Phenotypic and oncological insights in ANNA1 autoimmunity: Age stratification and biomarker analysis
Abstract
Objective
To describe the phenotypes, oncological associations, biomarker profiles, and outcomes across different age groups in patients with ANNA1 (anti-Hu) autoimmunity.
Methods
A retrospective review of patients with ANNA1-IgG in serum/CSF between January 1, 2001, and December 31,2019 was performed. Patients were classified into three groups based on the age of symptom onset. Phage immunoprecipitation sequencing (PhIP-Seq) and neurofilament light chain (NfL) measurements were done in patient sera/CSF with archived samples.
Results
Of 122 patients, 81 (66%), 20 (16%), and 21 (17%) patients belonged to older adults, young adults, and pediatric groups, respectively. Lung cancer and neuromuscular presentations were more common in older adults (p < 0.001), while limbic encephalitis and neuroblastoma were more common in pediatric patients (p < 0.005). Most young adults (75%) did not have cancer identified. Proportions of patients with a favorable response to immunotherapy were 20%, 30%, and 52% among older adults, young adults, and pediatric groups, respectively. PhIP-Seq demonstrated significant enrichment for ELAVL4 peptides especially for amino acids 240–289, in the majority of samples evaluated (36/67, 54%). ZIC and SOX2 peptides were significantly enriched in those with central nervous system presentations. Serum NfL levels were elevated in patients with cancer and those with poor long-term outcomes.
Interpretation
Young adults with ANNA1 autoimmunity phenotypically resembled older adults but rarely had an underlying cancer. Pediatric patients frequently presented with limbic encephalitis and neuroblastoma and often responded favorably to immunotherapy. Distinct antigenic signatures may underlie differences in clinical presentations. Serum NfL levels may be a biomarker of poor long-term outcomes in ANNA1 autoimmunity.
Introduction
Antineuronal nuclear antibody type-1 (ANNA1), also known as anti-Hu antibody, is strongly associated with small cell lung cancer and presents with various phenotypes including sensory neuronopathy, cerebellar degeneration, limbic encephalitis, dysautonomia, and/or gastrointestinal dysfunction.1-4 Phenotypes and oncological associations of ANNA1 autoimmunity across the age spectrum are not well described.5-7 The role of biomarkers like neurofilament light chain in ANNA1-IgG-mediated paraneoplastic neurological disorders is still underexplored.8, 9 We sought to explore the differences in clinical presentation, cancer association, and outcomes across three different age groups (pediatric, young adult, and older adult) in patients with ANNA1 autoimmunity. We also looked at the target antigens of interest using phage immunoprecipitation sequencing (PhIP-Seq), and the association of serum and cerebrospinal fluid (CSF) neurofilament levels with clinical outcomes, in patients with ANNA1 autoimmunity.
Methods
We reviewed the clinical details for all patients identified as positive for ANNA1-IgG in serum or CSF in the Mayo Clinic Neuroimmunology Laboratory between January 1, 2001, and December 31, 2019. ANNA1-IgG-positive cases with neurological immune checkpoint inhibitor related adverse events were not included.
Patients were divided into three age groups: less than 18 years (pediatric), 18–45 years (young adult), and greater than 45 years (older adult). Symptom onset was defined as acute (≤4 weeks), subacute (4–8 weeks), and chronic (>8 weeks). Mortality was noted at final follow-up and 5 years from the onset of neurological symptoms.
