Clinical characteristics of double negative atypical inflammatory demyelinating disease: A prospective study
Abstract
Objective
This study aimed to investigate the clinical characteristics and predictors of relapse in double negative atypical inflammatory demyelinating disease (IDD) and to explore potential antigenic targets by tissue-based assays (TBA) using rat brain indirect immunofluorescence.
Methods
We compared the clinical, laboratory, and MRI data of double negative atypical IDD with other IDD patients. Serum samples were collected for TBA. The predictors of relapse were examined over a minimum of 24 months follow-up.
Results
In our cohort of 98 patients with double negative atypical IDD, there was no significant female predominance (58.2%, 57/98). The lesions primarily affected the spinal cord and brain stem, with fewer cases of involvement in the area postrema (5.1%, 5/98) and longitudinally extensive transverse myelitis (43.9%, 43/98). A total of 62.5% (50/80) patients tested positive for anti-astrocyte antibodies based on rat brain TBA. Over a median duration of 39.5 months, 80 patients completed the entire follow-up, and 47.5% (38/80) patients exhibited monophasic course. A total of 36% (18/50) patients positively for anti-astrocyte antibodies had a monophasic course, which is significantly lower than patients negatively for anti-astrocyte antibodies (66.7%, 20/30) (p = 0.008). The presence of anti-astrocyte antibodies (hazard ratio (HR), 2.243; 95% CI, 1.087–4.627; p = 0.029) and ≥4 cerebrum lesions at first attack (HR, 2.494; 95% CI, 1.224–5.078; p = 0.012) were risk factors for disease relapse, while maintenance immunotherapy during remission (HR, 0.361; 95% CI, 0.150–0.869; p = 0.023) was protective factor.
Interpretation
Double negative atypical IDD are unique demyelinating diseases with a high relapse rate. Maintenance immunotherapy is helpful to the prevention of relapse, particularly in patients with anti-astrocyte antibodies or ≥4 cerebrum lesions at first attack.
Introduction
Central nervous system (CNS) inflammatory demyelinating disease (IDD) are a large group of autoimmune disorders characterized by inflammatory demyelination. This includes multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), acute disseminated encephalomyelitis, and Baló concentric sclerosis, and others.1, 2 IDD are classified on the basis of clinical manifestations, imaging features, course characteristics, and their responses to commonly used MS drugs. The typical IDD are all variants of MS, whereas atypical IDD encompass all other inflammatory demyelinating diseases.3
In clinical practice, we have encountered a subgroup of patients with atypical IDD in whom both serum and cerebrospinal fluid (CSF) test negative for aquaporin 4 (AQP4)-immunoglobulin G (IgG) and myelin oligodendrocyte glycoprotein (MOG)-IgG, as detected by cell-based assay (CBA). We refer to these patients as double-negative atypical IDD. Currently, there are well-established diagnostic criteria for MS, NMOSD, and MOGAD.2, 4, 5 However, double negative atypical IDD are not yet fully understood. Some double negative atypical IDD patients meet the 2015 NMOSD diagnostic criteria for AQP4-IgG-negative NMOSD. However, double negative NMOSD does not share the same pathogenesis as AQP4-IgG+ NMOSD.6, 7 Furthermore, in recent years, the discovery of glial fibrillary acidic protein (GFAP)-IgG in patients with steroid-responsive relapsing meningoencephalitis8 has raised the question of whether GFAP-IgG-related diseases should be classified as a distinct subtype of atypical IDD. Such findings underscore the importance of paying close attention to double negative atypical IDD and their unique characteristics. After the first episode, it can be challenging for clinicians to determine the likelihood of a monophasic course or the risk of relapse. Determining whether conventional immunosuppressive agents and glucocorticoids can effectively prevent disease relapse in individuals with double negative atypical IDD also presents a significant clinical challenge.
Potential antigenic targets in most double negative atypical IDD are yet to be identified. Tissue-based assay (TBA) using indirect immunofluorescence is similar to cell-based assay (CBA) and is widely used for antibody detection in the laboratory diagnosis of autoimmune diseases of the nervous system.9 Unlike CBA, TBA is not limited by known antigens and can determine the type of antibody based on fluorescence patterns.9 In double negative IDD, the presence and types of unknown antibodies are of great significance for diagnosis and treatment.
Due to double negative NMOSD is not considered a completely distinct disease entity, we did not exclude it from double negative atypical IDD in this study. Furthermore, given the absence of standardized diagnostic criteria for GFAP-IgG-associated disease, we have excluded patients whose serum or CSF tested positive for GFAP-IgG from our cohort. We compared the clinical characteristics of double negative atypical IDD patients with other IDD patients, and adopted TBA on serum samples at baseline and analyzed predictors of relapse over a minimum of 24 months of follow-up. We aimed to provide valuable insights into the pathogenesis, clinical characteristics, and treatment of double negative atypical IDD, with the goal of providing much more benefits for those patients.
Methods
Study design and population
This prospective longitudinal study included patients with CNS IDD who visited our research centers of nine tertiary hospitals in Hunan Province, China between July 2020 and December 2021. This study enrolled patients who visited our research centers for the first time and met the inclusion criteria. We rigorously screened patients with relapse and only considered those who were able to provide comprehensive medical records. The inclusion criteria for the double negative atypical IDD cohort were as follows: (1) Clinical symptoms and/or signs of optic nerve, spinal cord, brain stem, or brain involvement in the disease, with corresponding CNS demyelination lesions observed in imaging; (2) The clinical manifestations and clinical outcome consistent with atypical IDD;3 (3) Not meeting the 2017 McDonald diagnostic criteria for MS; (4) Negative results for AQP4-IgG, MOG-IgG, and GFAP-IgG by CBA, confirmed at least twice, with a minimum interval of 6 months. For patients diagnosed with MS, the 2017 McDonald diagnostic criteria were followed.4 Diagnoses of AQP4-IgG+ NMOSD were based on the 2015 diagnostic criteria for IPND.5 MOGAD diagnosis at inclusion was followed the 2018 international recommendations diagnosis of MOG encephalomyelitis,10 and all of those patients meet the diagnoses criteria of the 2023 international MOGAD panel.2
The exclusion criteria for this study were as follows: (1) Severe disturbance of consciousness and memory; (2) Comorbidities such as previous head trauma or infection, which neurological and imaging experts suspected would affect the judgment of CNS lesions; (3) Incomplete clinical data; (4) GFAP-IgG+ patients and double negative atypical IDD patients who were not available for serum samples or whose final diagnosis did not support IDD during follow-up; (5) Other potential causes such as vascular disease, infection, genetic diseases, metabolic disorders, poisoning, tumor, or system rheumatic immune disease-related CNS lesions were ruled out based on clinical characteristics, imaging, rheumatic immune parameters, histopathological examination, immunotherapy efficacy, and expert evaluations of neurologists. This includes: (1) Combined disease onset and progression characteristics, as well as examinations such as diffusion-weighted imaging (DWI), magnetic resonance angiography (MRA), and susceptibility-weighted imaging (SWI) to rule out vascular disease; (2) CSF cell count, protein, glucose, chloride levels, microbial cultures, and polymerase chain reaction (PCR) testing for viral nucleic acids were performed to exclude infectious diseases of CNS; (3) Any history of carbon monoxide (CO) exposure, drug and alcohol abuse, and biochemistry tests such as thyroid function, liver and kidney function, electrolytes, vitamins, blood and urine organic acids are considered along with necessary genetic testing to rule out genetic or metabolic diseases; (4) Cytological examination of the CSF, magnetic resonance spectroscopy (MRS), and pathological examination of CNS lesions are used when necessary to exclude CNS tumor; and (5) Combined the involvement of other systems or organs, autoimmune antibodies associated with rheumatic diseases, and pathological examination of CNS lesions as necessary to rule out systemic rheumatic diseases.
