value | Total | Argatroban plus alteplase (N = 32) | Alteplase alone (N = 41) | p Value | ||
| Age, y | 64 (57–70) | 65 (57–71) | 64 (56–70) | 0.51 | 65 (58–71) | 68 (61–71) | 62 (55–70) | 0.34 | 0.79 |
| Sex (F) | 79 (27.9) | 46 (32.6) | 33 (23.2) | 0.08 | 14 (19.2) | 7 (21.9) | 7 (17.1) | 0.61 | 0.13 |
| Current smoker | 119 (42.0) | 58 (41.1) | 61 (43.0) | 0.81 | 33 (45.2) | 15 (46.9) | 18 (43.9) | 0.70 | 0.63 |
| Current drinkera | 65/279 (23.3) | 34/138 (24.6) | 31/141 (22.0) | 0.16 | 15 (20.5) | 6 (18.8) | 9 (22.0) | 0.19 | 0.49 |
| Comorbiditiesb | |||||||||
| Hypertension | 178 (62.9) | 92 (65.2) | 86 (60.6) | 0.42 | 41 (56.2) | 19 (59.4) | 22 (53.7) | 0.63 | 0.29 |
| Diabetes | 66 (23.3) | 36 (25.5) | 30 (21.1) | 0.38 | 17 (23.3) | 8 (25.0) | 9 (22.0) | 0.76 | 0.99 |
| Previous strokec | 48 (17.0) | 28 (19.9) | 20 (14.1) | 0.20 | 8 (11.0) | 3 (9.4) | 5 (12.2) | 0.70 | 0.21 |
| Previous TIA | 1 (0.4) | 1 (0.7) | 0 (0.0) | 0.32 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 | 0.61 |
| Blood pressure at randomization, mmHg | |||||||||
| Systolic | 160 (142–176) | 160 (150–177) | 155 (140–175) | 0.64 | 158 (144–170) | 157 (147–164) | 158 (139–174) | 0.90 | 0.98 |
| Diastolic | 91 (82–100) | 95 (85–102) | 90 (81–99) | 0.02* | 90 (81–99) | 90 (82–99) | 90 (81–99) | 0.87 | 0.16 |
| FBG at randomization, mmol/L | 6.78 (5.69–9.20) | 6.75 (5.72–9.47) | 6.80 (5.58–9.16) | 0.95 | 6.64 (5.71–8.60) | 6.10 (5.34–7.85) | 6.92 (5.86–9.00) | 0.11 | 0.82 |
| NIHSS score at randomizationd | 9 (6–12) | 9 (7–12) | 8 (6–12) | 0.51 | 7 (6–10) | 8 (6–11) | 7 (6–10) | 0.34 | 0.02* |
| Estimated premorbid function (mRS score)e | |||||||||
| No symptoms (score, 0) | 221 (78.1) | 108 (76.6) | 113 (79.6) | 0.54 | 56 (76.7) | 26 (81.3) | 30 (73.2) | 0.42 | 0.80 |
| Symptoms without any disability (score, 1) | 62 (21.9) | 33 (23.4) | 29 (20.4) | 17 (23.3) | 6 (18.7) | 11 (26.8) | |||
| OTT, min | 153 (114–195) | 152 (115–202) | 155 (112–190) | 0.68 | 155 (119–210) | 168 (126–229) | 152 (110–206) | 0.73 | 0.60 |
| Endovascular therapy | 6 (2.1) | 2 (1.4) | 4 (2.8) | 0.41 | 2 (2.7) | 1 (3.1) | 1 (2.4) | 0.86 | 0.75 |
| Duration of hospitalization, d | 9 (7–12) | 9 (7–11) | 9 (6–13) | 0.85 | 9 (8–12) | 11 (8–13) | 9 (7–11) | 0.04* | 0.90 |
| Presumed stroke causef | |||||||||
| Undetermined | 116 (41.0) | 59 (41.8) | 57 (40.1) | 0.89 | 23 (31.5) | 5 (15.6) | 18 (43.9) | 0.04* | 0.51 |
| Large artery atherosclerosis | 102 (36.0) | 50 (35.5) | 52 (36.6) | 29 (39.7) | 13 (40.6) | 16 (39.0) | |||
| Small artery occlusion | 41 (14.5) | 21 (14.9) | 20 (14.1) | 12 (16.4) | 7 (21.9) | 5 (12.2) | |||
| Cardioembolic | 23 (8.1) | 11 (7.8) | 12 (8.5) | 8 (11.0) | 6 (18.8) | 2 (4.9) | |||
| Other | 1 (0.4) | 0 (0.0) | 1 (0.7) | 1 (1.4) | 1 (3.1) | 0 (0.0) | |||
- The data were shown with median (interquartile range) or number (percentage).