We included patients with limbic encephalitis, rhombencephalitis, nonlimbic encephalitis, and myelitis with no other neuromuscular or autonomic manifestations as isolated central nervous system (CNS) presentation. Encephalomyelitis referred to multifocal clinical dysfunction involving both the central and peripheral nervous systems.10 The diagnosis of limbic encephalitis included a compatible MRI, as ANNA1 autoimmunity may also cause nonlimbic encephalitis.11
Antibody testing
All patient specimens were tested in the Mayo Clinic Neuroimmunology Laboratory for neural autoantibodies as previously described and were positive for ANNA1-IgG by both a mouse tissue indirect immunofluorescence assay and western blot (native rat cerebellar Hu proteins)/antigen-specific blot (Euroimmun, Lübeck, Germany).12
Phage immunoprecipitation sequencing (PhIP-Seq)
Using previously published methods, 53 sera and 13 CSF samples of patients with ANNA1 were incubated with 1010 plaque-forming units per milliliter of whole human proteome phage display library.13 Using protein A/G beads (Invitrogen), antibody-bound phage particles were isolated and the genetic sequence for the peptide they express amplified using polymerase chain reaction (PCR). The amplified PCR products were prepared for next generation sequencing using Illumina Truseq Nano DNA library and sequenced on the Illumina NovaSeq platform using an SP flow cell. Sequenced reads were processed through an in-house developed bioinformatics pipeline to identify the antigen of interest (ELAVL4). Peptide enrichment scores were computed by taking the ratio of read counts in each sample to the mean count observed in control samples. Protein-level enrichment scores were calculated by summing peptide enrichment scores for the most common isoform of each protein.
Neurofilament light chain (NfL) analysis
NfL analysis was performed for 7 patients with paired serum and CSF samples, 44 patients with serum alone, and 10 patients with CSF alone using Ella multiplexed automated immunoassay system by Bio-Techne (Minneapolis, MN, USA) as per manufacturer's instructions.14 NfL concentrations were measured in duplicate and then averaged. Values were excluded if there was >20% difference between duplicates within the reported linear range. Fresh CSF samples from patients with normal pressure hydrocephalus were used as CSF controls, and samples from healthy controls were used as serum controls.
Statistical analysis
Categorical data were reported as frequencies and percentages, continuous data as medians and ranges. Fisher's exact test was used to compare categorical variables between subgroups. Kaplan–Meier curves were constructed to analyze the differences in survival between the three groups. Independent samples Mann–Whitney U test was used as appropriate to compare associations between groups using IBM SPPS, version 28. Heatmaps of the profile of autoantigens of interest identified using PhiP-Seq were generated using the seaborn visualization package in Python 5.4.3. The enrichment of each antigen between clinical presentations (CNS vs neuromuscular) was compared, using R version 4.0.1, by fitting a multivariate quasi-likelihood model configured to model the enrichment scores using cancer status. Next, another model was fit by adding the presentation variable as a covariate to the aforementioned model. The two models were compared using a F test. Antigens with a resulting P value <0.05 were considered to be significantly associated with clinical presentation.
Research ethics and informed consent
All patients consented for research participation, and the study was approved by the Institutional Review Board of Mayo Clinic, Rochester, Minnesota.
Results
We identified 122 patients; 61% were females. Of the 122 patients, 81 (66%), 20 (16%), and 21 (17%) belonged to older adult, young adult, and pediatric groups, respectively. Most adults (77%) were smokers. About 65% of patients were followed up at Mayo Clinic and the rest were non-Mayo patients. Among the patients evaluated at Mayo, 69 (87%), 6 (8%), and 4 (5%) patients belonged to the older adult, young adult, and pediatric groups, respectively. Overall, isolated neuromuscular presentation was most common, in 64 (53%) patients, followed by isolated CNS presentation in 36 (29%) patients. Encephalomyelitis was seen in 22 (18%) patients. Two patients had only cerebellar ataxia without encephalitis, and two patients had only refractory seizures but did not meet autoimmune encephalitis criteria and were included as autoimmune epilepsy. Presentations such as epilepsia partialis continua (n = 3), cranial neuropathy (n = 2), dysautonomia (n = 2), and neuromuscular junction disorder (n = 1) were all included among “others” (Fig. 1A). Some of the typical imaging findings in these patients are depicted in Figure 2.
