miR-133b as a potential regulator of a synaptic NPTX2 protein in Alzheimer's disease
Abstract
A synaptic protein, Neuronal Pentraxin 2 (NPTX2), has emerged as a pivotal biomarker for Alzheimer's dementia (AD). We identified candidate miRNAs targeting NPTX2 and performed association and mediation analyses using multi-omics data (N = 702). Among 44 candidate miRNAs, miR-133b was significantly associated with AD and Braak positivity. Higher miR-133b expression was also associated with higher NPTX2 gene expression and better cognition. Mediation analysis showed that miR-133b partially influences AD and cognition through the NPTX2 protein. Our integrated approach suggests a potential role of miR-133b in synaptic integrity and offers new insights into AD pathogenesis.
Introduction
Alzheimer's disease, which is a prevalent cause of dementia and a considerable strain on healthcare resources, is characterized by early synaptic changes before clinical symptoms are evident, highlighting the role of synaptic proteins as potential biomarkers for early-stage Alzheimer's disease.1 Among these, neuronal pentraxin 2 (NPTX2), a protein implicated in synaptic organization and function, has been identified as a potential biomarker for early detection of Alzheimer's disease.2 A recent study by Soldan et al. established a relation between reduced cerebrospinal fluid levels of NPTX2 and the onset of mild cognitive impairment, a prodromal stage of Alzheimer's disease.2
MicroRNAs (miRNAs), noncoding RNAs that play a crucial role in regulating gene expression, are increasingly recognized for their roles in neurological disorders, including Alzheimer's disease.3 Despite recognizing their broad biological significance, the interaction between miRNAs and synaptic proteins such as NPTX2 in the context of Alzheimer's disease is less understood. To address this knowledge gap, we investigated a potential regulatory relationship between miRNAs and NPTX2 by integrating brain tissue-based multi-omics data from the Religious Orders Study and the Rush Memory and Aging Project cohort (ROS/MAP). After identifying candidate miRNAs predicted to target NPTX2, we analyzed their associations with NPTX2 protein and gene expression levels, Alzheimer's dementia (AD), cognitive function, and neuropathological traits. Finally, we performed mediation analysis to assess the effect of the miRNA-NPTX2 association on AD diagnosis and cognition.
Methods
We used multi-omics datasets from the ROS/MAP cohort, including proteome (N = 400), RNA-Seq (N = 634), and miRNA profile (N = 702) data from brain tissue in the dorsolateral prefrontal cortex (DLPFC). MiRNAs profiling was performed using a microarray approach and subsequently validated through specific real-time reverse transcription PCR assays.4 RNA sequencing was performed on an Illumina HiSeq 2000 platform, using 101 base pair paired-end reads to achieve a target coverage of 50 million paired reads.4 Proteomic profiling was conducted using isobaric Tandem Mass Tag (TMT) peptide labeling, followed by high-pH offline fractionation and TMT mass spectrometry.5 Datasets for miRNA profiling, RNA-Seq, and proteomic profiling were downloaded from the Accelerating Medicines Partnership for Alzheimer's Disease Knowledge Portal on Synapse (syn3387325, syn8456638, and syn17015098) available at https://www.synapse.org.
We categorized individuals into AD and no cognitive impairment (NCI) groups based on a combination of clinical and neuropathological information to enhance the robustness of differentiation.6 AD was defined by Braak7 scores of 4 or higher (“Braak positive”), Consortium to Establish a Registry for Alzheimer's Disease (CERAD)8 scores of definite or probable Alzheimer's disease (“CERAD positive”), and cognitive diagnosis of probable Alzheimer's disease with no other causes. NCI was defined by Braak scores of 3 or lower (“Braak negative”), the CERAD scores of possible or no Alzheimer's disease (“CERAD negative”), and clinical diagnosis of no cognitive impairment.9 Cognitive performance was assessed using a composite Z score of global cognitive function, which averaged results from 19 tests across five cognitive domains, including episodic memory, working memory, semantic memory, perceptual speed, and visuospatial ability/perceptual orientation.10
Statistical analysis was performed as follows. First, we identified a set of candidate miRNAs predicted to target NPTX2 using two renowned TargetScan 7.211 and miRWalk 3.012 databases that compile both predicted and experimentally confirmed miRNA-gene target interactions. For improved analysis reliability, we selected candidate target miRNAs consistently identified in both databases. After candidate miRNAs predicted to target NPTX2 were selected, linear regression models using brain tissue-based proteome data were used to assess whether the predicted candidate miRNAs were associated with NPTX2 protein levels, with a multiple testing adjustment using the Benjamini–Hochberg false discovery rate (FDR) correction procedure.13 We also performed logistic and linear regression analyses using brain tissue-based multi-omics data to evaluate the association of the NPTX2-associated miRNA with NPTX2 gene expression levels, AD diagnosis, Braak and CERAD positivity, and composite Z scores of global cognition at the last visit. Finally, we performed mediation analysis of the NPTX2-associated miRNA which showed a significant correlation with AD diagnosis and global cognition to investigate whether the identified association was potentially mediated by NPTX2 protein levels.14 We used age, sex, study (ROS and MAP), RNA integrity numbers, postmortem interval, and years of education as covariates, and included apolipoprotein E ε4 (APOE ε4) carrier status as an additional covariate where appropriate. We conducted all statistical analyses using the R software, version 4.2.0, and determined statistical significance at a corrected P value below 0.05.