- ACS, anterior circulation stroke; FBG, fasting blood glucose; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; OTT, time from onset of symptom to intravenous thrombolysis; PCS, posterior circulation stroke; TIA, transient ischemic attack.
- a Defined as consuming alcohol at least once a week within 1 year prior to the onset of the disease.
- b The comorbidities were based on the patient or family report.
- c Previous stroke included ischemic and hemorrhagic stroke. Previous stroke referred only to the patients with pre-stroke mRS ≤1.
- d Patients with NIHSS scores of more than or equal to 6 were eligible for this study; NIHSS scores range from 0 to 42, with higher scores indicating more severe neurological deficit.
- e Scores on the modified Rankin Scale (mRS) of functional disability range from 0 (no symptoms) to 6 (death).
- f The presumed stroke cause was classified according to the TOAST (Trial of Org 10,172 in cute Stroke Treatment) using clinical findings, brain imaging, and laboratory test results. Other causes included nonatherosclerotic vasculopathies, hypercoagulable states, and hematologic disorder.
- * p value <0.05.
The comparisons of outcomes between treatment groups in each stroke subtype were shown in Table 3. Patients with 90-day mRS scoring 0 to 1 in the argatroban plus alteplase treatment group versus alteplase alone treatment group was 61.7% versus 62.7% in ACS, and 78.1% versus 61.0% in PCS, respectively (Fig. 2). Compared with alteplase alone, argatroban plus alteplase was significantly associated with a higher likelihood of 90-day excellent functional outcome in PCS (adjusted RD, 14.4%; 95% CI, 1.6% to 27.2%; p = 0.03), but not in the ACS group (adjusted RD, −2.4%; 95% CI, −10.1% to 5.2%; p = 0.54). There was an interaction trend between stroke territories and treatment effect on the outcome without significant difference (p = 0.08).
| Outcomes | Subgroups | No. (%) of events or median difference | Unadjusted | Adjusteda | pint value | |||
|---|---|---|---|---|---|---|---|---|
| Argatroban plus alteplase | Alteplase alone | Treatment difference (95% CI) | p value | Treatment difference (95% CI) | p value | |||
| Primary outcome | ||||||||
| mRS 0 to 1 within 90 db, c | ACS (N = 283) | 87/141 (61.7) | 89/142 (62.7) | −1.0 (−12.3 to 10.3) | 0.87 | −2.4 (−10.1 to 5.2) | 0.54 | 0.08 |
| PCS (N = 73) | 25/32 (78.1) | 25/41 (61.0) | 17.1 (−3.5 to 37.8) | 0.10 | 14.4 (1.6 to 27.2) | 0.03* | ||
| Secondary outcomes | ||||||||
| mRS 0 to 2 within 90 db, c | ACS (N = 283) | 107/141 (75.9) | 102/142 (71.8) | 4.1 (−6.2 to 14.3) | 0.44 | 4.2 (−2.7 to 11.1) | 0.23 | 0.17 |