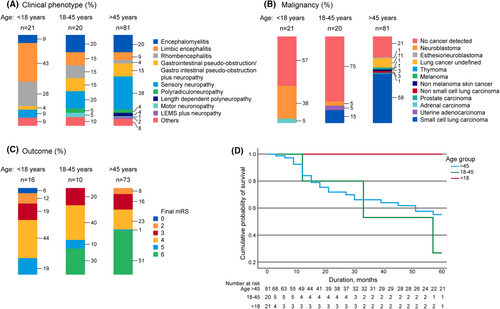
A subacute onset of symptoms was noticed in about two-thirds (68%) of the cohort. The phenotypic presentations, cancers detected, and outcomes are shown in Figure 1A–C. The distribution of neuropathy phenotypes in the cohort is shown in Figure S1. Co-existent high- and intermediate-risk neural antibodies were seen in 10 (8%) patients (all older adults) predominantly with underlying lung cancer (80%) as shown in Table S1.
Neuromuscular presentations predominated in adults while CNS presentations predominated in the pediatric group
Of the 81 patients in the older adults group, 71 (88%) were smokers and 53 (65%) had a predominantly neuromuscular presentation. Neuropathy was the initial clinical manifestation in 52 (64%) patients. The most common presentation was a sensory neuronopathy seen in 45 (37%) patients. Symptoms of weight loss (n = 56, 69%), appetite loss (n = 28, 34%), orthostatic intolerance (n = 24, 30%), falls (n = 21, 30%), and requirement of gait aid during follow-up (n = 44, 54%) were common in the older adults.
Young adults had a CNS presentation in seven (35%) patients and neuromuscular presentation in nine (45%) patients. They often had a focal weakness (n = 12, 60%) with asymmetric lower motor neuron findings (n = 11, 55%).
The pediatric group often had an isolated CNS presentation, predominantly limbic encephalitis in 10 (47%), with seizures in 13, memory loss in 11, and movement disorders in 10 (48%); movement disorders included cerebellar ataxia (n = 3), myoclonic jerks (n = 3), and opsoclonus myoclonus syndrome (n = 4).
Older adults more often had a neuromuscular presentation (65% vs 10%, p < 0.001), while pediatric patients more often had CNS presentations (81% vs 15%, p < 0.001). A detailed comparison of the various characteristics between groups is shown in Table 1. The distribution of neuropathy presentations is shown in Figure S1.
| Older adults, N | Young adults, N | p valuea | Pediatric group, N | p valueb | p valuec | |
|---|---|---|---|---|---|---|
| Females (%) | 51 (62.9) | 10 (50) | 0.312 | 13 (62) | 1.0 | 0.326 |
| Smoking (%) | 71 (88) | 7 (35) | <0.001 | 0 (0) | <0.001 | 0.003 |
| CNS presentation (%) | 12 (15) | 7 (35) | 0.044 | 17 (81) | <0.001 | 0.004 |
| Neuromuscular presentation (%) | 53 (65) | 9 (45) | 0.077 | 2 (10) | <0.001 | 0.015 |
| Encephalomyelitis (%) | 16 (19) | 4 (20) | 1.0 | 2 (10) | 0.354 | 0.410 |
| Limbic encephalitis (%) | 13 (16) | 5 (25) | 0.339 | 10 (48) | 0.006 | 0.197 |
| Rhombencephalitis | 13 (16) | 5 (25) | 0.339 | 7 (33) | 0.118 | 0.734 |
| Opsoclonus myoclonus syndrome (%) | 1 (1.2) | 0 (0) | 1.0 | 4 (19) | 0.006 | 0.107 |
| Gastrointestinal pseudo-obstruction (%) | 17 (20.9) | 3 (15) | 0.757 | 2 (10) | 0.349 | 0.476 |
| Seizure (%) | 12 (14.8) | 3 (15) | 1.0 | 13 (62) | <0.001 | 0.004 |
| Weight loss (%) | 56 (69.1) | 7 (35) | 0.009 | 3 (14) | <0.001 | 0.159 |
| Cancer association present (%) | 65 (80.2) | 5 (25) | <0.001 | 9 (43) | 0.002 | 0.326 |
| Lung cancer (%) | 58 (71.6) | 4 (20) | <0.001 | 0 (0) | <0.001 | 0.048 |
| Neuroblastoma (%) | 0 (0) | 0 (0) | – | 8 (38) | <0.001 | <0.001 |
