Retinal thinning differentiates treatment effects in relapsing multiple sclerosis below the clinical threshold
Abstract
Objective
To investigate retinal layer thinning as a biomarker of disease-modifying treatment (DMT) effects in relapsing multiple sclerosis (RMS).
Methods
From an ongoing prospective observational study, we included patients with RMS, who (i) had an optical coherence tomography (OCT) scan within 6 to 12 months after DMT start (rebaseline) and ≥1 follow-up OCT ≥12 months after rebaseline and (ii) adhered to DMT during follow-up. Differences between DMT in thinning of peripapillary-retinal-nerve-fiber-layer (pRNFL) and macular ganglion cell-plus-inner plexiform-layer (GCIPL) were analyzed using mixed-effects linear regression. Eyes suffering optic neuritis during follow-up were excluded.
Results
We included 291 RMS patients (mean age 30.8 years [SD 7.9], 72.9% female, median disease duration 9 months [range 6–94], median rebaseline-to-last-follow-up-interval 32 months [12–82]).
Mean annualized rates of retinal layer thinning (%/year) in reference to DMF (n = 84, GCIPL 0.28, pRNFL 0.53) were similar under TERI (n = 18, GCIPL 0.34, pRNFL 0.59), GLAT (n = 24, GCIPL 0.32, pRNFL 0.56), and IFNb (n = 13, GCIPL 0.33, pRNFL 0.60) were slightly lower under S1PM (n = 27, GCIPL 0.19, pRNFL 0.42) and CLA (n = 23, GCIPL 0.20, pRNFL 0.42), and were significantly lower under NTZ (n = 47, GCIPL 0.09, pRNFL 0.24; both p < 0.001) and antiCD20 (n = 55, GCIPL 0.10, pRNFL 0.23; both p < 0.001). In patients achieving NEDA-2, observed thinning rates were lower overall, but still significantly lower under NTZ and antiCD20.
Interpretation
Applying a rebaselining concept, retinal layer thinning differentiates DMT effects even in clinically stable patients and, thus, might be a useful biomarker to monitor DMT efficacy on subclinical neuroaxonal degeneration—at least on a group level.
Introduction
Multiple sclerosis (MS) is a chronic autoinflammatory disease comprising a largely overlapping continuum of acute focal inflammation and progressively accumulating neuroaxonal damage.1, 2 The degree of neuroaxonal damage—albeit mostly remaining clinically ineloquent in early stages—is the main factor determining long-term prognosis.1, 2 Thus, it is critical to identify subclinical neuroaxonal damage in the early disease phase and measure its accumulation over time in order to improve prognostication and enable early treatment intervention.2
Optical coherence tomography (OCT) provides high-resolution in-vivo imaging and thickness measurement of distinct retinal layers with excellent reproducibility.3 Thinning of both the peripapillary retinal nerve fiber layer (pRNFL) and the combined macular ganglion cell and inner plexiform layer (GCIPL) has been proven as a reliable surrogate marker of neuroaxonal damage in MS associated with clinical correlates of neuroaxonal damage such as disability worsening and progression independent of relapse activity (PIRA).4-8 Rates of retinal layer thinning also vary according to disease-modifying treatments (DMT), with high-efficacy DMTs reducing inner retinal layer atrophy.9-11 While these findings have made OCT a promising option for monitoring DMT effects on neuroaxonal damage, the degree of variation in relation to detectable effect sizes remains a concern.12 It was recently shown that applying a rebaselining concept, that is, setting the starting point of monitoring 6–12 months after DMT start as established for MRI, improves differentiation of DMT effects by eliminating influence on retinal atrophy measurement stemming from disease processes occurring before DMT initiation.13, 14
Here, we aimed to investigate the potential of retinal layer thinning to differentiate DMT effects in relapsing MS (RMS) when employing a rebaselining concept.