| PCS (N = 73) | 28/32 (87.5) | 30/41 (73.2) | 14.3 (−3.4 to 32.1) | 0.11 | 14.4 (3.6 to 25.1) | 0.009** | ||
| mRS distribution at 90 db | ACS (N = 283) | – | – | 0.99 (0.66 to 1.51) | 0.98 | 0.98 (0.74 to 1.30) | 0.87 | 0.33 |
| PCS (N = 73) | – | – | 1.35 (0.58 to 3.14) | 0.48 | 1.20 (0.71 to 2.04) | 0.49 | ||
| Early neurological improvement within 48 hc, d | ACS (N = 283) | 101/141 (71.6) | 97/142 (68.3) | 3.3 (−7.4 to 14.0) | 0.54 | 3.0 (−4.3 to 10.2) | 0.42 | 0.93 |
| PCS (N = 73) | 22/32 (68.8) | 27/41 (65.9) | 2.9 (−18.8 to 24.5) | 0.79 | −0.2 (−13.9 to 13.5) | 0.98 | ||
| Early neurological deterioration within 48 hc, e | ACS (N = 283) | 4/141 (2.8) | 5/142 (3.5) | −0.7 (−4.8 to 3.4) | 0.74 | −1.0 (−3.7 to 1.7) | 0.47 | 0.63 |
| PCS (N = 73) | 1/32 (3.1) | 3/41 (7.3) | −4.2 (−14.2 to 5.8) | 0.41 | −6.9 (−12.5 to −1.2) | 0.02* | ||
| Change in NIHSS score at 14 df, g | ACS (N = 283) | −0.40 (−0.69 to −0.15) | −0.35 (−0.82 to −0.11) | 0.02 (−0.07 to 0.11) | 0.65 | 0.03 (−0.03 to 0.09) | 0.28 | 0.52 |
| PCS (N = 73) | −0.37 (−0.81 to −0.12) | −0.23 (−0.63 to −0.14) | 0.09 (−0.10 to 0.27) | 0.35 | 0.12 (0.08 to 0.24) | 0.04* | ||
| Stroke or other vascular events within 90 dh | ACS (N = 283) | 0/141 (0.0) | 0/142 (0.0) | N/A | N/A | N/A | N/A | NA |
| PCS (N = 73) | 0/32 (0.0) | 0/41 (0.0) | N/A | N/A | N/A | N/A | ||
| Safety outcomes | ||||||||
| Symptomatic intracranial hemorrhagec | ACS (N = 283) | 5/141 (3.5) | 1/142 (0.7) | 2.8 (−0.5 to 6.2) | 0.10 | 2.6 (0.4 to 4.7) | 0.02* | 0.99 |
| PCS (N = 73) | 0/32 (0.0) | 1/41 (2.4) | −2.4 (NA to NA) | 0.37 | −4.1 (NA to NA) | 0.09 | ||
| Parenchymal hematoma type 2c | ACS (N = 283) | 3/141 (2.1) | 1/142 (0.7) | 1.4 (−1.3 to 4.2) | 0.31 | 1.3 (−0.5 to 3.1) | 0.16 | 0.99 |
| PCS (N = 73) | 0/32 (0.0) | 2/41 (4.9) | −4.9 (NA to NA) | 0.21 | −8.5 (NA to NA) | 0.05 | ||
| Major systemic bleedingc | ACS (N = 283) | 0/141 (0.0) | 0/142 (0.0) | N/A | N/A | N/A | N/A | N/A |
| PCS (N = 73) | 0/32 (0.0) | 0/41 (0.7) | N/A | N/A | N/A | N/A | ||
| Sensitivity analyses for primary outcome | ||||||||
| Propensity score matchingi | ACS (N = 73) | 18/35 (51.4) | 28/38 (73.7) | −22.3 (−43.9 to −0.6) | 0.04* | −22.3 (−45.0 to 0.4) | 0.05 | 0.01* |
| PCS (N = 73) | 25/32 (78.1) | 25/41 (61.0) | 17.1 (−3.5 to 37.8) | 0.10 | 14.4 (1.6 to 27.2) | 0.03* | ||
- pint value means the p value for interaction.
- ACS, anterior circulation stroke; CI, confidence interval; mRS, modified Rankin Scale; N/A, not applicable; NIHSS, National Institute of Health Stroke Scale; PCS, posterior circulation stroke.
