A comparison of natalizumab and ocrelizumab on disease progression in multiple sclerosis
Abstract
Objective
No direct comparisons of the effect of natalizumab and ocrelizumab on progression independent of relapse activity (PIRA) and relapse-associated worsening (RAW) events are currently available. We aimed to compare the risk of achieving first 6 months confirmed PIRA and RAW events and irreversible Expanded Disability Status Scale (EDSS) 4.0 and 6.0 in a cohort of naïve patients treated with natalizumab or ocrelizumab from the Italian Multiple Sclerosis Register.
Methods
Patients with a first visit within 1 year from onset, treated with natalizumab or ocrelizumab, and ≥3 visits were extracted. Pairwise propensity score-matched analyses were performed. Risk of reaching the first PIRA, RAW, and EDSS 4.0 and 6.0 events were estimated using multivariable Cox proportional hazards models. Kaplan–Meier curves were used to show cumulative probabilities of reaching outcomes.
Results
In total, 770 subjects were included (natalizumab = 568; ocrelizumab = 212) and the propensity score-matching retrieved 195 pairs. No RAW events were found in natalizumab group and only 1 was reported in ocrelizumab group. A first PIRA event was reached by 23 natalizumab and 25 ocrelizumab exposed patients; 7 natalizumab- and 10 ocrelizumab-treated patients obtained an irreversible EDSS 4.0, while 13 natalizumab- and 15 ocrelizumab-treated patients reached an irreversible EDSS 6.0. No differences between the two groups were found in the risk (HR, 95%CI) of reaching a first PIRA (1.04, 0.59–1.84; p = 0.88) event, an irreversible EDSS 4.0 (1.23, 0.57–2.66; p = 0.60) and 6.0 (0.93, 0.32–2.68; p = 0.89).
Interpretation
Both medications strongly suppress RAW events and, in the short term, the risk of achieving PIRA events, EDSS 4.0 and 6.0 milestones is not significantly different.
Introduction
The overlapping of focal recurring inflammation, more widespread inflammatory processes, and neurodegeneration underlines the course of multiple sclerosis (MS) during all stages of the disease, challenging the classic described phenotypes.1, 2 There is a mutual contribution of progression independent of relapse activity (PIRA) and relapse-related worsening (RAW) events to progressive disability accrual since the disease onset, requiring an optimal timing of diagnosis and the appropriate disease-modifying therapy (DMT) to reduce as much as possible the disability burden.3-6 Relapses remain a crucial determinant of disability accumulation, playing a role both in the short and long term,7, 8 and age is configured as the main risk factor associated with the occurrence of PIRA events.9
Exposure to new highly effective (HE) DMTs has been proven to have greater efficacy in reducing the accumulation of disability resulting from PIRA and RAW,2, 9 in comparison with moderate effective treatments. However, reliable comparative effectiveness studies between HE DMTs are scarce in the MS literature landscape10 and their effect on PIRA needs to be better clarified. Ocrelizumab (OCR) and natalizumab (NTZ) demonstrated a strong anti-inflammatory activity as HE DMTs, changing the prognosis of MS patients.11 Studies based on large cohorts from disease registries demonstrated to be essential in exploring potential disease outcomes and DMT effectiveness over the medium and long term in real-life settings.12
In a large real life-cohort of naïve relapsing–remitting MS patients (RRMS) from the Italian MS and Related Disorders Register (I-MS&RD),13 we compared the risk of achieving the first 6 months confirmed PIRA and RAW events and irreversible Expanded Disability Status Scale (EDSS) 4.0 and 6.0 between patients treated with NTZ and those treated with OCR.
Materials and Methods
Data extraction
This was a study based on data extracted from the I-MS&RD. The I-MS&RD was approved by the ethical committee at the “Azienda Ospedaliero – Universitaria – Policlinico of Bari” (Study REGISTRO SM001 – approved on 8 July 2016) and by local ethics committees in all participating centers. Patients signed an informed consent that allows to collect and use their clinical data for research purposes. According to the Registry rules, the Scientific Committee of the I-MS&RD granted the approval to conduct this project and extract and use the registry data. Data extraction was executed in May 2023. A minimum dataset of demographic and clinical characteristic from the I-MS&RD was considered.
Study population and outcome definitions
RRMS patients with a first visit within 1 year from disease onset, a first DMT prescription with NTZ or OCR after 31 December 2017, and ≥3 EDSS score evaluations were included in the analysis.
- Confirmed disability accrual (CDA) was defined as a confirmed 6-month disability increase from study baseline, measured by EDSS (increase ≥1.5 points with baseline EDSS = 0; ≥1.0 point with baseline EDSS >1.0, and <5.5; ≥0.5 point with baseline EDSS >6.0). Date of CDA was assigned at the first EDSS when an increase was registered.