Methods
Patients
From an ongoing prospective observational study conducted at the Departments of Neurology of the Medical Universities of Vienna and Innsbruck, we included patients diagnosed with RMS according to 2017 McDonald criteria, who were newly initiated on a DMT.4, 15 Inclusion criteria comprised (i) availability of OCT scans within 6 to 12 months after DMT start (rebaseline), (ii) at least one follow-up OCT with a minimum interval of 12 months to rebaseline, and (iii) continuous adherence to the DMT during follow-up (Fig. 1).
Definitions
For the purpose of this study, a relapse was defined as patient-reported symptoms with objectively observed signs typical of an acute CNS inflammatory demyelinating event with a duration of at least 24 hours in the absence of fever or infection, separated from the last relapse by at least 30 days.16 Disability worsening was defined as an increase in the Expanded Disability Status Scale (EDSS) of ≥1.5/1.0/0.5 points from scores of 0/1.0–5.5/≥6.0 confirmed after 3 months.17 We then classified disability worsening mutually exclusive as either relapse associated worsening (RAW), defined as disability worsening occurring within 3 months after a relapse, or PIRA, defined as disability worsening occurring in the absence of a relapse >3 months.18
NEDA-2 (no evidence of disease activity) was defined as an absence of relapse and disability worsening as per the definitions described above.
Optical coherence tomography
OCT imaging was performed by experienced neuro-ophthalmologists at the Department of Ophthalmology of the same institution using the same spectral-domain OCT (Spectralis®, Heidelberg Engineering, Heidelberg, Germany; software Heidelberg eye explorer software version 6.9a) without pupil dilatation in a dark room. For pRNFL measurement, a custom 12° (3.4 mm) ring scan centered on the optic nerve head was used (1536 A-scans, automatic real-time tracking [ART]: 100 averaged frames). For GCIPL measurement, a 20° × 20° macular volume scan (512 A-scans, 25 B-scans, vertical alignment, ART: 16) centered on the macula was performed. The follow-up function was activated to ensure that longitudinal scans were obtained at the same locations. GCIPL thickness was defined as the mean layer thickness of the four inner (3 mm ring) and outer quadrants (6 mm ring) of the circular grid centered around the foveola as defined by the Early Treatment Diabetic Retinopathy Study.19 Semiautomated image processing was conducted using the built-in proprietary software for automated layer segmentation with manual correction of obvious errors. All examinations were performed in accordance with the OSCAR-IB quality control criteria and described according to the APOSTEL 2.0 criteria.20, 21
By default, thicknesses of GCIPL and pRNFL were calculated as the mean of the values for both eyes. Patients with a history of unilateral optic neuritis (ON) <6 months before rebaseline were excluded from the study. Eyes with a history of ON ≥6 months before rebaseline were eligible for inclusion, as further retinal thinning does not differ between eyes with and without prior ON.22 Eyes suffering ON between rebaseline and last follow-up were excluded and only the values of eyes without ON during the observation period were used for calculation of retinal layer thinning.4, 7 To identify subclinical ON during the observation period, we used interocular asymmetry in retinal thinning (i.e., inter-eye difference in GCIPL/pRNFL thickness reduction compared to the prior OCT) with cut-off values of ≥4 μm for GCIPL and ≥5 μm for pRNFL.23, 24 In these cases, we used only the eye with the higher value for layer thinning calculations. Thus, all parameters used for statistical analyses are not underlying inter-eye interactions. The investigators performing the OCT were blinded to clinical parameters and vice versa. Exclusion criteria comprised previous diagnoses of ophthalmological (i.e., myopia greater than −6 diopters, optic disc drusen, glaucoma, other abnormalities of the optic nerve head or the macula not attributable to MS), neurological, or drug-related causes of vision loss.20
Statistics
Statistical analysis was performed using SPSS 26.0 (SPSS Inc, Chicago, IL, USA) and R-Statistical Software (Version 4.0.0).
