Dopaminergic therapy disrupts decision-making in impulsive-compulsive Parkinsonian patients
Abstract
Impulsive-compulsive behaviors (ICB) are common non-motor symptoms of Parkinson's disease (PD) often associated with dopaminergic drugs (DD) therapy. We investigated the acute effects of DD on decision-making in PD patients with ICB (ICB+) and without it (ICB−), and in healthy controls (HC). Participants performed a risk-based decision-making task twice, with PD patients tested before (DD OFF) and after (DD ON) DD intake. In DD OFF, all groups developed a risk-averting strategy. In DD ON, ICB+ patients (but not ICB− nor HC) reverted to riskier choices. We conclude that DD has a specific strong acute effect on ICB+ patients' decision-making.
Introduction
Parkinson's disease (PD) is primarily characterized by motor dysfunctions such as bradykinesia, rigidity, and tremor, but presents also several non-motor symptoms. Impulsive-compulsive behaviors (ICB) such as pathological gambling, binge eating, hypersexuality, and compulsive buying occur in PD patients with a frequency close to 15%.1 ICB has been linked to the intake of dopaminergic drugs (DD) aimed at improving PD motor symptoms.2 Several works showed dopamine neurons' involvement in learning and decision processes,3, 4 which are altered in PD patients following DD therapy.5 Indeed, long-term correlation between DD intake and the onset of ICB was thoroughly investigated.6 Recent studies focused on the acute effect of DD during behavioral tasks,7-9 but the presence of a differential acute susceptibility to DD in ICB remains unclear. While DD is an important factor in inducing ICB, there is evidence supporting the need for a sensitive neural substrate to develop these disorders: demographic, neural, and genetic risk factors have been associated with ICB.2, 10-14
Here, we investigate the hypothesis of a differential effect of DD in ICB patients by studying the acute effects of DD intake in PD patients, both with (ICB+) and without (ICB−) ICB, in an economic risk-decision task.
Materials and Methods
Participants
This study was approved by the ethical committee of Careggi Hospital (Florence, Italy) and in accordance with the Declaration of Helsinki. Fourteen Parkinsonian patients diagnosed with ICB (ICB+) and 16 without ICB (ICB−) were recruited by the Parkinson Unit of the Careggi Hospital. Only patients with a Mini-Mental State Examination (MMSE) >24 were included. There were no differences between groups regarding age, sex, education, disease duration, MMSE, and L-dopa-equivalent daily dose (LEDD) (Table 1). Moreover, groups showed no difference in motor severity, assessed with part three of the Unified Parkinson's Disease Rating Scale (UPDRS) (Table 1). Groups differed only in the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Diseases-Rating Scale (QUIP-RS, Table 1). We also tested 12 healthy controls (HC) matched with Parkinsonian patients for age, sex, and education (Table 1).
| Values | Test p-values | |||||
|---|---|---|---|---|---|---|
| HC | ICB− | ICB+ | HC vs ICB− | HC vs ICB+ | ICB− vs ICB+ | |
| Dunn test | ||||||
| Age | 67.50 ± 12.75 | 68.00 ± 6.25 | 69.00 ± 13.75 | 1.0 | 1.0 | 1.0 |
| Education (years) | 13.00 ± 5.00 | 13.00 ± 1.75 | 13.00 ± 4.25 | 0.82 | 1.0 | 1.0 |
| Fisher exact test | ||||||
| Sex M (F) | 7 (5) | 10 (6) | 9 (5) | 1.0 | 1.0 | 1.0 |
| Mann–Whitney U test | ||||||
| Disease duration (years) | – | 12 ± 9.75 | 9 ± 7.75 | – | – | 0.41 |
| MMSE | 28.35 ± 3.07 | 26.70 ± 3.80 | 0.32 | |||
| QUIP-RS | – | 0.00 ± 4.25 | 8.00 ± 13.25 | – | – | 0.011 |
| UPDRS III DD OFF | – | 36.50 ± 28.25 | 26.00 ± 14.5 | – | – | 0.49 |
| UPDRS III DD ON | – | 14.50 ± 20.25 | 18.50 ± 14.00 | – | – | 0.56 |
| LEDD (mg) | – | 582.00 ± 321.5 | 572.5 ± 596.5 | – | – | 1.0 |
- Demographic data: Dunn test and Fisher exact test with Bonferroni correction were used respectively for continuous and categorical variables. Clinical data: The Mann–Whitney U test was used. Values of variables are shown in terms of median and interquartile range, except for sex where the number of males (number of females) are reported.