- RAW was defined as a CDA event in which the initial disability increase from study baseline occurred within 90 days or earlier after or 30 or earlier days before the onset of a relapse.
- PIRA was defined as a CDA event occurring more than 90 days after and more than 30 days before the onset of a relapse.
- Irreversible EDSS 4.0 and 6.0: achievement of EDSS scores greater than or equal to 4.0 or 6.0 followed by never lower EDSS ratings in all the subsequent follow-up visits.
Study method and statistical analysis
The following variables were included in the dataset: age (in years), gender (male and female), type of onset (monofocal/multifocal/not known), number of relapses (0, 1, ≥2), IgG oligoclonal bands (OCBs) in the CSF (presence/absence/not known), number of EDSS evaluations with complete information regarding functional scores (FS), and start and end dates of all the administered DMTs.
The inclusion of the “not known” category was necessary to ensure the inclusion of all the selected patients in the multivariate analysis. Furthermore, indicating the number of EDSS assessments is functional to matching subjects with a comparable number of visits.
In descriptive analyses, categorical data were expressed as frequency and proportion. Continuous data were expressed as mean (SD).
To mitigate the impact of potential biases, pairwise propensity score (PS)-matched analyses 1:1 based on baseline covariates at the start of the first NTZ or OCR dose, were performed, without replacement. The quality of the procedure in each pair of matched cohorts was assessed with absolute standardized mean difference (SMD), considering SMD less than 10% acceptable, as absolute value.
PS-matched analysis derived from a multivariable logistic regression model to estimate patient's probability of being assigned to OCR treatment. Considering the matched cohort with balanced characteristics, the risk of reaching the first PIRA and RAW events and irreversible EDSS 4.0 and 6.0 were estimated using multivariable Cox proportional hazards models (CHM). Results of CHM were represented as hazard ratio and 95% confidence interval, HR (95% CI). Kaplan–Meier (KM) curves were used to show the cumulative probabilities of reaching the outcomes.
Patients included in the cohort were followed from the start of NTZ or OCR to the moment of the occurrence of the outcome considered, or to the last visit, or to the treatment switch.
A p-value <0.05 was considered statistically significant. Analyses were performed using SAS Software Release 9.4 (SAS Institute, Cary, NC).
Results
Clinical data of 81,123 patients were available in the I-MS&RD at the time of data extraction. After applying the inclusion criteria, we retrieved a cohort of 770 RRMS patients, of which 568 were treated with NTZ and 212 with OCR. The PS matching procedure subsequently resulted in 195 pairs of patients. (Fig. 1) Baseline characteristics of the cohort before and after PS matching are reported in Table 1. Patients treated with NTZ, before the PS matching, were younger at DMT start (mean (SD): 30.19 (10.15)10-15 years vs. 36.22 (11.23)), less disabled (mean (SD) EDSS score: 1.92 (1.31) vs. 2.51 (1.51)), more frequently females (65.49% vs. 58.02%), compared to patients treated with OCR in term of SMD values. After the matching procedure, considering the SMD values, the groups resulted balanced and comparable, with an age at DMT start of about 35 years, female gender in 60% of patients, monofocal onset in about 72% patients, OCBs presence in about 91% patients, EDSS score of about 2.5, number of EDSS evaluations of about 4.5, and at least one relapse in about 32% patients.
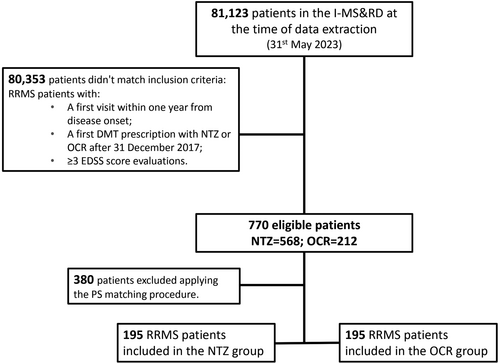
| Variable | NTZ (n = 568) | OCR (n = 212) | SMD | NTZ (n = 195) | OCR (n = 195) | SMD |
|---|---|---|---|---|---|---|
| Age at DMT start, mean (SD), years | 30.19 (10.15) | 36.22 (11.23) | 56.4 | 35.03 (10.61) | 35.21 (10.65) | 1.7 |
| Female, n (%) | 372 (65.49) | 123 (58.02) | −15.4 | 114 (58.46) | 117 (60.00) | 3.1 |
| Type of clinical onset of the disease | ||||||
| Monofocal | 425 (74.82) | 150 (70.75) | −9.2 | 144 (73.85) | 140 (71.79) | −4.6 |
| Multifocal | 93 (16.37) | 36 (16.98) | 26 (13.33) | 34 (17.44) | ||
| Not known | 50 (8.80) | 26 (12.26) | 25 (12.82) | 21 (10.77) | ||
| OCBs presence in CSF, n (%) | ||||||
| Absent | 19 (3.35) | 11 (5.19) | 9.1 | 9 (4.62) | 10 (5.13) | 2.4 |
| Present | 219 (38.56) | 106 (50.00) | 93 (47.69) | 95 (48.72) | ||
| Not known | 330 (58.10) | 95 (44.81) | 93 (47.69) | 90 (46.15) | ||
| EDSS at DMT start, mean (SD) | 1.92 (1.31) | 2.51 (1.51) | 41.6 | 2.35 (1.43) | 2.32 (1.32) | −1.7 |
| Number of EDSS score evaluations, mean (SD) | 4.67 (2.55) | 4.47 (2.40) | −8.1 | 4.44 (2.45) | 4.48 (2.42) | 1.9 |
| Number of relapses prior DMT start, n (%) | ||||||
| 0 | 376 (66.20) | 145 (68.40) | 4.7 | 121 (62.05) | 132 (67.69) | 11.8 |
| 1 | 152 (26.76) | 55 (25.94) | 61 (31.28) | 51 (26.15) | ||
| ≥2 | 40 (7.04) | 12 (5.66) | 13 (6.67) | 12 (6.15) |
- Abbreviations: DMT, disease-modifying therapy; OCBs, oligoclonal bands; CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale.