We employed linear mixed-effects regression models determining change rates in pRNFL and GCIPL over the available measurements from the rebaseline scan, with treating time (unit: days) as a continuous predictor variable and subjects as random effects while accounting for the nested data structure. To account for flooring/ceiling effects known to occur in MS associated retinal atrophy, log-linear transformation was utilized to normalize each absolute pRNFL and GCIPL metric. DMT was defined as an independent variable with dimethyl fumarate (DMF) used as the reference substance due to the available sample size providing a robust reference group. The regression models were adjusted for age, sex, and disease duration. To facilitate interpretability, we extracted annualized percent change (%/year [%/y] with higher percentages indicating a greater loss) from rebaseline (termed aLpRNFL and aLGCIPL) by multiplying the fixed coefficient for change per day from these models.
We conducted a pre-planned subgroup analysis in patients achieving NEDA-2 during the observation period using the same model set-up in order to investigate whether retinal layer thinning would depict differences between DMT substances in patients without clinical disease activity, that is, the subclinical accumulation of neuroaxonal damage.
To check for a confounding influence of the study center, we conducted sensitivity analyses by conducting the described analyses in subgroups according to center.
To check for a potential confounding influence of ON history, we performed sensitivity analyses by excluding eyes with any ON history.
We tested all variables for normal distribution by Lilliefors-test and for collinearity by variance inflation factor (VIF) and excluded all variables from the regression analyses if the VIF was >2.0 corresponding to an R2 of ≥0.50. A two-sided p-value <0.05 was considered statistically significant. As this was an exploratory analysis, we did not perform correction for multiple testing.
Standard protocol approvals, registrations, and patient consents
The study was approved by the ethics committees of the Medical Universities of Vienna and Innsbruck (EK No: 2323/2019 and AM3743-281/4). Written informed consent was obtained from all study participants.
Results
Of 452 patients included in the ongoing observational study at database closure for the present study (30-JAN-2024), 291 patients were included in the present study. The detailed inclusion/exclusion process is shown in Figure 2.

Detailed characteristics of the study cohort are shown in Table 1. Of the 291 patients included, 84 (28.9%) received DMF, 24 (8.2%) glatiramer acetate (GLAT), 18 (6.2%) teriflunomide (TERI), 13 (4.5%) interferon-beta preparations (IFNb), 27 (9.3%) sphingosine-1-phosphate receptor modulators ([S1PM], fingolimod [n = 20], ozanimod [n = 4], ponesimod [n = 3]), 23 (7.9%) cladribine (CLA), 47 (16.2%) natalizumab (NTZ), and 55 (18.9%) antiCD20 monoclonal antibodies (ocrelizumab [n = 13], ofatumumab [n = 7], rituximab [n = 35]) over a median 32 months from rebaseline to last follow-up (range: 12–82).
| Total n = 291 | |
|---|---|
| Femalea | 213 (73.2) |
| Age at rebaseline (years)b | 31.9 (9.0) |
| MS disease duration at rebaseline (months)c | 9 (6–94) |
| EDSS at rebaselinec | 2.0 (0–4.5) |
| DMT naïvea | 143 (49.1) |
| DMT during observation perioda | 291 (100.0) |
| Dimethyl fumarate | 84 (28.9) |
| Interferon beta | 13 (4.5) |
| Glatiramer acetate | 24 (8.2) |
| Teriflunomide | 18 (6.2) |
| S1PM | 27 (9.3) |
| Cladribine | 23 (7.9) |
| AntiCD20 | 55 (18.9) |
| Natalizumab | 47 (16.2) |
| Rebaseline-to-last-follow-up-interval (months)c | 32 (12–82) |
| Number of OCT scans (per patient)c | 3 (2–11) |
| Inter-eye difference pRNFL at rebaseline (μm)c | 0 (0–4) |
| Inter-eye difference GCIPL at rebaseline (μm)c | 0 (0–3) |
- AntiCD20, anti-CD20 monoclonal antibodies (ocrelizumab, ofatumumab, rituximab); DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale; GCIPL, ganglion cell and inner plexiform layer; OCT, optical coherence tomography; pRNFL, peripapillary retinal nerve fiber layer; S1PM, sphingosine-1-phosphate receptor modulators (fingolimod, ozanimod, ponesimod).
- a Absolute number and percentage.
- b Mean and standard deviation.
- c Median and range.