After a rigorous screening process, 262 patients were included in the study (Fig. 1). Baseline information was recorded, including demographic, clinical, laboratory, and magnetic resonance imaging (MRI) data. Demographic and clinical data included sex, age of first attack, attack phenotype, precursor infection, number of relapses, duration of disease, clinical symptoms, first attack and highest expanded disability status scale (EDSS) scores, autoimmune history, remission treatment, and length of remission. Laboratory data included oligoclonal band, antinuclear antibody, anti-SSA antibody, anti-SSB antibody, thyroglobulin antibody (TGAb), anti-thyroid peroxidase antibody (TPOAb), CSF leukocyte count, CSF protein levels, and CSF IgG levels.
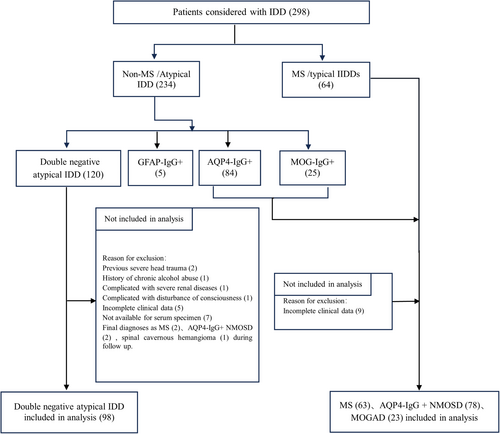
The end point of this study was a follow-up period of over 24 months for all enrolled double negative atypical IDD patients (until February 2024), and 80 patients completed the entire follow-up. During the follow-up period, EDSS scores, attack form, remission treatment, and length of remission in double negative atypical IDD patients were collected. As this was an observational study, we refrained from intervening or influencing the treatment plans implemented by the attending doctors.
Determination of serum autoantibodies by indirect immunofluorescence
We collected peripheral blood samples at baseline from the double negative atypical IDD patients for TBA using a rat brain indirect immunofluorescence. To assess the specificity and sensitivity of the TBA, we collected samples for TBA from patients who had tested seropositive for AQP4-IgG (22), MOG-IgG (15), and GFAP-IgG (8) using CBA. We also included patients with neurologic diseases who tested seronegative for AQP4-IgG, MOG-IgG, and GFAP-IgG by CBA, including 32 MS patients, 22 noninflammatory encephalopathy (NIE) patients, 8 noninflammatory peripheral neuropathy (NIPN) patients, 11 CNS tumor patients, and 10 noninflammatory myelopathy (NIM) patients.
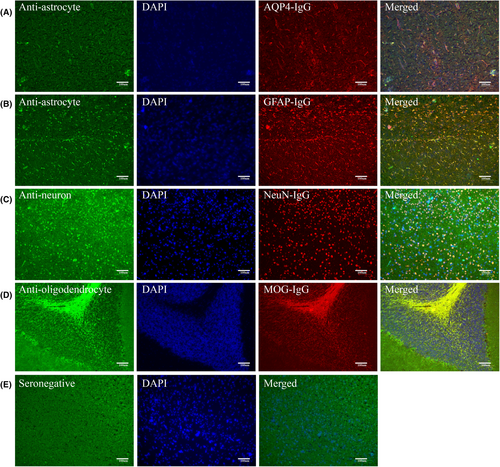
Frozen slices of fresh brain tissue from Sprague–Dawley rats were fixed with acetone after air drying. Serum samples were diluted at a ratio of 1:10 and incubated with tissue sections for 60 min. Fluorescein isothiocyanate (FITC)-labeled goat-antihuman antibody (1:200 dilution) (Jackson ImmunoResearch, West Grove, PA, USA) was applied for 30 min after washing. Nuclei were visualized by counterstaining with DAPI (1:10,000 dilution) (SolarBio, Tongzhou District, Beijing, China) for 2 min. The sections were examined in a dark room using ZOE Fluorescent Cell Imager (Bio-Rad, Hercules, California, USA). Based on preliminary staining results, double immunostaining was performed at room temperature using commercial rabbit antibodies (Proteintech, Wuhan, Hubei, China) (1:200 dilution) to confirm that serum antibodies bound to neural cells. For neuronal binding, anti-neuronal nuclear antibody was used. For oligodendrocyte binding, MOG-IgG was used. For astrocyte binding, AQP4-IgG and GFAP-IgG were used as the primary antibodies. AlexaFluoro 594-conjugated goat-anti-rabbit IgG antibodies (1:200 dilution) (Jackson ImmunoResearch) were used as the secondary antibodies. We further employed cynomolgus monkey brain tissue slices for TBA detection to validate the results of anti-astrocyte antibodies based on rat brain tissue of 64 double negative atypical IDD samples. We also used commercial rabbit AQP4-IgG and GFAP-IgG as the primary antibodies (1:200 dilution) to confirm that serum antibodies bound to astrocyte, and AlexaFluoro 594-conjugated goat-anti-rabbit IgG antibodies (1:200 dilution) were used as the secondary antibodies. Immunofluorescence was evaluated by two independent senior laboratory technicians to ensure specific fluorescent staining.
In this study, TBA refers to tissue-based assays using a rat brain indirect immunofluorescence. However, when it is necessary to differentiate between TBA using a rat and monkey brain indirect immunofluorescence, they will be referred to as “rat brain TBA” and “monkey brain TBA” respectively.
GFAP detection
Serum GFAP of double negative atypical IDD patients was quantified using commercial Human GFAP DuoSet ELISA (R&D Systems Minneapolis, MN, USA) by an independent investigator who was blinded to the result of TBA.