Median follow-up (IQR) after the treatment start was 1.63 (0.87–2.72) for NTZ group and 1.60 (0.80–2.68) years for OCR group.
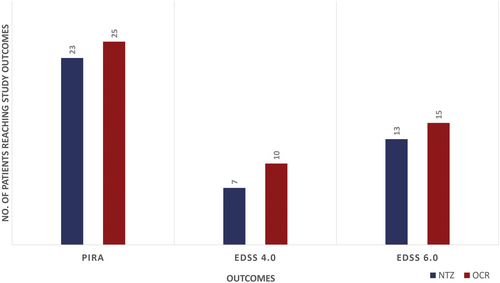
A first PIRA event was reached by 23 (11.79%) NTZ-exposed patients and 25 (12.82%) OCR exposed patients. No RAW events were found in the NTZ group and only 1 RAW event was reported in the OCR group. An irreversible EDSS 4.0 was reached by 7 (4.14%) NTZ-treated patients and 10 (5.85%) OCR-treated patients, and an irreversible EDSS 6.0 was reached by 13 (6.67%) NTZ-treated patients and 15 (7.69%) OCR-treated patients. (Fig. 2).
KM curves for the probabilities of reaching the outcomes are shown in the Figure 3. KM for RAW events were not outlined because there were no events to display.
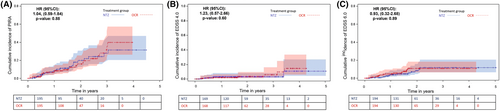
No differences between the two groups were found in the risk (HR, 95%CI) of reaching a first PIRA (Fig. 3A) (1.04, 0.59–1.84; p = 0.88) event, and irreversible EDSS 4.0 (Fig. 3B) (1.23, 0.57–2.66; p = 0.60) and EDSS 6.0 (0.93, 0.32–2.68; p = 0.89) (Fig. 3C).
Finally, 27 (13.85%) and 8 (4.10%) (p = 0.001) patients switched to another DMT, in NTZ- and OCR-treated groups, respectively. In the NTZ-treated switcher group, 9 patients (33.33%) switched to OCR.
In the OCZ-treated group, 3 patients switched to glatiramer acetate for pregnancy planning, 1 to NTZ for disease progression, and 4 to dimethyl fumarate due to a de-escalation strategy. In the NTZ group, we identified 20 patients switched for adverse events, with 2 cases of elevated levels of serum transaminases and 18 cases of suspension due to JCV antibody positivity, and within this group 8 patients switched to cladribine, 7 to OCR, 1 to siponimod, 1 to dimethyl fumarate, 1 to teriflunomide, 1 to interferon beta-1a, and 1 to glatiramer acetate; 4 patients suspended therapy due to allergic reactions, switching to cladribine, OCR, alemtuzumab and dimethyl fumarate; 1 patient switched to siponimod due to secondary progressive MS conversion, 1 to dimethyl fumarate for pregnancy planning and 1 to OCR for patient choice.
Discussion
To our knowledge, this is the first study aimed at comparing the effect of NTZ and OCR on the risk of achieving the first 6-month confirmed PIRA and RAW events and severe EDSS disability milestones in a large real-life cohort of naïve RRMS patients.