| Hospitalization (%) | 57 (70) | 5 (25) | <0.001 | 5 (24) | <0.001 | 1.0 |
| MRI abnormal (%) | 24/57 (42) | 6/10 (60) | 0.729 | 10/17 (59) | 0.173 | 0.698 |
| CSF abnormal (%) | 29/38 (76) | 8/10 (80) | 1.0 | 7/11 (64) | 0.451 | 0.635 |
| Pleocytosis, >5 cells/mcl (%) | 6 (7) | 5 (25) | 0.039 | 2 (10) | 0.667 | 0.238 |
| Elevated protein, >50 mg/dL (%) | 27 (33) | 3 (15) | 0.171 | 2 (10) | 0.033 | 0.663 |
| Gait aid (%) | 43 (53) | 6 (30) | <0.001 | 7 (33) | 0.016 | 0.520 |
| mRS >3 at first visit (%) | 36/73 (49) | 8/11 (72.7) | 0.198 | 9/16 (56) | 0.784 | 0.448 |
| mRS >3 at final visit (%) | 55/73 (75) | 8/10 (80) | 1.0 | 10/16 (63) | 0.365 | 0.420 |
| Median follow-up duration, months (range) | 29 (3–257) | 32 (10–81) | 0.838 | 44.5 (3–122) | 0.913 | 0.806 |
| Immunotherapies utilized | ||||||
| Steroids (%) | 45 (56) | 8 (40) | 0.317 | 11 (52) | 0.810 | 0.536 |
| IVIG (%) | 21 (26) | 7 (35) | 0.578 | 10 (48) | 0.066 | 0.530 |
| Plasma exchange (%) | 8 (10) | 3 (15) | 0.452 | 2 (10) | 1.0 | 0.663 |
| Cyclophosphamide (%) | 6 (7) | 3 (15) | 0.376 | 3 (14) | 0.386 | 1.0 |
| Rituximab (%) | 3 (4) | 2 (10) | 0.256 | 3 (14) | 0.100 | 1.0 |
| Mycophenolate (%) | 4 (5) | 2 (10) | 0.340 | 2 (10) | 0.600 | 1.0 |
| Azathioprine (%) | 2 (3) | 2 (10) | 0.175 | 0 (0) | 1.0 | 0.232 |
| Improvement after immunotherapy (%) | 16 (20) | 6 (30) | 0.365 | 11 (52) | <0.001 | 0.080 |
| Improvement/stabilization at last follow-up (%) | 20/68 (29) | 1/5 (20) | 1.0 | 3/4 (75) | 0.089 | 0.206 |
| Mortality at final follow-up (%) | 37 (46) | 3 (15) | 0.020 | 0 (0) | <0.001 | 0.107 |
| Mortality by 5 years of symptom onset (%) | 25 (31) | 3 (15) | 0.263 | 0 (0) | 0.002 | 0.107 |
- – indicates statistics not computable as no events in both groups.
- a Statistical comparison between older and young adults.
- b Statistical comparison between older adults and pediatric group.
- c Statistical comparison between young adults and pediatric group.
Most young adults did not have an underlying malignancy, while neuroblastoma and lung cancer were common in pediatric and older adults, respectively
Cancer was detected in 79 (65%) patients in the cohort, and cancer therapy was administered to 50 (63%) of them. In 64 (81%) patients, the neurological syndrome preceded cancer detection. At the time of cancer diagnosis, 69 patients had limited-stage disease and 10 patients had advanced-stage disease. At the last follow-up, 35 patients had their cancer in remission, nine patients were not treated for cancer, and seven patients died while planning or undergoing therapy. Five patients were still undergoing therapy at the last follow-up. Cancer had relapsed in eight patients, progressed in seven patients, and remained static in three patients. There was a higher frequency of cancer detection in older adults (80%), most commonly lung (n = 58, 72%). Only 5 (25%) young adults had a cancer detected (lung cancer = 4, uterine adenocarcinoma = 1) with median age at onset of 43 years. Nine (43%) patients in the pediatric age group had cancer, all but one of which were neuroblastoma, which was different from the older adults (38% vs 0%, p < 0.001).