This study was conducted in accordance with the tenets of the 2013 revision of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Xiangya Hospital of Central South University (NO. 201904147–2).
Statistical analysis
Analyses were performed using SPSS v. 25.0 (IBM Corp., Armonk, NY, USA). Categorical variables were described as counts and percentages and continuous variables were described as medians and ranges or means and standard deviations. The demographic, clinical, laboratory, and MRI characteristics of patients with different types of IDD were compared using a student's t-test, one-way analysis of variance, Wilcoxon test, Kruskal–Wallis test, chi-square test, or Fisher's exact test, as appropriate. A p <0.05 was considered statistically significant. Univariate Cox regression analysis was conducted to examine the relationship between variables and the time to first relapse. Variables for which the HR had a p ≤0.1 were included in a multivariate analysis. The Kaplan–Meier method was used to create survival curves for those factors found to be associated with a relapsing disease course. A survival curve comparison was performed using a log-rank test.
Results
The demographic and clinical features of double negative atypical IDD
Female-to-male ratios in double negative atypical IDD, NMOSD, MOGAD, and MS groups were 1.4:1 (57/41), 12:1 (72/6), 0.9:1 (11/12), and 1.6:1 (39/24), respectively. Similar with MOGAD, our data showed no significant female predominance in double negative atypical IDD. The age at first attack (in years) for patients with double negative atypical IDD had a median of 41 with a range of 14–69, which was closer to patients with NMOSD rather than MOGAD and MS. Double negative atypical IDD patients had a higher proportion of precursor infection (29.6%, 29/98) compared to other IDD (p < 0.001), and they were seldom concomitant with other systemic autoimmune diseases (3.1%, 3/98) compared to NMOSD (19.2%, 15/78). Double negative atypical IDD patients also had higher first attack EDSS scores (4.3 ± 2.4) and highest EDSS scores (4.8 ± 2.4) compared to MOGAD and MS, indicating a higher disease burden. The clinical symptoms at first attack are shown in Table 1. The most common symptoms among double negative atypical IDD patients were limbs or trunk paresthesia (61.2%, 60/98), limb weakness (54.1%, 53/98), and rectal or bladder disorder (30.6%, 30/98). Hypopsia (20.4%, 20/98) was also common but less frequent than NMOSD patients (41.0%, 32/78). Double negative atypical IDD patients had a lower proportion of headache (9.2%, 9/98) and seizure (2.0%, 2/98) compared to MOGAD patients, as well as a lower proportion of dizziness (11.2%, 11/98) and unstable walking (12.2%, 12/98) compared to MS patients at first attack.
| Double negative atypical IDD (N = 98) | AQP4-IgG + NMOSD (N = 78) | MOGAD (N = 23) | MS (N = 63) | p-value | |
|---|---|---|---|---|---|
| Female, n (%) | 57 (58.2%) | 72 (92.3%) | 11 (47.8%) | 39 (61.9%) | <0.001 |
| Age at first attack, median (range), year | 41 (14–69) | 51 (13–82) | 29 (12–69) | 30 (8–70) | <0.001 |
| Precursor infection, n (%) | 29 (29.6%) | 9 (11.5%) | 4 (17.4%) | 3 (4.8%) | <0.001 |
| Concomitant autoimmune diseases, n (%) | 3 (3.1%) | 15 (19.2%) | 1 (4.3%) | 1 (1.6%) | <0.001 |
| Number of attacks, median (range) | 1 (1–4) | 2 (1–12) | 2 (1–7) | 3 (1–9) | <0.001 |
| Disability Status Scale, mean ± SD | |||||
| EDSS score at first attack | 4.3 ± 2.4 | 4.0 ± 2.1 | 3.0 ± 2.1 | 2.8 ± 1.7 | <0.001 |
| Highest EDSS score | 4.8 ± 2.4 | 5.7 ± 2.4 | 3.6 ± 1.9 | 3.5 ± 1.9 | <0.001 |
| Initial attack symptom, n (%) | |||||
| Hypopsia | 20 (20.4%) | 32 (41.0%) | 9 (39.1%) | 15 (23.8%) | 0.012 |
| Seizure | 2 (2.0%) | 1 (1.3%) | 7 (30.4%) | 0 (0.10%) | <0.001 |
| Headache | 9 (9.2%) | 5 (6.4%) | 13 (56.5%) | 6 (9.5%) | <0.001 |
| Dizziness | 11 (11.2%) | 8 (10.3%) | 3 (13.0%) | 20 (31.7%) | 0.002 |
| Memory impairment | 3 (3.1%) | 1 (1.3%) | 1 (4.3%) | 8 (12.7%) | 0.017 |
| Nausea or vomiting | 9 (9.2%) | 14 (17.9%) | 2 (8.7%) | 3 (4.8%) | 0.073 |
| Diplopia | 10 (10.2%) | 5 (6.4%) | 0 (0.0%) | 10 (15.9%) | 0.098 |
| Bulbar paralysis | 13 (13.3%) | 3 (3.8%) | 0 (0.0%) | 5 (7.9%) | 0.057 |
| Limbs or trunk paresthesia | 60 (61.2%) | 43 (55.1%) | 5 (21.7%) | 34 (54.0%) | 0.008 |
| Limb weakness | 53 (54.1%) | 39 (50.0%) | 8 (34.8%) | 36 (57.1%) | 0.297 |
| Unstable walking | 12 (12.2%) | 5 (6.4%) | 4 (17.4%) | 22 (34.9%) | <0.001 |
| Rectal or bladder disorder | 30 (30.6%) | 17 (21.8%) | 4 (17.4%) | 10 (15.9%) | 0.143 |
| MRI findings, n (%) | |||||
| Optic nerve T2 hyperintensity | 16 (16.3%) | 35 (44.9%) | 11 (47.8%) | 19 (30.2%) | <0.001 |
| Bilateral lesion | 7 (7.1%) | 26 (33.3%) | 6 (26.1%) | 4 (6.3%) | <0.001 |
| Chiasmal lesion | 4 (4.1%) | 22 (28.2%) | 2 (8.7%) | 3 (4.8%) | <0.001 |
| Longitudinally extensive lesion | 3 (3.1%) | 19 (24.4%) | 4 (17.4%) | 0 | <0.001 |
| Cerebrum lesion | 33 (33.7%) | 31 (39.7%) | 16 (69.6%) | 55 (87.3%) | <0.001 |
| Diencephalon lesion | 7 (7.1%) | 18 (23.1%) | 3 (13.0%) | 7 (11.1%) | 0.019 |
| Brain stem lesion | 46 (46.9%) | 36 (46.2%) | 8 (34.8%) | 32 (50.8%) | 0.627 |
| Area postrema | 5 (5.1%) | 13 (16.7%) | 1 (4.3%) | 3 (4.8%) | 0.020 |
| Cerebellum lesion | 9 (9.2%) | 5 (6.4%) | 2 (8.7%) | 17 (27.0%) | 0.001 |