During this observation period, 61 (21.0%) patients suffered a relapse with 23 (7.9%) resulting in RAW and 14 (4.8%) had PIRA. NEDA-2 status was retained in 218 patients (74.9%).
Retinal layer atrophy rates differ depending on DMT
Starting from the rebaseline OCT scan acquired 6–12 months after DMT initiation, mean annualized retinal layer atrophy rate was 0.21%/y for GCIPL (0.15–0.29) and 0.42%/y for pRNFL (0.31–0.59).
Patients receiving DMF, who served as the reference group, displayed a mean aLGCIPL of 0.28%/y and a mean aLpRNFL of 0.53%/y (Fig. 3). By comparison, observed atrophy rates were similar under TERI (aLGCIPL 0.34%/y, aLpRNFL 0.59%/y), GLAT (aLGCIPL 0.32%/y, aLpRNFL 0.56%/y) and IFNb (aLGCIPL 0.33%/y, aLpRNFL 0.60%/y). Under treatment with S1PM (GCIPL 0.19%/y [p = 0.093], pRNFL 0.42%/y [p = 0.097]) and CLA (GCIPL 0.20%/y [p = 0.095], pRNFL 0.42%/y [p = 0.099]), atrophy rates were numerically slightly lower compared to the DMF group, although above the set level of statistical significance. Patients receiving NTZ (GCIPL 0.09%/y, pRNFL 0.24%/y; both p < 0.001) and antiCD20 (GCIPL 0.10%/y, pRNFL 0.23%/y; both p < 0.001, respectively) displayed significantly less retinal layer atrophy than the DMF reference group.
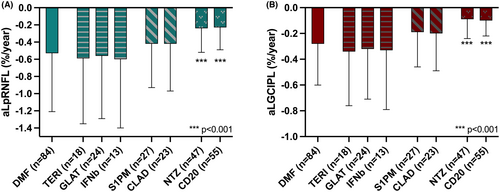
There was no difference in aLpRNFL or aLGCIPL from the rebaseline scan between treatment-naïve patients and those receiving another DMT prior to the one observed.
While aLpRNFL was slightly associated with both higher age and longer disease duration (0.09%/y [0.03–0.18] per decade, p = 0.013 and 0.10%/y [0.02–0.20] per decade, p = 0.020), aLGCIPL was not significantly associated with either of them. Patients suffering a relapse during the observation period had significantly higher aLpRNFL and aLGCIPL (0.23%/y [0.06–0.33]; p = 0.002 and 0.20%/y [0.14–0.34]; p < 0.001). The same was observed for patients displaying RAW (0.70%/y aLpRNFL [0.59–0.82]; p < 0.001 and 0.50%/y aLGCIPL [0.35–0.52]; p < 0.001) and PIRA (0.95%/y aLpRNFL [0.82–1.04]; p < 0.001 and 0.64%/y aLGCIPL [0.46–0.74]; p < 0.001).
Retinal layer atrophy rates still differ in patients achieving clinical stability
In the subgroup of patients achieving NEDA-2 (n = 218) during the observation period, mean aLGCIPL was 0.15%/y for GCIPL (0.06–0.32) and 0.33%/y for pRNFL (0.18–0.62).
Compared to the DMF reference group maintaining NEDA-2 (n = 48, aLGCIPL 0.26%/y, aLpRNFL 0.45%/y), retinal layer atrophy rates were similar to TERI (n = 10, aLGCIPL 0.30%/y, aLpRNFL 0.54%/y), GLAT (n = 13, aLGCIPL 0.28%/y, aLpRNFL 0.51%/y) and IFNb (n = 7, aLGCIPL 0.28%/y, aLpRNFL 0.56%/y) (Fig. 4).