- a Adjusted for covariates compared between argatroban plus alteplase and alteplase alone treatment groups with p value <0.1 in each subgroup.
- b mRS scores range from 0 to 6: 0 = no symptoms, 1 = symptoms without clinically significant disability, 2 = slight disability, 3 = moderate disability, 4 = moderately severe disability, 5 = severe disability, and 6 = death. The analyses were performed by ordinal logistic regression and presented by odds ratio. No violation of the proportional odds assumption occurred.
- c Calculated using generalized linear model and presented by risk difference.
- d Early neurological improvement was defined as a decrease between baseline and 48 h of 2 on the NIHSS score.15
- e Early neurological deterioration was defined as an increase between baseline and 48 h of 4 on the NIHSS score, but not as a result of cerebral hemorrhage.16
- f NIHSS scores range from 0 to 42, with higher scores indicating greater stroke severity. The log (NIHSS+1) was analyzed using generalized linear model.
- g Calculated using generalized linear model and presented by geometric mean ratio.
- h Calculated using Cox regression model and presented by hazard ratio.
- i Propensity score matching was conducted by matching age, gender, time from symptom onset to thrombolysis, premorbid function, history of stroke or transient ischemic attack, and potential confounders with p value <0.1 between two subgroups with the ratio 1:1, the caliper of 0.05, and a nearest-neighbor matching strategy.
- * p value <0.05.
- ** p value <0.01.
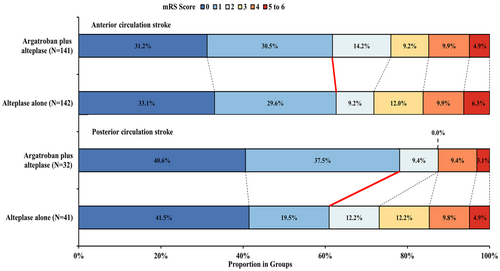
With respect to secondary outcomes, adjusted analyses found that there is a significantly higher likelihood of mRS 0–2 (adjusted RD, 14.4%; 95% CI, 3.6% to 25.1%; p = 0.009), lower risk of 48-h early neurological deterioration and more decrease in 14-day NIHSS score (adjusted GMR, 0.12; 95% CI, 0.08 to 0.24; p = 0.04) were associated with argatroban plus alteplase treatment group in PCS. In addition, with respect to safety outcomes, more symptomatic intracranial hemorrhage was found to be associated with argatroban plus alteplase treatment in ACS (3.5% versus 0.7%; adjusted RD, 2.6%; 95% CI, 0.4% to 4.7%; p = 0.02), while less in PCS (0.0% versus 2.4%; adjusted RD, −4.1%; p = 0.09). Other outcomes were not significantly different between treatment groups in any stroke subtype. Furthermore, we found no significant interaction among secondary outcomes.
After propensity score matching, 146 of 356 selected patients were matched including 73 in ACS and 73 in PCS. The results of sensitivity analysis were shown in Table 3, which were consistent with those in the primary analyses. Moreover, we found a significant interaction between treatment effects and stroke territories in matched population (pint = 0.01).
Discussion
To our best knowledge, the current study is the first attempt to investigate the efficacy of argatroban plus alteplase in patients with ACS vs PCS. Based on this secondary analysis of ARAIS trial, we aimed to explore the effect of stroke territories on clinical outcomes among patients with acute ischemic stroke who received argatroban plus alteplase treatment.