| Spinal cord lesion | 71 (72.4%) | 67 (85.9%) | 5 (21.7%) | 45 (71.4%) | <0.001 |
| LETM | 43 (43.9%) | 57 (73.1%) | 1 (4.3%) | 0 (0.0%) | <0.001 |
| Cervical | 54 (55.1%) | 54 (69.2%) | 5 (21.7%) | 43 (68.3%) | <0.001 |
| Thoracic | 41 (41.8%) | 44 (56.4%) | 2 (8.7%) | 23 (36.5%) | <0.001 |
| Lumbar/conus | 6 (6.1%) | 4 (5.1%) | 0 (0.0%) | 5 (7.9%) | 0.649 |
| Serum autoantibodies, n (%) | |||||
| ANA | 20 (20.4%) | 44 (56.4%) | 3 (13.0%) | 19 (30.2%) | <0.001 |
| Anti-SSA | 4 (4.1%) | 23 (29.5%) | 3 (13.0%) | 4 (6.3%) | <0.001 |
| Anti-SSB | 0 (0.0%) | 7 (9.0%) | 0 (0.0%) | 1 (1.6%) | 0.004 |
| TGAb | 12 (12.2%) | 19 (24.4%) | 2 (8.7%) | 6 (9.5%) | 0.042 |
| TPOAb | 17 (17.3%) | 19 (24.4%) | 2 (8.7%) | 7 (11.1%) | 0.131 |
| Unmatched OB, n (%) | 13 (13.3%) | 17 (21.8%) | 5 (21.7%) | 48 (76.2%) | <0.001 |
| CSF analysis, mean ± SD | N = 86 | N = 71 | N = 22 | N = 59 | |
| Leucocyte count | 7.9 ± 20.3 | 9.1 ± 30.9 | 50.4 ± 114.7 | 2.2 ± 3.7 | 0.009 |
| Protein, g/L | 0.39 ± 0.33 | 0.42 ± 0.22 | 0.43 ± 0.25 | 0.31 ± 0.12 | 0.003 |
| IgG, g/L | 0.04 ± 0.06 | 0.07 ± 0.08 | 0.06 ± 0.06 | 0.05 ± 0.03 | 0.001 |
- ANA, antinuclear antibodies; AQP4, anti-aquaporin-4; CSF, cerebrospinal fluid; EDSS, expanded disability status scale; IDD, inflammatory demyelinating disease; MOGAD, myelin oligodendrocyte glycoprotein antibody-associated disease; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders; OB, oligoclonal band; SSA, Sjogren syndrome A antibody; SSB, Sjogren syndrome B antibody;LETM, longitudinally extensive transverse myelitis; TGAb, thyroglobulin antibody; TPOAb, anti-thyroid peroxidase antibody.
The MRI and laboratory features of double negative atypical IDD
The MRI and laboratory features of double negative atypical IDD are summarized in Table 1. Abnormal MRI findings primarily involved the spinal cord (72.4%, 71/98), brain stem (46.9%, 46/98), and cerebrum (33.7%, 33/98) in double negative atypical IDD patients. These findings were more similar to NMOSD patients than to MOGAD or MS patients. Notably, double negative atypical IDD patients had a lower proportion of area postrema (5.1%, 5/98) longitudinally extensive transverse myelitis (LETM) lesions (43.9%, 43/98), compared to NMOSD patients. Double negative atypical IDD patients had a lower proportion of optic nerve lesion (16.3%, 16/98) compared to NMOSD (44.9%, 35/78), MOGAD (47.8%, 11/23), and MS (30.2%, 19/63). In particular, the proportion of bilateral optic nerve lesion (7.1%, 7/98) and longitudinally extensive optic nerve lesion (lesion involving more than 50% of the optic nerve length11, 12) (3.1%, 3/98) involvement were lower than NMOSD and MOGAD, and the proportion of chiasmal lesion (4.1%, 4/98) involvement was lower than in NMOSD. Diencephalic involvement was less common in double negative atypical IDD (7.1%, 7/98) than in NMOSD (23.1%, 18/78), and cerebrum involvement (33.7%, 33/98) was less common than MOGAD (69.6%, 16/23) and MS (87.3%, 55/63). The proportion of double negative atypical IDD patients with ANA (20.4%, 20/98), anti-SSA antibodies (4.1%, 4/98), anti-SSB antibodies (0, 0/98), and TGAb (12.2%, 12/98) was significantly lower than in NMOSD patients, but not significantly different from the other groups. Double negative atypical IDD patients had a significantly lower proportion of unmatched OB (13.3%, 13/98) compared to MS patients (76.2%, 48/63). Double negative atypical IDD patients had fewer CSF leukocyte cells (7.9 ± 20.3/L) compared to MOGAD patients (50.4 ± 114.7/L), and CSF IgG levels were lower in double negative atypical IDD patients (0.04 ± 0.06 g/L) compared to NMOSD (0.07 ± 0.08 g/L) and MS (0.05 ± 0.03 g/L) patients. Although not statistically significant, there was a trend of higher CSF protein levels in double negative atypical IDD (0.39 ± 0.33 g/L) compared to MS patients (0.31 ± 0.12 g/L) and lower levels compared to NMOSD patients (0.42 ± 0.22 g/L).
The attack features and maintenance treatment of double negative atypical IDD
After a minimum follow-up of 24 months, 80 patients completed the entire follow-up, and the cohort had a median disease duration of 39.5 (range: 25–124) months. Among the 80 patients with double negative atypical IDD, there were a total of 153 recorded attacks. The median time between the first and second attacks was 6 months, ranging from 2 to 96 months. The attack phenotype of double negative atypical IDD was classified as ON (optic neuritis), TM (transverse myelitis), BS (brain stem syndrome), CS (cerebrum syndrome), and Mixed (multifocal CNS involvement). TM was the most frequent first attack phenotype, accounting for 51.3% (41/80) of patients, followed by mixed involvement (21.3%, 17/80) and BS (13.8%, 11/80). Among the 42 s attacks, TM (52.4%, 22/42) and ON (21.4%, 9/42) were the most common phenotypes. Throughout the disease course, TM (47.7%, 73/153) remained the most common phenotype, followed by ON (16.3%, 25/153) and Mixed (15.0%, 23/153). Since three Mixed attacks also included ON attacks, there were a total of 28 ON attacks, of which 5/28 (17.9%) were both sides affected. The monophasic course was more common in TM (51.2%, 21/41) and BS (72.7%, 8/11) cases. However, only 35.3% (6/17) of Mixed involvement, 25.0% (1/4) of isolated CS, and 28.6% (2/7) of isolated ON tended to have a monophasic course (Fig. 2A).