Results
In the ROS/MAP cohort, a total of 702 participants with available miRNA profile data were included as detailed in Table 1. The median age at death was 88.5 years, with 64.1% being females. For the analysis with AD diagnosis, we used a subset of 279 participants, including both 102 NCI and 177 AD patients. For analyses with NPTX2 protein and gene expression levels, we used a subset of 448 and 199 participants, respectively.
| NCI (n = 102) | AD (n = 177) | Others (n = 423) | p valueb | |
|---|---|---|---|---|
| Female (%) | 54 (52.9) | 126 (71.2) | 269 (63.6) | 0.009 |
| Age at death in yearsa | 85.0 (78.8–89.0) | 90.0 (87.4–90.0) | 88.1 (83.7–90.0) | <0.001 |
| Education in yearsa | 16 (13–19) | 16 (13–18) | 16 (14–19) | 0.704 |
| Study | 0.680 | |||
| ROS (%) | 53 (52.0) | 93 (52.5) | 235 (55.6) | |
| MAP (%) | 49 (48.0) | 84 (47.5) | 187 (44.2) | |
| Composite Z score of global cognition at the last visita | 0.19 (−0.02–0.40) | −2.20 (−2.82 – −1.41) | −0.60 (−1.13 – −0.10) | <0.001 |
| RNA integrity numbersa | 7.3 (6.0–8.0) | 6.7 (5.4–7.4) | 7.0 (5.6–7.7) | 0.005 |
| Postmortem interval in hoursa | 6.3 (4.8–8.6) | 5.8 (4.2–8.3) | 5.7 (4.2–8.3) | 0.202 |
- Values are n (%), unless indicated otherwise. Among a total of 702 participants with miRNA profiling data, 177 participants met criteria for AD and 102 met criteria for NCI based on neuropathological and clinical data.
- AD, Alzheimer's dementia; MAP, Memory and Aging Project; miRNAs, microRNAs; NCI, no cognitive impairment; ROS, Religious Orders Study.
- a Data are presented as median (interquartile range).
- b The Kruskal–Wallis H test or chi-square test was used to determine the P value for comparisons between NCI, AD, and others groups, as appropriate.
From the TargetScan and miRWalk databases, we identified 462 and 1,413 candidate miRNAs targeting NPTX2, respectively. Subsequently, we selected 282 miRNAs that were commonly predicted to target NPTX2 across both databases. Among 282 miRNAs, there was an overlap of 44 candidate miRNAs with those included in the ROS/MAP dataset, which comprised 309 miRNA.
Association analysis of the 44 candidate miRNAs identified miR-133b as significantly associated with NPTX2 protein levels after multiple comparison correction (β [standard error (SE)], 0.21 [0.056]; FDR-corrected p = 0.014) (Fig. 1A and Table S1). The association between miR-133b levels and NPTX2 protein remained significant even after adjusting for the APOE e4 carrier status as an additional covariate. In addition, miR-133b was less expressed in AD (odds ratio (OR) = 0.21, p < 0.001) and the Braak positive group (OR = 0.67, p = 0.032) and marginally associated with CERAD positivity (OR = 0.69, p = 0.054) (Fig. 1B). Higher expression levels of miR-133b were also associated with higher expression levels of the NPTX2 gene (β [SE], 0.34 [0.078]; p < 0.001) and better global cognition at the last visit (β [SE], 0.55 [0.098]; p < 0.001) (Fig. 1C).
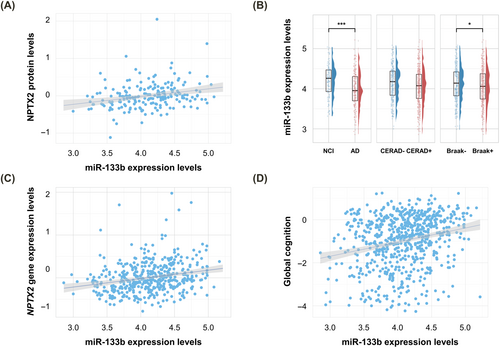
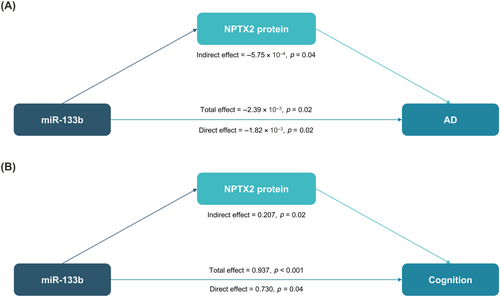
Mediation analysis showed that miR-133b expression levels had both direct and indirect effects on AD through NPTX2 protein levels (direct effect: β = −1.82 × 10−3; p = 0.02; indirect effect: β = −5.75 × 10−4; p = 0.04) (Fig. 2A). Additionally, miR-133b expression levels were found to affect global cognition at the last visit through both direct and indirect effects mediated by NPTX2 protein levels (direct effect: β = 0.430; p = 0.04; indirect effect: β = 0.207; p = 0.02) (Fig. 2B).