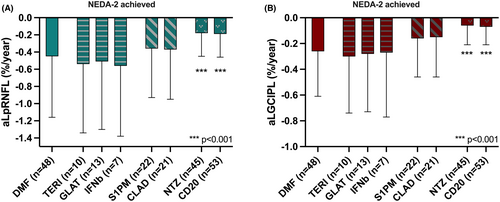
As in the overall cohort, layer atrophy rates were significantly lower in groups achieving NEDA-2 with NTZ (n = 45, GCIPL 0.06%/y, pRNFL 0.18%/y; both p < 0.001) and antiCD20 (n = 53, GCIPL 0.07%/y, pRNFL 0.19%/y; both p < 0.001), whereas they were numerically lower with S1PM (n = 22, GCIPL 0.16%/y, pRNFL 0.36%/y) and CLA (n = 21, GCIPL 0.15%/y, pRNFL 0.37%/y) without reaching statistical difference.
Discussion
Here, we aimed to investigate the potential of retinal layer thinning for differentiating DMT effects in RMS employing a rebaselining concept, that is, setting the starting point of monitoring 6–12 months after DMT start.
We found that using a rebaseline, retinal layer atrophy is significantly lower under treatment with high-efficacy DMT, that is, NTZ and antiCD20. Importantly, these differences remained significant in patients without evidence of clinical disease activity or disability progression (NEDA-2) strongly suggesting that OCT may indeed enable differentiation of the degree of subclinical neuroaxonal degeneration developing under DMT.
Our study is in line with a broad body of evidence establishing GCIPL and pRNFL atrophy as a surrogate of MS associated neuroaxonal damage, which is occurring less pronounced in patients with clinical stability and in those treated with high-efficacy DMT.4, 7, 9, 11, 25-27
Retinal atrophy is correlated to MRI based brain atrophy and both are often viewed to reflect accumulation of diffuse neuroaxonal damage.27-29 However, MRI brain atrophy may reflect many different pathological processes, whereas OCT retinal atrophy can be reliably attributed to loss of neuronal cell bodies/dendrites in the GCIPL and axons in RNFL.2, 6 OCT also provides some methodological advantages providing a higher degree of standardization and reliability at lower cost and infrastructural expenditure.27-30 As the degree of variation in retinal atrophy in relation to detectable effect sizes has remained a concern, we could recently show that setting a rebaseline significantly improved reliability by eliminating influence from disease processes occurring before DMT initiation and potentially accounting for delayed onset of therapeutic biological effect on neuroaxonal damage.14, 26 While previous studies reported larger effect sizes for GCIPL than pRNFL, atrophy rates for pRNFL were slightly higher than for GCIPL—although with a wider range—which may reflect a strength of the re-baselining approach.4, 7, 9, 14, 25
Here, we provide evidence that retinal layer atrophy is not only significantly lowered in patients receiving high-efficacy DMT compared to those treated with less efficacious treatment, but beyond that even displays differences between DMTs in patients without any signs of clinical disease activity (NEDA-2).
Thus, OCT may indeed allow to measure MS associated subclinical neuroaxonal damage. Strengthening this hypothesis, it also appears that PIRA is associated with higher levels of atrophy than RAW (0.95%/y vs. 0.70%/y aLpRNFL and 0.64%/y vs. 0.50%/y aLGCIPL) and RAW with higher levels than any relapse (0.70%/y vs. 0.23%/y aLpRNFL and 0.50%/y vs. 0.20%/y aLGCIPL). This is in line with previous studies, which found that disability progression is closely related with retinal neuroaxonal degeneration, particularly when occurring in absence of relapse (PIRA).10, 25, 28, 31-33 In turn, current evidence suggests that high-efficacy DMT reduces the risk of disability progression and PIRA, although still far from preventing it, potentially by—among other effects—preventing the accumulation of neuroaxonal damage.2, 18, 34 As our cohort mainly comprises patients with early disease (median disease duration 9 months), in whom the degree of neuroaxonal damage is—while often pronounced subclinically—mostly clinically ineloquent (median EDSS 2.0), and with a high proportion of patients receiving high-efficacy DMT, the rates of disability progression and PIRA observed over a median follow-up of 32 months were unsurprisingly low.1, 2, 35 The fact that retinal layer atrophy rates significantly differ in such a cohort even when only looking at those maintaining clinical stability, enticingly suggests that OCT might provide a tool for monitoring decline above and beyond the level of clinical threshold. However, the effect sizes of retinal layer atrophy currently remain close to the range of reported test–retest-reliability, and thus, OCT is not yet suitable for application in clinical practice to monitor individual patients.3, 27, 36