Pediatric group had a more favorable response to immunotherapy
Only 20% and 30% of patients showed improvement after immunotherapy among older and young adults, respectively. The improvement after immunotherapy was higher in pediatric patients than in older adults (52% vs 20%, p < 0.001). At final follow-up, 20/68 (29%) older adults, 1/5 (20%) young adults, and 3/4 (75%) pediatric patients showed sustained stabilization or improvement.
Patients with cancer had a distinct clinical profile compared to those without cancer
When stratified based on the presence or absence of cancer, we found that patients with cancer were often older adults who were smokers, had a neuromuscular presentation with weight loss, responded less often to immunotherapy, and had increased disability, hospitalizations, and mortality at the final follow-up, as shown in Table 2.
| Characteristic | No cancer detected, n = 43 (%) | Cancer detected, n = 79 (%) | p value |
|---|---|---|---|
| Sex | 0.443 | ||
| Males | 19 (44) | 29 (37) | |
| Females | 24 (56) | 50 (63) | |
| Age group | <0.001 | ||
| >45 years | 16 (37) | 65 (82) | |
| 19–45 years | 15 (35) | 5 (6) | |
| ≤18 years | 12 (28) | 9 (11) | |
| Smoking | 17 (40) | 61 (77) | <0.001 |
| CNS presentation | 18 (42) | 18 (23) | 0.037 |
| Neuromuscular presentation | 17 (40) | 47 (6) | 0.039 |
| Encephalomyelitis | 8 (19) | 14 (18) | 1.0 |
| Limbic encephalitis | 11 (26) | 17 (22) | 0.655 |
| Rhombencephalitis | 11 (26) | 14 (18) | 0.351 |
| Gastrointestinal pseudo-obstruction | 5 (12) | 17 (22) | 0.222 |
| Sensory neuronopathy | 12 (28) | 33 (42) | 0.170 |
| Weight loss | 14 (33) | 52 (66) | <0.001 |
| Seizure | 12 (28) | 16 (20) | 0.372 |
| Neuropathic pain | 8 (19) | 38 (48) | 0.002 |
| Immunotherapy given | 26 (61) | 46 (58) | 0.849 |
| Improvement after immunotherapy | 17 (40) | 16 (20) | 0.032 |
| MRI abnormal | 16 (57) | 22 (41) | 0.171 |
| CSF abnormal | 19 (79) | 25 (71) | 0.558 |
| Gait aid required | 15 (35) | 41 (52) | 0.113 |
| mRS >3 at first visit | 17 (52) | 36 (64) | 1.0 |
| mRS >3 at final follow-up | 19 (59) | 54 (81) | 0.030 |
| Hospitalization | 16 (37) | 51 (65) | 0.005 |
| Mortality at final follow-up | 5 (12) | 35 (44) | <0.001 |
| Co-existent antibodies | 2 (5) | 8 (10) | 0.492 |
The median duration of follow-up was 29 months (range 3–257) in older adults, 32 months in young adults (range 10–81), and 44.5 months in pediatric patients (range 3–122). At final follow-up, mortality was noted in 37 (46%) older adults and 3 (15%) young adults, among whom 5-year mortality was noted in 25 older adults and all three young adults. No mortality was noted in the pediatric group. Of the 40 patients who had mortality, 20 (50%) died due to cancer, seven (17%) patients died due to neurological disability, two (5%) patients succumbed to infectious complications, and no cause was noted in 10 (25%) patients. The survival curves of the three groups at 5 years are shown in Figure 1D. Other characteristics of the cohort, such as MRI and electrodiagnostic results, are shown in Table S2.
PhIP-Seq detected ELAVL4 in more than 80% of ANNA1 patient samples, with majority having a common epitope
Using PhIP-Seq data, we found that ELAVL4 peptides were enriched across the cohort in 54/67 (81%) samples, especially for the amino acids 240–289 in 33/54 (61%) serum samples (Fig. 3A) and 3/13 (23%) CSF samples (not shown).