Experimental procedure
All participants performed two sessions of economic risk-decision-making tasks on the same day. PD patients performed the first task session after overnight DD withdrawal and the second task session after ~1 h from DD intake. HC patients repeated the same task after the same waiting time. Each session consisted of 40 trials in which participants had to choose between two options: a low-risk (LR) option with a high probability (80%) of obtaining a low reward (6€) and a 20% probability of obtaining no reward (expected value = 4.8); and a high-risk (HR) option with a low probability (20%) of obtaining a high reward (18€) and an 80% probability of obtaining no reward (expected value = 3.6). In this task, the LR option is the optimal choice to maximize reward.
Subjects were aware of the reward amount for both options and unaware of the reward probabilities since they had to learn this aspect empirically during the task. After each decision, the outcome of both options was displayed (see Supplementary Materials for details).
Statistical analysis
We measured each subject's post-learning risk aversion (RA) as the fraction of LR choices in the last 20 trials.7 We used one-sample t-test to assess for each group and session the difference from chance level (RA = 0.5), applying FDR correction (Benjamini–Hochberg). We used one-way ANOVA test to compare differences in RA between groups, followed by post hoc comparisons (t-test, Bonferroni correction). Statistics are reported as median ± interquartile range unless otherwise specified. Normality and homogeneity of variance were assessed, respectively, with Shapiro–Wilk's test and Levene's test.
To characterize the learning phase, we computed the fraction of LR choices in each group for each trial, obtaining three curves per session. We used logarithmic regression analysis for each session to assess the effect of groups (HC, ICB−, ICB+) (see Supplementary Materials). We used the model intercept to measure the initial bias toward one of the two options, while the effect of trials was employed to quantify the slope of the learning curves. T-test was used to assess the significance of bias and slope, applying Bonferroni correction separately for each parameter and session. The same procedure was applied for the pairwise comparison of bias and slope interactions.
Next, we evaluated how DD affected the slope of different subjects. To estimate individual subjects' slopes, we employed a generalized linear mixed-effects model with the logit function as a link function and the binomial distribution for decision distribution. This model was fitted only on Parkinsonian patients, and, to avoid introducing a group effect on slope estimation, the group was not included as a regressor. Finally, we investigated the effect of LEDD, as well as demographic and clinical scores (see Supplementary Materials), and group on both estimated slopes and RA in drugs ON condition, employing linear regression analysis.
Results
We evaluated behavioral differences between ICB− and ICB+ groups of PD patients in an economic risk-based decision-making task before and after the prescribed DD dose intake (see Methods). Results were compared with those of an age-matched HC group.
All groups started without significant bias toward one of the two choices (see Supplementary Material). As the session progressed, we observed a significant increase in LR choices for all groups (slope, HC: CI: [0.01, 0.10], p = 0.0055, Fig. 1A; ICB−: CI: [0.049, 0.14], p = 4e-6, Figure 1B; ICB+: CI: [0.004, 0.095], p = 0.028, Fig. 1C; t-test, Bonferroni correction; slope-group interaction p > 0.05, t-test, Bonferroni correction). No significant difference was found between groups in terms of final strategy (RA) (Fig. 1D, F(2,38) = 2.36, p = 0.1, one-way ANOVA test). However, while both HC and ICB− groups displayed a significant preference for LR options (RA >0.5) at the end of the session, the ICB+ group did not (Fig. 1D, HC: RA = 0.775 ± 0.15, t (11) = 6.13, p = 0.0002; ICB−: RA = 0.775 ± 0.225, t (15) = 4.77, p = 0.0004; ICB+: RA = 0.65 ± 0.3, t (12) = 2.08, p = 0.07, t-test, FDR correction).
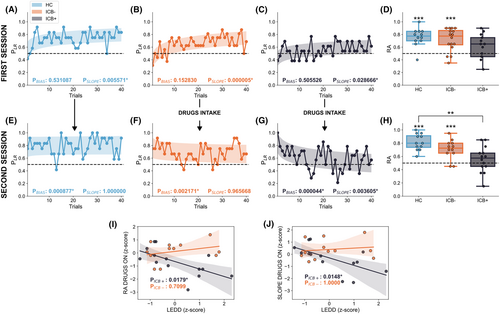
In the second session, all groups started with a bias toward LR (RA >0.5, see Supplementary Materials) thanks to the experience of the first session. As expected, HC retained their risk-averting strategy (slope CI: [−0.056, 0.069], p = 1.0, t-test Bonferroni correction, Figure 1E). Interestingly, the same occurred for ICB− in the DD ON condition (slope, CI: [−0.088, 0.037], p = 0.96, t-test Bonferroni correction, Figure 1F). ICB+ patients, instead, during the DD ON session gradually reduced the fraction of LR choices, showing a significant negative slope (Fig. 1G, slope, p = 0.0036, CI: [−0.149, −0.023], t-test Bonferroni correction), significantly lower than the one of HC (interaction, p = 0.039, t-test, Bonferroni correction). The final strategy indeed was associated with a RA significantly above 0.5 for HC and ICB− but not for ICB+ (Fig. 1H, HC: RA = 0.8 ± 0.17, t (11)=8.9, p = 1.38e-5; ICB−: RA = 0.725 ± 0.15, t (11)=4.89, p = 7.13e-4; ICB+: 0.575 ± 0.2, t (13)=1.01, p = 0.32, t-test, FDR correction) and a significant difference between HC and ICB+ (p = 0.0016, Cohen's d = 1.570, t-test, Bonferroni correction).