In line with previous studies, both age and disease duration had a small impact on atrophy of pRNFL but not significantly on GCIPL.14, 32 Of note, sensitivity analyses did not indicate an influence of ON occurring >6 months before baseline on retinal layer atrophy, which is in line with most studies.22, 27, 31, 37
Several limitations to this study have to be acknowledged. First, it needs to be emphasized that the sample size for some DMT groups is relatively small and our exploratory findings require external validation in an independent cohort, which was not available for this study. Second, inclusion criteria and endpoints were defined retrospectively, potentially inducing selection bias. Specifically, only 64.4% of the original cohort (n = 452) fulfilled inclusion criteria (see Fig. 2). However, the full prospectively followed study cohort did not significantly differ from the presented cohort in any of the variables analyzed. Also, the present study cohort includes mainly young patients with short disease duration and active MS, limiting assumptions on generalizability to older cohorts with longer disease duration and less active disease. The minimal required follow-up period of 12 months is relatively short for determining neuroaxonal damage. However, we conducted a separate analysis requiring at least 24 months of follow-up, which this did not significantly change the results, which we would hypothesize is primarily due to the use of a rebaseline. Further, the choice of DMT was independent of the study and was made neither blinded nor randomized, causing indication bias. Thus, the rates of relapse and disability progression need to be interpreted within this context. Regarding the rates of retinal layer atrophy, it seems likely that the indication bias might have even reduced the mean group differences as patients with more active disease are inherently more likely to receive high-efficacy DMT. OCT scans were performed at slightly inconsistent intervals (every 6–12 months). However, the mixed-effects models utilized are robust to irregularly spaced measurements mitigating potentially arising bias.38 OCT scans were conducted at two different centers theoretically raising concerns regarding inter-rater variability. However, both centers used the same OCT device (Heidelberg Spectralis) including identical software configurations, and sensitivity analyses regarding effect of study center did not show a significant impact. Applicability of results is limited to populations without confounding comorbidities (e.g., severe myopia, optic disc drusen, any retinal damage not attributable to MS). Due to the inclusion/exclusion criteria reducing biological variability and the meticulous quality control performed for this study limiting measurement errors, variation of retinal atrophy measures is likely increased in a real-world setting with varying OCT protocols and devices.
MRI parameters (new T2 and contrast-enhancing lesions) of disease activity, which may also be related with retinal layer atrophy over time, were not available in a sufficiently standardized manner to be included in this analysis. Thus, we were also unable to employ NEDA-3 for an additional subgroup analysis. Finally, it needs to be acknowledged that retinal layer atrophy, as is the case with MRI brain atrophy, is not specific for MS and may also occur due to a variety of other conditions. Thus, application of OCT for disease monitoring in MS requires the exclusion of any relevant comorbidities by regular and thorough ophthalmological examinations.
In conclusion, our study shows that retinal layer atrophy, when monitored starting from a rebaseline OCT scan 6–12 months after DMT initiation, enables differentiation of DMT effects even in patients achieving NEDA-2, that is, clinically stable MS. Considering the established value of retinal layer atrophy in combination with the methodological advantages of OCT providing standardized non-invasively acquired quantitative measures, retinal layer atrophy may—upon external validation—meet the urgent need for a biomarker allowing longitudinal monitoring of subclinical neuroaxonal damage at least on a group level.
Funding Information
This study was partially funded by the Austrian MS Research Society.