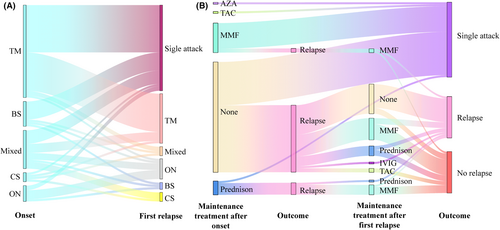
No patients experienced disease progression during remission. The use of maintenance treatment and its relationship with the first and second relapse were recorded in Figure 2B. Among double negative atypical IDD patients, 71.3% (57/80) received no maintenance treatment after first attack, and of those, 61.4% (35/57) experienced relapse. Of these 35 relapsed patients, 42.9% (15/35) still did not receive maintenance treatment after the first relapse, and 80.0% (12/15) experienced a second relapse. In contrast, 28.8% (23/80) of patients received maintenance treatment after first attack, with only 30.4% (7/23) experiencing relapse. Mycophenolate mofetil (MMF) was used as maintenance treatment in 18.8% (15/80) of double negative atypical IDD patients after first attack, with a relapse rate of only 13.3% (2/15). Prednisone as monotherapy was used as maintenance therapy in 7.5% (6/80) of double negative atypical IDD patients after first attack, and the relapse rate was 83.3% (5/6) for these patients. Azathioprine (AZA) and tacrolimus (TAC) were each used as maintenance therapy in 1.3% (1/80) of patients, and no relapse was recorded for these patients.
All patients underwent MRI at the time of disease relapse, and all had enlarged or new lesions. A total of 103 MR images were recorded in 80 patients with long-term follow-up, with a median duration of 11.5 (range: 3–25) months from the previous acute attack. MRI was repeated at least once during remission in 69 patients. Among them, 19/69 (27.5%) showed no change in MRI lesions, 44/69 (63.8%) showed shrinkage of lesions, 5/69 (7.2%) showed disappearance of lesions, and 1/69 (1.4%) had enlargement of the original MRI lesion without disease aggravation. Twenty-six patients showed brain lesions, 10/26 (38.5%) showed no change in MRI lesions, and 16/26 (61.5%) showed shrinkage of lesions during follow-up MRI examination.
The results of TBA suggested unknown anti-astrocyte antibodies in patients with double negative atypical IDD
We further employed TBA to investigate the potential autoimmune patterns of double negative atypical IDD patients. We first determined the specificity and sensitivity of the TBA examination by the specimens with AQP4 antibody and GFAP antibody positivity or negativity determined by CBA. In samples positive for AQP4-IgG and GFAP-IgG, 81.8% (18/22) and 75.0% (6/8), respectively, tested positive for anti-astrocyte antibodies by TBA, resulting in an overall anti-astrocyte antibody positivity rate of 80.0% (24/30) (Fig. 3, left). This result indicates that TBA has a relatively high sensitivity for detecting anti-astrocyte antibodies in serum samples. However, no antibodies against oligodendrocytes were detected in the samples positive for MOG-IgG, indicating that our experimental rat brain tissue may not be suitable for detecting MOG-IgG. Moreover, despite MS being classified as an autoimmune disease, it did not show predominant positive results of anti-astrocyte antibody (12.5%, 4/32) and anti-neuron antibody (6.3%, 2/32) (Fig. 3, right). In samples from patients with NIM, NIPN, NIE, and CNS tumors, the anti-astrocyte antibody negativity rate was 80.0% (8/10), 75% (6/8), 90.9% (20/22) and 72.7% (8/11) separately (Fig. 3, right). CBA tested seronegative samples showed an overall anti-astrocyte antibody positivity rate of only 13.3% (11/83), indicating that TBA has relatively high specificity in identifying previously unidentified anti-astrocyte antibodies in serum samples.
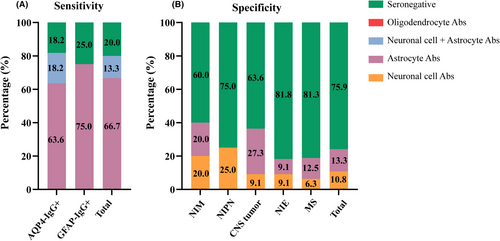
Based on the above results, we conducted rat brain TBA on the samples from patients with double negative atypical IDD and found that 78.8% (63/80) of the samples had antibodies against neurons and glial cells. Among the positive samples, 79.4% (50/63) tested positive for anti-astrocyte antibodies, 10/63 (15.8%) samples were positive for both neurons and astrocytes, 12/63 (19.0%) were positive for neurons only, and only 1/63 (1.6%) was positive for oligodendrocytes (Fig. 4). These results suggested that there was an unknown anti-astrocyte antibody pattern in double negative atypical IDD. We further employed cynomolgus monkey brain tissue for TBA detection to validate the results. Sixty-four double negative atypical IDD samples are available for monkey brain TBA detection (Table S1, Fig. S1). Among the 64 samples, 39 tested positives for anti-astrocyte antibodies by rat brain TBA. Of these 39 positive samples, 34 were also found to be positive by monkey brain TBA, with an agreement rate of 87.2%. Among the 25 samples that tested negative for anti-astrocyte antibodies by rat brain TBA, all were negative by monkey brain TBA. Serum GFAP was also performed in these 64 samples, and GFAP levels were higher in the anti-astrocyte antibody positive group (225.9 ± 284.0 pg/mL) than in the negative group (133.2 ± 138.6 pg/mL), but there was no significant difference (p = 0.220) (Fig. S2). These results further suggest that anti-astrocyte antibodies detected in rat brain TBA exhibits relatively high specificity and sensitivity.