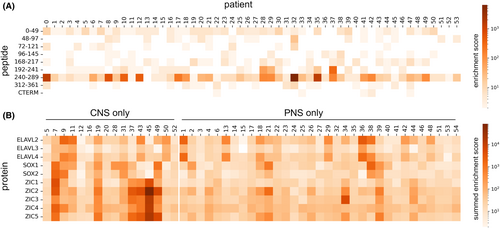
Anti-Zic and anti-SOX2 antibodies were enriched in sera of patients with CNS-predominant presentation
We used a multivariate quasi-likelihood model adjusted for the presence of underlying small cell lung cancer and found that SOX2 (p = 0.0006) and all ZIC peptides [ZIC1 (p < 0.00001), ZIC2 (p < 0.00001), ZIC3 (p = 0.0009), ZIC4 (p < 0.00001), ZIC5 (p < 0.00001)] were significantly higher in patients with CNS presentation compared to those with a neuromuscular presentation (Fig. 3B).
Serum NfL levels were elevated in patients with cancer and poor outcomes at last follow-up
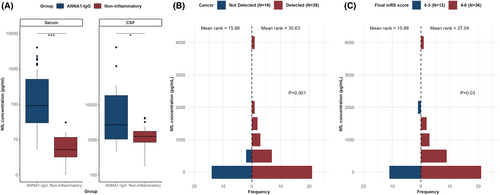
Serum and CSF levels of NfL were significantly higher in patients with ANNA1-IgG than in noninflammatory controls (Fig. 4A). However, since we did not have age-appropriate cutoffs for serum NfL levels, we used previously published age-appropriate cutoff values (>95% percentile)15; for CSF, the 95th percentile of noninflammatory disease controls (normal pressure hydrocephalus) was used as the cutoff. Elevated NfL was detected in 94% of serum samples and 50% of CSF samples. Serum NfL levels were higher in patients with cancer than in those with no cancer detected (p = 0.001), and in those with poor outcomes (mRS >3) at final follow-up compared to those with more favorable outcomes (mRS ≤3; p = 0.029; Fig. 4B1,B2). However, we did not find similar associations between CSF NfL levels and cancer or outcomes at final follow-up. There was no correlation between serum or CSF NfL levels with clinical phenotypes or age groups.
Discussion
In this study, we investigated the clinical characteristics, cancer associations, and biomarker profiles of ANNA1 autoimmunity across pediatric, young adult, and older adult patients. Our findings reveal distinct manifestations across these age groups. Pediatric patients primarily presented with CNS involvement, such as seizures and movement disorders, and responded more favorably to immunotherapy, often with an underlying neuroblastoma. In contrast, young adults showed varied phenotypes and had a lower association with lung cancer. Older adults, predominantly smokers, most commonly presented with sensory neuronopathy and had a higher incidence of lung cancer, reflecting a distinct clinical profile. PhIP-Seq analysis revealed a common amino acid sequence of the ELAVL4 peptide enriched in the majority of patient sera. In addition, patients with CNS presentations showed increased enrichment for ZIC and SOX2 peptides, suggesting a unique antigenic signature associated with CNS involvement. Beyond clinical presentations and immunoprofiles, neural injury biomarkers provided additional insights. Serum NfL levels were significantly elevated in patients with cancer and were correlated with poor long-term outcomes.
Our cohort was predominantly female, consistent with findings from other American studies,1, 3 though this contrasts with European cohorts where the gender distribution has varied.2, 16 Smoking may be an underlying confounding factor to explain this association. Sensory neuronopathy was the most common neuromuscular manifestation in our cohort, as well as in previous studies.1, 16, 17 The increased mortality in older adults and poor response to immunotherapy contrasts markedly with the pediatric group. Consistent with previous reports,16 our study showed that presence of cancer per se leads to worse outcome as shown by the increased disability rates, hospitalizations, and mortality.