Conflict of Interest
Gabriel Bsteh: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Lilly, MedWhizz, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis. He serves as an Executive Committee Member of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Harald Hegen: has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Bristol Myers Squibb, Horizon, Merck, Novartis, Sanofi-Genzyme, Siemens, and Teva, and received honoraria for consulting Biogen, Bristol Myers Squibb, Novartis, Roche, Sanofi-Genzyme, and Teva. He is associate editor of Frontiers in Neurology. Nik Krajnc: has participated in meetings sponsored by, received speaker honoraria or travel funding from Alexion, BMS/Celgene, Janssen-Cilag, Merck, Novartis, Roche, and Sanofi-Genzyme and held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Fabian Föttinger: nothing to disclose. Patrick Altmann: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Merck, Roche, Sanofi-Genzyme, and Teva, and received honoraria for consulting from Biogen. He received a research grant from Quanterix International and was awarded a combined sponsorship from Biogen, Merck, Sanofi-Genzyme, Roche, and Teva for a clinical study. Michael Auer: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Merck, Novartis, Sanofi Genzyme, and Horizon Therapeutics. Klaus Berek: has participated in meetings sponsored by and received travel funding from Biogen, Roche, Sanofi-Genzyme, and Teva. Barbara Kornek: has received honoraria for speaking and for consulting from Biogen, BMS-Celgene, Johnson&Johnson, Merck, Novartis, Roche, Teva, and Sanofi-Genzyme outside of the submitted work. No conflict of interest with respect to the present study. Fritz Leutmezer: has participated in meetings sponsored by, received speaker honoraria or travel funding from Actelion, Almirall, Biogen, Celgene, Johnson&Johnson, MedDay, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva, and received honoraria for consulting Biogen, Celgene, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. Stefan Macher: declares no conflict of interest relevant to this study. Tobias Monschein: has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. Markus Ponleitner: Has participated in meetings sponsored by, received speaker or consulting honoraria or travel funding from Amicus, Merck, Novartis, and Sanofi-Genzyme. Paulus Rommer: has received honoraria for consultancy/speaking from Alexion/Astra Zeneca, Allmiral, Amgen/Horizon, Amicus, Biogen, Merck, Novartis, Roche, Sandoz, and Sanofi. He has received research grants from Amicus, Biogen, Merck, Roche. Christiane Schmied: declares no conflict of interest relevant to this study. Karin Zebenholzer: received speaking honoraria or travel grants from Biogen, Celgene/BMS, Novartis, and Sanofi-Genzyme. Gudrun Zulehner: has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. Tobias Zrzavy: has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. Florian Deisenhammer: has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, Celgene, Merck, Novartis, Roche, and Sanofi-Genzyme. His institution received scientific grants from Biogen and Sanofi-Genzyme. Franziska Di Pauli: has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Biogen, Celgene BMS, Horizon, Johnson&Johnson, Merck, Novartis, Sanofi-Genzyme, Teva, and Roche. Her institution has received research grants from Roche. Berthold Pemp: has received honoraria for consulting from Novartis, has received honoraria for advisory boards/consulting from Chiesi and GenSight, and has received speaker honoraria from Novartis, Chiesi, and Santen. Thomas Berger: has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, BMS/Celgene, Genesis, GSK, GW/Jazz Pharma, Horizon, Janssen-Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi-Genzyme, Teva, and UCB. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Roche, Sanofi-Genzyme, Teva), and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Teva.
Author Contributions
Gabriel Bsteh: Study concept and design, patient recruitment, acquisition of data, statistical analysis and interpretation of data, drafting of manuscript, study supervision. Harald Hegen: Patient recruitment, acquisition of data, assisted in statistical analysis, interpretation of data, critical revision of manuscript for intellectual content. Nik Krajnc: Patient recruitment, acquisition of data, interpretation of data, critical revision of manuscript for intellectual content. Patrick Altmann, Michael Auer, Klaus Berek, Barbara Kornek, Fritz Leutmezer, Stefan Macher, Tobias Monschein, Markus Ponleitner, Paulus Rommer, Christiane Schmied, Karin Zebenholzer, Gudrun Zulehner, Tobias Zrzavy, Florian Deisenhammer, Franziska Di Pauli, and Berthold Pemp: Patient recruitment, acquisition of data, critical revision of manuscript for intellectual content. Thomas Berger: Study concept and design, patient recruitment, interpretation of data, critical revision of manuscript for intellectual content.
Acknowledgments
None.
Open Research
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the ethics committee and the data-clearing committee of the Medical University of Vienna.