The clinical features of double negative atypical IDD patients with and without anti-astrocyte autoantibodies
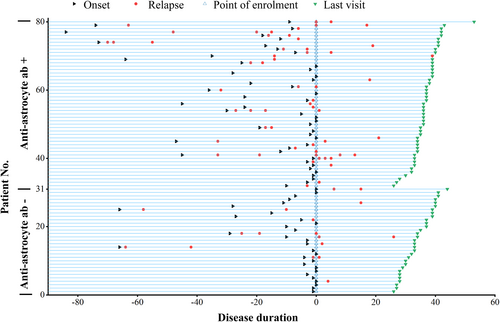
We classified double negative atypical IDD patients based on anti-astrocyte antibody detection by TBA and compared clinical characteristics (Table 2). Among patients negative for anti-astrocyte autoantibodies, 36.7% (11/30) were seropositive for ANA, higher than those with anti-astrocyte autoantibodies (p = 0.009). Additionally, 66.7% (20/30) of these patients had experienced a single attack, higher than those with anti-astrocyte autoantibodies (18/50, 36.0%) (p = 0.008) (Fig. 5). However, the proportion of anti-astrocyte autoantibody-positive patients with cerebrum lesions was 44.0% (22/50), significantly higher than the antibody-negative group (4/30, 13.3%) (p = 0.005). Moreover, the antibody-positive group had a significantly higher number of attacks compared to the antibody-negative group (p = 0.017). The highest EDSS score was comparable between the anti-astrocyte autoantibodies-negative group (4.7 ± 2.4) and the anti-astrocyte autoantibodies-positive group (4.6 ± 2.3). Although there was no statistically significant difference, the anti-astrocyte autoantibodies-negative group showed higher first attack EDSS scores (4.4 ± 2.5) and lower last visit EDSS score scores (2.6 ± 1.0) compared to anti-astrocyte autoantibodies-positive group, which suggests that patients with anti-astrocyte autoantibodies may experience a more pronounced disease burden. Other clinical characteristics were similar between the groups. In addition, we presented the clinical and MRI data of patients of autoantibodies-negative group, anti-neuron autoantibodies-positive group and anti-oligodendrocyte autoantibodies positive group in Table S2. However, no significant differences were found in the clinical and MRI data of these three groups, which may be attributed to the small sample size.
| Anti-astrocyte autoantibodies-negative group (N = 30) | Anti-astrocyte autoantibodies-positive group (N = 50) | p-value | |
|---|---|---|---|
| Female, n (%) | 21 (70.0%) | 26 (52.0%) | 0.113 |
| Age at disease first attack, median (range), years | 41 (20, 69) | 40 (14, 69) | 0.877 |
| Disease duration at time of serum sampling, median (range), months | 4 (0.5, 74) | 7.5 (0, 84) | 0.382 |
| Immunotherapy at time of serum sampling, n (%) | 21 (70.0%) | 35 (70.0%) | 1.000 |
| Delay since last attack, median (range), months | 31 (8, 73) | 34 (0, 94) | 0.739 |
| Precursor infection, n (%) | 11 (36.7%) | 13 (26.0%) | 0.313 |
| Patients with single attack, n (%) | 20 (66.7%) | 18 (36.0%) | 0.008 |
| Concomitant autoimmune diseases, n (%) | 1 (3.3%) | 2 (4.0%) | 1.000 |
| Number of attacks, median (range) | 1 (1, 4) | 2 (1, 6) | 0.017 |
| Disability Status Scale, mean ± SD | |||
| EDSS score at first attack | 4.4 ± 2.5 | 3.8 ± 2.2 | 0.205 |
| Highest EDSS score | 4.7 ± 2.4 | 4.6 ± 2.3 | 0.753 |
| Last visit EDSS score | 2.6 ± 1.0 | 3.1 ± 1.2 | 0.093 |
| Initial attack symptom, n (%) | |||
| Hypopsia | 4 (13.3%) | 12 (24.0%) | 0.248 |
| Seizure | 0 | 2 (4.0%) | 0.525 |
| Headache | 1 (3.3%) | 6 (12.0%) | 0.246 |
| Dizziness | 2 (6.7%) | 6 (12.0%) | 0.703 |
| Memory impairment | 0 | 2 (4.0%) | 0.525 |
| Nausea or vomiting | 1 (3.3%) | 6 (12.0%) | 0.246 |
| Diplopia | 3 (10.0%) | 6 (12.0%) | 1 |
| Bulbar paralysis | 3 (10.0%) | 7 (14.0%) | 0.736 |
| Limbs or trunk paresthesia | 21 (70.0%) | 29 (58.0%) | 0.344 |
| Limb weakness | 16 (53.3%) | 27 (54.0%) | 0.954 |
| Unstable walking | 3 (10.0%) | 6 (12.0%) | 0.246 |
| Rectal or bladder disorder | 10 (33.3%) | 15 (30.0%) | 0.755 |
| MRI findings, n (%) | |||
| Optic nerve T2 hyperintensity | 3 (10.0%) | 10 (20.0%) | 0.351 |
| Bilateral lesion | 1 (3.3%) | 6 (12.0%) | 0.246 |
| Chiasmal lesion | 0 | 3 (6.0%) | 0.288 |
| Longitudinally extensive lesion | 1 (3.3%) | 1 (2.0%) | 1 |
| Cerebrum lesion | 4 (13.3%) | 22 (44.0%) | 0.005 |
| Diencephalon lesion | 2 (6.7%) | 3 (6.0%) | 1.000 |
| Brain stem lesion | 12 (40.0%) | 26 (52.0%) | 0.298 |
| Area postrema | 1 (3.3%) | 4 (8.0%) | 0.645 |
| Cerebellum lesion | 2 (6.7%) | 7 (14.0%) | 0.471 |
| Spinal cord lesion | 23 (76.7%) | 35 (70.0%) | 0.518 |
| LETM | 12 (40.0%) | 23 (46.0%) | 0.600 |
| Cervical | 15 (50.0%) | 30 (60.0%) | 0.383 |
| Thoracic | 14 (46.7%) | 19 (38.0%) | 0.446 |
| Lumbar and/or Conus medullaris | 3 (10.0%) | 2 (4.0%) | 0.358 |
| Serum autoantibodies, n (%) | |||
| ANA | 11 (36.7%) | 6 (12.0%) | 0.009 |
| Anti-SSA | 0 | 3 (6.0%) | 0.288 |
| Anti-SSB | 0 | 0 | – |
| TGAb | 6 (20.0%) | 4 (8.0%) | 0.164 |
| TPOAb | 6 (20.0%) | 7 (14.0%) | 0.539 |
| Unmatched OB, n (%) | 2 (6.7%) | 9 (18.0%) | 0.195 |
| CSF analysis, mean ± SD | N = 26 | N = 44 | |
| Leucocyte count | 2.4 ± 3.8 | 11.2 ± 27.5 | 0.932 |
| Protein, g/L | 0.41 ± 0.38 | 0.36 ± 0.25 | 0.814 |
| IgG, g/L | 0.05 ± 0.08 | 0.04 ± 0.03 | 0.808 |
- ANA, antinuclear antibodies; A-TPO, anti-thyroid peroxidase antibody; LETM, longitudinally extensive transverse myelitis; OB, oligoclonal band; TGA, thyroglobulin antibody.