Finally, we examined if the behavior of ICB groups during the DD ON session was related to the specific DD therapy followed by the patient, summarized by the LEDD. A linear regression model showed a significant effect of LEDD on RA on ICB+ but not on ICB− (Fig. 1I, interaction LEDD-group: p = 0.016; LEDD effect: ICB+: p = 0.017; ICB−: p = 0.70, t-test, Bonferroni correction). Consistently, we found a significant effect of LEDD on learning slope for ICB+ but not for ICB− (Fig. 1J, interaction LEDD-group p = 0.033; LEDD effect: ICB+: p = 0.014; ICB−: p = 1.0, t-test, Bonferroni correction). RA and learning slope displayed instead no relevant condition-dependent relationship with clinical or demographic scores (see Supplementary materials).
Discussion
Our results show that after DD assumption, ICB+ patients (but not ICB−) progressively worsen their performance during a decision-making task. This suggests that DD intake has a strong acute effect on ICB+ patients' decision-making processes, to the point of disrupting previously developed efficient strategies. During the first DD OFF session of the task, ICB+ patients progressively increased the fraction of LR choices, as ICB− and HC groups did, indicating correct learning of the options' expected values. However, after DD intake, ICB+ patients progressively decreased the probability of choosing the LR option, eventually reaching a nonsignificant RA. Conversely, ICB− patients, similarly to HC, did not alter their strategy. Notably, for ICB+ patients, but not for ICB−, the LEDD correlated with the increased tendency for risky choices at the individual level, indicating an interplay between chronic therapy and acute effects which again is specific to ICB+ subjects.
DD-induced modifications have been observed in various nodes of the decision-making neural circuitry, including the ventral-medial prefrontal and orbitofrontal cortex,15, 16 dorsal and ventral striatum,9, 17 and subthalamic nucleus.18, 19 These alterations are hypothesized to affect outcome evaluation, reducing the weight given to losses5, 8, 16, 20 and increasing that given to gains.9, 21 Our results are consistent with this mechanism, which might cause the ICB+ individuals to drift toward suboptimal choices with high potential gains but also high risks. However, the DD effect alone does not account for the behavioral data, as ICB− patients have taken comparable doses without noticeable behavioral alterations. Our results support the hypothesis that DD per se does not cause learning and decision-making impairment but rather seems to act as a trigger when it finds a dopamine-hypersensitive neural substrate. In particular, if the cognition-related ventral region of the STN of a PD patient is particularly preserved in terms of local activity10, 12 and connectivity,14 a dopaminergic treatment could lead to hyperdopaminergic dysregulation of behavior. Further investigation is needed to assess how this substrate might be related to genetic11, 13 and demographic1, 2 factors associated with ICB onset.
Acknowledgements
FT was supported by THE (“Tuscany Health Ecosystem”) Project funded by the Italian Ministry of University and Research – PNRR – Next Generation EU Projects Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 – Call for tender No. 341 of 15/03/2022 of Italian Ministry of University and Research funded by the European Union. AV, AK, ER, and AM were supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) – A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022). EO was supported by the AGE-IT PE8 SPOKE1 Project funded by the Italian Ministry of University and Research (MUR), PNRR Mission 4 Component 2 Investment 1.3, Next Generation “Partenariati Estesi 8” – B83C22004800006 (PE00000015).
Author Contributions
FT: Study design, execution, statistical analysis, writing of the first draft, editing of the manuscript; EO: Study design, execution, writing of the first draft, editing of the manuscript; AG: Study design, execution, editing of the manuscript; FP: Execution, editing of the manuscript; SV: Execution, editing of the manuscript; LC: Execution, editing of the manuscript; AK: Statistical analysis, editing of the manuscript; AAV: Study design, execution, editing of the manuscript; ER: Study design, statistical analysis, editing of the manuscript; AR: Study design, editing of the manuscript; CR: Study design, execution, writing of the first draft, editing of the manuscript; SR: Study design, execution, writing of the first draft, editing of the manuscript; AM: Study design, execution, statistical analysis, writing of the first draft, editing of the manuscript.
Conflict of Interest
The authors have no conflict of interest to report.
Open Research
Data Availability Statement
Data are available on request due to privacy/ethical restrictions.