Of the 10 pediatric patients with limbic encephalitis, 5 (50%) had an underlying neuroblastoma (median age 8, range 4–15); no tumors were detected in the remaining cases (median age 12, range 1–16). This contrasts with a French series of ANNA1-positive pediatric limbic encephalitis patients where only 25% had neuroblastoma,6 but similar to other case reports where an association with neuroblastoma was reported.18-20 We also found a higher frequency of cancer in pediatric patients (43%) compared to previously reported studies.5-7 Although not statistically significant, 47% of pediatric patients showed improvement or stabilization following immunotherapy compared to 26% of older adults. The lack of response to immunotherapy is well documented in paraneoplastic neurologic syndromes.1, 2, 21, 22 Interestingly, young adults resembled the older adults in terms of clinical presentation despite having less of a cancer association. Although a large French cohort reported a substantially lower median age in the group with no cancer detected, the age ranges of patients with and without cancer overlapped and there was no age stratification as done in our paper.16
About one-third of our patients did not have any cancer detected. It is possible that an occult cancer was missed in preliminary screening or that patients were lost to follow-up before workup was completed. Since patients with or without cancer have been reported to have similar clinical profiles, periodic screening is warranted.23
Although not pathogenic by itself, ANNA1 antibodies are biomarkers of T-cell-mediated autoimmunity. The ANNA1 antigen (ELAVL4 or HuD) is expressed intracellularly in neurons of the dorsal root ganglia, myenteric plexus, cortex, cerebellum, and brainstem, as well as in the neuroendocrine cells of small cell lung carcinoma. Its presence in the above varied locations may explain the multifocal nature of its neurologic autoimmunity. In patients with ANNA1 autoimmunity, there occurs a loss of immune tolerance secondary to tumor-related genetic alterations of the onconeural protein.24 The protein is degraded by an immunoproteasome and cryptic peptide fragments not recognized as “self” are expressed via MHC-1 molecules on the cell surface in response to cytokines such as interferon gamma, which then triggers an autoimmune response.24 Using PhIP-Seq, we found significant enrichment for ELAVL4 peptides, especially for amino acids positions 240–289, which are known to be the target T-cell epitope in ANNA1 patients.25, 26 Interestingly, this peptide region has been shown to have high affinity for HLA-A1 and HLA-A2/B18 and also to activate CD8+ T cells in ANNA1-IgG patients, supporting the antigen-specific T-cell pathophysiology of this disease.26, 27 We observed a considerably higher number of patients with immunoprofiles enriched for ELAVL4 peptides in PhIP Seq (81% vs 25%) compared to a prior study on epitope mapping of the anti-Hu antigen. In that study, there was a marked convergence in patient samples across a 17-residue sequence at amino acid positions 276–290 which is common to ELAVL2, ELAVL3, and ELAVL4 and also shared peptides with the epitope detected in our analysis.25
IgG responses to SOX2 and ZIC peptides were significantly enriched in patients with CNS presentation compared to those with neuromuscular presentation, which is a novel finding. Although the presence of ZIC and SOX family enrichment was noted in patients with ANNA1-IgG previously, the correlation with phenotypic expression in these patients was not attempted.25 ZIC4 antibodies were shown to co-occur with ANNA1 or CRMP5 antibodies in about 27% of patients with paraneoplastic neurological syndromes due to an underlying small cell lung carcinoma.28 Subsequently, ZIC antibodies (ZIC1, ZIC2, and ZIC4) were observed in seronegative paraneoplastic cerebellar degeneration due to underlying small cell lung cancer, with the antibodies probably recognizing common antigenic epitopes.29 The ZIC gene family and SOX2 are evolutionarily conserved genes that serve to regulate normal neural development. The ZIC family consists of five genes that, when mutated, can lead to holoprosencephaly, cerebellar malformations, and neural tube defects.30 Similarly, SOX2 mutations can lead to intellectual impairments and problems with vision and motor control.31 Our results suggest that differences in the antigenic signature could contribute to differences in the clinical phenotype of patients with ANNA1-IgG. Further studies looking into the pathophysiological basis of this association and validation of these antibodies using other assays are required to draw definitive conclusions.