Factors associated with a relapsing course of double negative atypical IDD
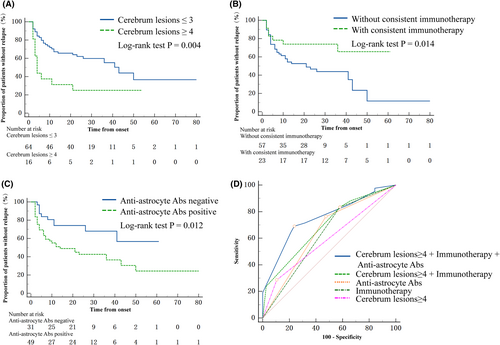
We analyzed data from 80 patients with double negative atypical IDD followed up for over 24 months. COX regression analysis evaluated various variables (Table 3). The results showed that a cerebrum lesion ≥4 on MRI at first attack was associated with an increased risk of second attack (Univariate HR, 2.598; 95% CI, 1.315–5.131; p = 0.006; Multivariate COX HR, 2.494; 95% CI, 1.224–5.078, p = 0.012). Similarly, the presence of anti-astrocyte autoantibodies detected by TBA was associated with a higher risk of second attack (Univariate HR, 2.340; 95% CI, 1.145–4.780; p = 0.020; Multivariate COX HR, 2.243; 95% CI 1.087–4.627; p = 0.029). Conversely, consistent immunotherapy during remission was linked to a reduced risk of second attack (Univariate HR, 0.373; 95% CI 0.162–0.859; p = 0.021; Multivariate COX HR, 0.361; 95% CI 0.150–0.869, p = 0.023) (Fig. 6A–C). Although LETM on MRI at first attack initially showed an increased risk of second attack (OR, 1.917; 95% CI, 1.031–3.566; p = 0.040), this association became insignificant in the multivariate analysis. Further analysis demonstrated that the combination of cerebrum lesions, anti-astrocyte antibodies, and consistent immunotherapy provided the best performance for predicting the relapse of double negative atypical IDD compared to cerebrum lesions alone (p = 0.008), anti-astrocyte antibodies alone (p = 0.040), and consistent immunotherapy alone (p = 0.002). The area under the curve (AUC) was 0.752 (95% CI, 0.646–0.859), with a sensitivity of 0.690 and specificity of 0.763 (Fig. 6D).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Relapse HR (95% CI) | p-value | Relapse HR (95% CI) | p-value | |
| Gender (female) | 0.760 (0.411–1.407) | 0.383 | ||
| EDSS at first attack ≥4 | 0.731 (0.372–1.436) | 0.363 | ||
| Age at first attack | ||||
| ≥50 | 0.738 (0.369–1.475) | 0.389 | ||
| ≤30 | 1.693 (0.821–3.494) | 0.154 | ||
| Precursor infection | 0.684 (0.334–1.399) | 0.298 | ||
| Unmatched oligoclonal band | 1.741 (0.802–3.780) | 0.161 | ||
| ANA | 0.564 (0.237–1.342) | 0.196 | ||
| TGAb | 0.727 (0.259–2.045) | 0.546 | ||
| TPOAb | 0.816 (0.342–1.945) | 0.646 | ||
| Anti-astrocyte Ab | 2.340 (1.145–4.780) | 0.020 | 2.243 (1.087–4.627) | 0.029 |
| Consistent immunotherapy during remission | 0.373 (0.162–0.859) | 0.021 | 0.361 (0.150–0.869) | 0.023 |
| First attack phenotype | ||||
| ON | 1.765 (0.688–4.528) | 0.237 | ||
| CE | 1.974 (0.698–5.584) | 0.200 | ||
| BS | 0.285 (0.069–1.182) | 0.084 | 0.459 (0.105–2.004) | 0.300 |
| TM | 0.779 (0.421–1.442) | 0.427 | ||
| Mixed | 1.390 (0.661–2.925) | 0.386 | ||
| MRI lesion of first attack | ||||
| Cerebrum lesion | ||||
| ≥4 | 2.598 (1.315–5.131) | 0.006 | 2.494 (1.224–5.078) | 0.012 |
| <4 | 0.529 (0.072–3.863) | 0.530 | ||
| Brain stem lesion | 1.192 (0.642–2.214) | 0.577 | ||
| Cerebellum lesion | 2.654 (0.810–8.695) | 0.107 | ||
| Spinal cord | ||||
| LETM | 1.917 (1.031–3.566) | 0.040 | 1.321 (0.687–2.541) | 0.404 |
| Cervical cord | 1.608 (0.856–3.020) | 0.140 | ||
| Thoracic cord | 1.450 (0.778–2.705) | 0.242 | ||
| Lumbar cord | 0.975 (0.235–4.052) | 0.972 | ||
- ANA, antinuclear antibodies; A-TPO, anti-thyroid peroxidase antibody; BS, brain stem syndrome; CS, cerebrum syndrome; EDSS, expanded disability status scale; LETM, longitudinally extensive transverse myelitis; Mixed, multifocal CNS involvement; ON, optic neuritis; TGA, thyroglobulin antibody; TM, transverse myelitis.
Discussion
The discovery and understanding of AQP4-IgG and MOG-IgG in atypical IDD has led to the reclassification of these diseases based on specific pathogenic antibodies.13-15 However, research on double negative atypical IDD is limited, primarily focusing on idiopathic optic neuritis, idiopathic myelitis, and double-antibody-negative NMOSD, with few comprehensive studies of their clinical characteristics. We observed that the clinical features of double negative atypical IDD were distinct from those of MS, AQP4-IgG+ NMOSD, and MOGAD. Serum analysis of double negative atypical IDD patients provided further evidence of autoimmunity and yielded a preliminary identification of potential antigen targets. We have identified three predictors associated with a relapsing course in double negative atypical IDD patients: immunotherapy during remission, the number of cerebrum lesions, and the presence of anti-astrocyte antibodies.
Unlike AQP4-IgG+ NMOSD or MS but similar to MOGAD, there was no significant sex bias in double negative atypical IDD.16, 17 The median age of onset of double negative atypical IDD was similar to that of AQP4-IgG+ NMOSD, rather than MS or MOGAD.18, 19 Interestingly, patients with double negative atypical IDD showed a higher rate of precursor infection before first attack, suggesting immune dysfunction as a potential factor in disease pathogenesis. Double negative atypical IDD are most commonly characterized by impairments of the spinal cord, brain stem, and visual function, which is consistent with their MRI features. We found that double negative atypical IDD exhibited lower proportions of autoantibodies such as ANA, Anti-SSA, TGA, and A-TPO, as well as a lower incidence of area postrema lesions and LETM compared to those with AQP4-IgG+ NMOSD, despite sharing several clinical and MRI characteristics. A comparison of the highest EDSS scores showed double negative atypical IDD to carry a greater disease burden than MS and MOGAD. We observed a higher rate of pulmonary, urinary, and deep vein thrombosis in double negative atypical IDD patients during the acute phase compared with MS and MOGAD (data not shown), which may be associated with more severe spinal cord damage. More than half of double negative atypical IDD patients in our cohort experienced relapse, which was significantly less than in MS and NMOSD but similar to MOGAD.19-21 The high disease burden and the possibility of relapse suggest that immune maintenance therapy is warranted in patients with double negative atypical IDD. More than half of the patients who did not receive immune maintenance therapy suffered relapse, but the relapse rate was significantly lower in those who received immune maintenance therapy. Our findings also indicate that, similar to AQP4-IgG+ NMOSD, immunosuppressive agents such as mycophenolate mofetil (MMF) and tacrolimus (TAC) can effectively reduce the risk of relapse in double negative atypical IDD.22, 23 However, the effectiveness of prednisone monotherapy showed no significant relapse-prevention effects in double negative atypical IDD patients.