Discussion
In this study, a significant association between miR-133b expression levels and NPTX2 protein levels, alongside potential implications of miR-133b for AD diagnosis and cognition, reveals a potential role in AD pathogenesis. Mediation analysis highlights both direct and indirect effects of miR-133b on AD diagnosis and cognition via the NPTX2 protein, emphasizing a regulatory role that miRNAs may play in modulating synaptic proteins, which are essential for sustaining neural network integrity and functionality.2, 15
In this study, lower expression levels of miR-133b were associated with AD and poor cognition, aligning with previous findings.16 Overexpression of miR-133b has been found to alleviate the effects of Aβ-induced cell viability reduction and apoptosis, while enhancing neurite remodeling and brain plasticity, all of which benefit functional recovery.16, 17 These findings suggest neuroprotective effects of miR-133b in Alzheimer's disease, indicating that modulating miR-133b levels could serve as a promising therapeutic strategy. Furthermore, miR-133b enriched in the midbrain influences both the differentiation and degeneration of dopaminergic neurons, and its dysregulation in Parkinson's disease highlights its critical role in neurodegeneration.18
While several studies have explored roles of miRNAs in Alzheimer's disease,3, 15 few have elucidated their impact on specific synaptic proteins like NPTX2 and the subsequent effects on cognition within a well-characterized cohort.15 The strength of our study lies in the comprehensive analysis including brain proteome, RNA-Seq, and miRNA profile data from a well-characterized cohort, providing supporting evidence for the role of miR-133b in influencing synaptic integrity by regulating NPTX2 protein levels and highlighting its potential contributions to clinical and neuropathological manifestations. Additionally, mediation analysis provides a detailed understanding of the direct and indirect effects of miR-133b on AD, providing insights that have not been extensively explored in previous studies.2, 15
Our study has several limitations. Firstly, this study is a cross-sectional design, restricting our capacity to infer causality or observe the progression of miRNA and NPTX2 interactions throughout the course of Alzheimer's disease. Secondly, although the ROS/MAP cohort provides a large and well-characterized dataset, it may not fully represent the broader AD population, particularly in terms of ethnic and genetic diversity. Lastly, this study primarily identified associations between miR-133b, NPTX2 protein levels, AD pathology, and cognition. Functional experiments are necessary to confirm the direct regulatory effects of miR-133b on NPTX2 and to elucidate the underlying mechanisms.
In conclusion, our integrative study highlights a potential role of miR-133b as a regulator of a synaptic NPTX2 protein in AD, providing valuable insights into molecular mechanisms of the synaptic biomarker. Further investigations focusing on the functional aspects of miR-133b-mediated NPTX2 dysregulation may enhance our understanding of AD pathogenesis and unveil novel therapeutic implications.
Acknowledgements
We would like to thank the databases from the ROS/MAP cohort for this study. ROSMAP resources can be requested at https://www.radc.rush.edu.
Funding Informartion
ROSMAP is supported by P30AG10161, P30AG72975, R01AG15819, R01AG17917. U01AG46152, U01AG61356. Additional support for data analysis was provided by NLM R01 LM012535, NIA R01 AG19771, NIA P30 AG10133, NIA P30 AG072976, NLM R01 LM011360, NIA U01 AG068057, NIA U01AG072177, R01 AG057739, NIA U01 AG024904, NLM R01 LM013463, NIA R01 AG068193, T32 AG071444, NIA U19AG074879, and NIA R01 AG069901. This work was supported by the National Research Foundation of Korea grant funded by the Korean government (Ministry of Science and ICT; No. 2020R1C1C1013718), the Bio&Medical Technology Development Program of the National Research Foundation funded by the Korean government (MSIT) (No. RS-2023-00223501), and Hallym University Medical Center Research Fund.
Conflict of Interest
All the authors report that they have no disclosures or conflicts of interest relevant to the manuscript.
Author Contributions
SWH contributed to the concept and design of the work, data acquisition, interpretation of data, drafting of the manuscript, statistical analysis, and critical revision of the manuscript for important intellectual content. YHP and KN contributed to the concept and design of the work, data acquisition, interpretation of data, drafting of the manuscript, statistical analysis, critical revision of the manuscript for important intellectual content, and supervision. SYK, AJS, and PJB contributed to the interpretation of data and manuscript reviewing. DAB contributed by obtaining funding, study supervision, data acquisition, and manuscript reviewing. All authors read and approved the final manuscript.
Consent for Publication
Not applicable.
Open Research
Data Availability Statement
The ROS/MAP cohort data will be freely available at the AMP-AD database (www.synapse.org).