Patients with a cancer detected and those with poor outcomes at last follow-up had significantly elevated serum NfL levels compared to those without an underlying cancer and good outcomes. However, we did not find this association with CSF NfL levels. No association was observed between serum or CSF NfL levels and clinical presentation. A previous study of patients with autoimmune neurological syndromes had shown elevated CSF NfL levels in patients with cancer and poor long-term outcomes compared to those without underlying cancer.8 The discordant findings could be attributed to differences in patient population (only 8% in that study had ANNA1 antibodies) and cutoffs for the NfL levels used. Moreover, the controls we used to establish the CSF NfL cutoffs were patients with normal pressure hydrocephalus who are known to have higher NfL levels than healthy controls.32 Our results emphasize that serum NfL is often elevated in those with cancer and may be useful as a biomarker to identify patients with poor long-term outcomes. Further studies with serial NfL measurements might shed light on its correlation with disease activity.
Some limitations of our study include its retrospective nature with missing follow-up data for some patients, the majority of whom were non-Mayo pediatric and young adult patients. Among the Mayo patients, there were 69 older adults, 6 young adults, and 4 pediatric patients who had detailed follow-up data. We had limited archived sera/CSF of young adults for use in PhIP-Seq (only 10% of samples) and NfL studies (20% of samples) compared to 50% of older adults and pediatric patients. Workup for cancer was not uniform for patients managed at other hospitals which could have limited the true “no cancer” status. Additionally, no normal values were established for NfL measurements in CSF, and normal pressure hydrocephalus samples were used as controls. Previously published, age-based cutoffs for serum NfL were established using SiMoA assays, and our NfL measurements were performed on the Ella platform. However, it has been shown that the choice of assay used for plasma NfL measurements (Ella or SiMoA) did not influence quantification, with good correlation noted between the assays.33
We present a spectrum of ANNA1 autoimmunity in pediatric and young adult patients that contrasts with older adult patients, and which can potentially influence clinical practice in terms of oncological screening and administration of immunotherapies. Our study also provides comprehensive immunoprofiling data including association of humoral responses to other neural-specific antigens with the neurologic phenotype. Serum NfL may serve a biomarker for long-term outcome prediction in ANNA1 autoimmunity.
Author Contributions
Concept and design: NKP and DD. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: NKP and DD. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: NKP, CK, and SD. Supervision: DD.
Acknowledgements
We thank Sara Vinje for administrative assistance and media support for their help in creating the figures. We are deeply grateful to the patients and their families for participation in this study.
Funding Information
The study was partly funded by Roche/Genentech.
Conflict of Interest
NKP, MM, AZ, CK, HK, SRS, SD, AMK, GM, and JRM report no conflicts interest. A.Z. has patents submitted for Tenascin-R-IgG, PDE10A-IgG, and DACH1-IgG as biomarkers of paraneoplastic neurological autoimmunity. She has received research funding from Roche not relevant to this work. She has consulted, without personal compensation, for Alexion Pharmaceuticals. A.M. is funded by grants from NIH (RO1NS126227, U01NS120901) and has consulted for Janssen and Roche Pharmaceuticals without personal compensation. Dr. McKeon has patents for Septin-5-IgG and Septin-7-IgG licensed to Euroimmun, a patent for GFAP-IgG issued, a patent for MAP1B-IgG with royalties paid to himself and licensed to Ravo Diagnostika, and patents for CAMKV-IgG, KLCHL11-IgG, and PDE10A-IgG pending. S.J.P. reports receiving grants, personal fees paid to Mayo Clinic, and nonfinancial support from Alexion Pharmaceuticals, Inc. and MedImmune, Inc./Viela Bio; receiving personal fees from Genentech/Roche, UCB, and Astellas, outside the submitted work; holding patent 8,889,102 (application 12–678350) issued and patent 9,891,219B2 (application 12–573942) issued. D.D. has consulted for UCB, Immunovant, Argenx, Arialys, and Astellas pharmaceuticals. All compensation for consulting activities is paid directly to Mayo Clinic. He is a named inventor on filed patent that relates to KLHL11 as marker of autoimmunity and germ cell tumor. He has patents pending for LUZP4-IgG, cavin-4-IgG and SKOR2-IgG as markers of neurological autoimmunity. He has received funding from the DOD (CA210208 & PR220430), David J. Tomassoni ALS Research Grant Program and UCB.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