It was regretful that the ARAIS trial did not demonstrate the benefit of argatroban as an adjunct to alteplase for acute ischemic stroke. However, in the current study, we found argatroban plus alteplase showed significantly higher likelihood of functional outcome at 90 days in patients with PCS, which was not identified in those with ACS. The results suggested that PCS patients who received intravenous alteplase may be target population benefited from argatroban. This finding was further supported by the interaction detected between stroke territories and efficacy of argatroban. Moreover, this finding indicates that the neutral results of the ARAIS trial may be partially attributed to the mixed population. In parallel with functional outcomes at 90 days, argatroban plus alteplase also significantly prevented the occurrence of early neurological deterioration within 48 h and reduced the NIHSS score at discharge compared with baseline in the PCS group. Given that the close association of early neurological deterioration and persistent neurological deficit with poor long-term prognosis after intravenous thrombolysis,21, 22 we interpreted that the improvement in early neurological function may be attributed to 90-day functional outcome. Compared with ACS group, relatively higher proportions of large artery atherosclerosis and cardioembolic stroke were found in the PCS group. Large artery atherosclerosis was identified as an independent risk factor of early neurological deterioration,13 and argatroban was associated with decreased risk of early neurological deterioration in the current study, which was similar with previous study.23 Given that anticoagulation also could improve early neurological function in acute cardioembolic stroke,24 the better functional outcome in patients with PCS may be associated with the relatively higher proportion of cardioembolic stroke as well as large artery atherosclerosis in the PCS group. Generally, these findings demonstrated that patients with PCS may benefit from argatroban plus alteplase compared with alteplase alone, and the treatment effect may be related to the difference in stroke etiology of PCS versus ACS, especially large artery atherosclerosis or cardioembolic stroke.
In the ARAIS trial, the risk of any intracranial hemorrhage between two treatments was similar. However, in the current study, symptomatic intracranial hemorrhage was higher in the ACS (2.1% [6/283]) than PCS (1.4 [1/73]) group. This finding was consistent with previous study that patients with PCS had a lower risk of intracranial hemorrhage following intravenous thrombolysis treatment.25, 26 Interestingly, argatroban plus alteplase was also found associated with significantly higher risk of symptomatic intracranial hemorrhage compared with alteplase alone in ACS group, which may be attributed to the higher risk of symptomatic intracranial hemorrhage in all the patients with ACS. The high risk of symptomatic intracranial hemorrhage in ACS may be associated with the fact that PCS symptoms were usually not well recognized by NIHSS score that was used to judge symptomatic intracranial hemorrhage.
Although the current findings were important, we admitted several limitations. First, given the symptoms may not localize the stroke territories very well, we included the patients who finished vessel examination in the current study as well as the ARAIS trial. Thus, due to the lack of responsible vessel examination, a large proportion of patients without determined stroke territories judicated by responsible vessel were excluded, which may introduce potential selection bias and reduce the sample size. But, the ratio of PCS versus ACS (about ¼) was similar with the real world data.11 Second, imbalanced and relatively small sample size in each stroke subtype would affect statistical power. Although we conducted sensitivity analysis to address the imbalance by matching propensity score, small sample sizes after matching still reduced the power. Thus, the efficacy of argatroban plus alteplase in PCS warrant further investigation in clinical trial with large sample size. Third, lack of vessel examination at baseline and following argatroban treatment limited the interpretation of whether the better functional outcomes in patients with PCS were attributed to its effect on reducing early neurological deterioration due to reducing the re-occlusion of responsible vascular. Fourth, the results need to be validated in a non-Chinese population. Generally, we interpreted our findings with caution due to the exploratory nature of post hoc analysis and negative result of the ARAIS trial. This finding warrant further confirmation.
Conclusions
Our study demonstrated that, in comparison with alteplase alone, argatroban combined with alteplase was associated with better functional outcomes and safety profile in patients with PCS. Considering potential selected bias due to large patients being excluded, this important finding warrants further investigation in the future.
Acknowledgements
We thank all the participating hospitals and clinician investigators.
Funding Information
This study was supported by grants from the Science and Technology Project Plan of Liaoning Province (2023-MSLH-348, 2019JH2/10300027). The funders of the study had no role in the study design, data acquisition and analysis, or the report writing.
Conflict of Interest
The authors report no competing interests.
Author Contributions
H.-S.C. contributed to conception and design of the study, and critically revised the manuscript; Y.C. contributed to acquisition, analysis of data, and wrote the original draft.
Trial Registration
ClinicalTrial.gov identifier: NCT0370958.
Open Research
Data Availability Statement
Data supporting findings are available from corresponding author on reasonable request.