Meanwhile, we have observed that some double negative atypical IDD patients remain relapse-free without receiving maintenance immunotherapy. This indicates the need for further evidence to guide which patients should undergo immunotherapy after their initial attack to prevent relapse. Therefore, our study explores whether TBAs can serve as a biomarker to provide additional guidance for the treatment of double negative atypical IDD.24-27 In most of our double negative atypical IDD patients, TBA detected unknown antibodies. These primarily targeted nonmyelinating cells such as astrocytes and neurons, with few antibodies directly targeting oligodendrocytes. Interestingly, MOG-IgG-positive blood specimens did not yield positive results in rat brain TBA, which is consistent with previous findings that rodent brain tissue does not recognize human MOG-IgG well.28 Notably, patients negative for anti-astrocyte antibodies had significantly fewer attacks, a higher rate of single attacks, and fewer cerebrum lesions, indicating that anti-astrocyte antibodies may serve as important predictors of the relapse course of double negative atypical IDD. Due to a higher proportion of patients in the anti-astrocyte antibody-positive group receiving immunotherapy during the later stages of follow-up, there was no significant difference in delay since the last attack between the anti-astrocyte autoantibodies-negative group and -positive group. This also suggests that maintaining immunotherapy can reduce the risk of recurrence.
It is important to note that silent or asymptomatic MRI lesions are one of the characteristics of MS patients and can support the diagnosis of MS.4 Absence of radiological activity is a key target for disease-modifying therapy, which is now widely employed.29, 30 However, silent MRI lesions were not included in the diagnostic criteria for NMOSD and MOGAD.2, 5 In one study that included 182 MOGAD and 222 AQP4-IgG+ NMOSD patients, new silent lesions were identified in 3.0% and 2.6% of MRI during remission respectively and may herald impending relapse.31 Similarly, in the double negative atypical IDD cohort, silent MRI activity (1.4%) was rarely detected during remissions, suggesting that silent MRI lesions are not a significant feature of double negative atypical IDD.
Despite the lack of specific antigens being identified, both TBA using rat brain tissue and monkey brain tissue have confirmed that an immune response against astrocytes plays a crucial role in the pathophysiology of double negative atypical IDD (Figs. 3 and 4, Figs. S1 and S2). While there was no significant difference in serum GFAP levels between the anti-astrocyte antibody-positive and -negative groups, the elevated GFAP in the serum of double negative atypical IDD patients suggests the presence of astrocyte damage. Previous research has indicated elevated serum GFAP in NMOSD patients during relapses.32 Interestingly, elevated serum GFAP has also been observed in non-astrocyte antibodies mediated IDD, such as MS and MOGAD.33, 34 This suggests that serum GFAP levels could be elevated in anti-astrocyte antibody-negative IDDs, potentially explaining the lack of significant difference between the two groups in our study. This result may also be related to the fact that GFAP is mainly present in CSF, while the levels of GFAP in the serum are lower.32, 35 We will further focus on CSF GFAP levels in double negative atypical IDD patients with and without anti-astrocyte autoantibodies in subsequent studies.
During the follow-up period, two patients in our double negative atypical IDD cohort were diagnosed with MS, two with AQP4-IgG+ NMOSD, and one with spinal cavernous hemangioma (all of whom excluded from the final analysis). Previous longitudinal studies have also found that a small number of patients initially diagnosed with optic neuritis or myelitis may later be re-diagnosed with MS.36-38 However, patients with no clinical features of MS at the start of such studies rarely go on to receive an MS diagnosis.39, 40 Our strict inclusion criteria ensured that this study had a low percentage of MS re-diagnoses. All patients whose previous demyelinating event resulted in MRI features consistent with typical MS were excluded. In previous studies, some seronegative IDD, particularly AQP4-IgG-negative NMOSD, have changed to AQP4-IgG seropositivity during follow-up.41-43 Therefore, periodic retesting for demyelinating antibodies in patients with double negative atypical IDD is important for accurate diagnosis.
Certainly, this study still had several limitations. First, while it was conducted prospectively, treatment decisions regarding immunotherapy were developed based on the clinical experience of the physician and patient's circumstances. Therefore, there were no standardized treatment. Second, despite strict inclusion criteria and relatively long follow-up, the cohort of double negative atypical IDD included in our study still exhibited some heterogeneity. Third, there was a limited number of patients. Further studies with larger cohorts and longer follow-ups are needed to validate our findings. In addition, we will assess the changes in anti-astrocyte antibody titers using TBA in our future investigations. This procedure may offer valuable insights into disease activity and the efficacy of immunotherapy.
Conclusions
In conclusion, this study provides systematic description of double negative atypical IDD patients, revealing a high relapse rate after the initial episode. TBA plays a crucial role in distinguishing double negative atypical IDD from other types of IDD. Cerebrum lesions ≥4 at onset and the presence of anti-astrocyte antibodies are independent risk factors for relapse, but maintenance immunotherapy prevents relapse in double negative atypical IDD patients. These findings have important implications for guiding treatment decisions and predicting relapse.
Acknowledgments
This work was funded by Natural Science Foundation of Hunan province, China (Grant Number: 2023JJ30868, 2023JJ40502, 2022JJ40724, and 2023JJ30062), National Natural Science Foundation of China (Grant Number: 82171399 and 82371413), Scientific Research Fund of Hunan Provincial Education Department (Grant Number: 22B0358), Science and Technology Innovation Guidance Project of Hunan Province (Grant Number: 2020SK53009), Changsha Municipal Natural Science Foundation (Grant Number: kq2007037), and Ministry of Education Humanities and Social Sciences (22YJA840015).
Conflicts of Interest
The authors declared no potential conflict of interest.
Author Contributions
FJ performed patients screening, specimen collection, and data collection and analysis; HL, WY, SO, JH, ET, KF, and JY participated in screening enrolled patients and data collection; FJ and HC performed TBA, and HY and QZ assessed fluorescent patterns; HY and QZ, as co-corresponding authors, played a crucial role in designing the study and meticulously screening the enrolled patients; JY guided the language writing and polishing; and FJ and QZ wrote the manuscript. All the authors have read and approved the final manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