All the newly approved HE DMTs have demonstrated an excellent profile of effectiveness in reducing clinical and radiological disease activity, over the years.11 Some recent meta-analyses have developed an effectiveness ranking for all available DMTs, placing NTZ and OCR at comparable levels.14, 15 NTZ proved to be highly effective in RRMS treatment both in clinical trials and real-world settings with a well-established safety profile, leading to a significant improvement in the control of disease activity.16, 17 Growing evidence suggests that OCR has a strong anti-inflammatory action, with an almost complete suppression of clinical and radiological disease activity, and a significant effect in slowing the progression of disability accrual in RR and progressive MS.18, 19
A recent study20 compared, in a real-world PS-matched cohort of RRMS patients, the effectiveness of NTZ and OCR using the NEDA-3 as outcome. After 30 months of follow-up, NEDA-3 was reached by 53.1% in the OCR group and by 36.1% in the NTZ group, the treatment with OCR was associated with a lower risk of relapse, while the reduction of serum NFL levels did not differ between the two treatment groups. Another study showed that, in RRMS who switched from NTZ to dimethyl fumarate or fingolimod or OCR, the switch to OCR was associated with the lowest ARR and discontinuation rates and the longest time to first relapse.21
To date, the assessment of DMT effect on PIRA events has not been sufficiently investigated.22, 23
PIRA events, especially those that occurred early in the disease course, have been demonstrated to be predictors of unfavorable long-term disability prognosis.24 Therefore, a timely and prolonged treatment with the appropriate DMT is also primarily aimed at reducing the occurrence of PIRA events.2, 9 The superiority of OCR over interferon beta 1a in reducing PIRA, has already been reported in OPERA 1 and 2 trials.4 OCR proved an all-rounder beneficial effect on cognitive function, fatigue, and quality of life of MS patients, considering composite clinical measures and magnetic resonance imaging (MRI) and fluid biomarkers to assess disease progression.19
Currently, the pathophysiological substrate of PIRA remains incompletely characterized.25, 26 The effect on PIRA of NTZ and OCR, with completely different mechanisms of action but a common strong anti-inflammatory effect, could be due to the suppression of inflammatory pathological processes in the “smoldering” MS lesions.2, 6 Despite predicted effects on inflammatory networks related to microglia in chronic active lesions, anti-CD20 therapies failed to fully resolve paramagnetic rim lesions after a 2-year MRI follow-up in a recent study.27 These findings highlight the necessity of better identifying PIRA's pathological substrates to further evaluate appropriate treatment strategies.
Our results demonstrate that both OCR and NTZ strongly suppress RAW events and have a similar impact on PIRA in naïve RRMS patients, confirming that an early access to HE DMTs28-30 may result in a beneficial effect on the accumulation of neurological damage, dependent and independent from relapses, early in the disease course.31, 32
In OPERA I/II, in patients treated with OCR, PIRA events were the main contributors to both 12-week and 24-week CDA after 96 weeks (147 out of 167 [88%] and 115 out of 129 [89%]) and only a minority presented RAW events (29.7%).4 However, this pooled analysis of two randomized clinical trials (RCTs) used a composite CDA definition, including EDSS alongside with timed 25-feet walk and 9-hole peg test evaluations. Furthermore, considering the limitations of a comparison between our cohort and Kappos's, baseline data resulted similar in terms of age, sex, EDSS and number of relapses; however, in the pooled analysis only 73.3% of patients were treatment naïve.
Currently, to our knowledge there is no data about PIRA in RCTs populations of NTZ-treated patients.
A few limitations of our study should be taken into account. As with previous retrospective observational studies, incomplete or inaccurate data entered in the register must be acknowledged.
The lack of long-term follow-up has to be considered and the validation of these results over the long-term will constitutes a crucial point of future analysis. Although patients with less than 1 year of follow-up might not have greatly contributed to the 6-month confirmed PIRA evaluation, it is important to underline that we selected a cohort of closely monitored patients with at least three EDSS evaluations.
Moreover, as part of the data interpretation process, we also ran an analysis of the power calculation for equivalency study to assess limitations of this comparison of the two DMTs. Although the sample size calculation is not required in observational studies, we hypothesized several scenarios and calculated the necessary patient count to obtain the higher statistical power of 0.80. The number of patients to include per group would be 443 for a difference and 608 for an equivalence analysis, 439 for a superiority and 281 for a non-inferiority analysis. In our study, considering a cumulative incidence of 0.31 and 0.40 respectively for NTZ and OCR cohort, a power of 0.46 have been ensured with a sample size of 195 patients per group. With this cohort, the power is 0.38 for an equivalence study, 0.66 for a superiority, and 0.50 for a non-inferiority analysis. Therefore, a possible explanation of the absence of differences in PIRA outcomes between OCR and NTZ could be related to the sample size, too small to detect a difference.
In the definition of CDA events, we considered solely the EDSS score: although MRI features are considered a crucial prognostic factor and radiological activity is a cornerstone in defining the disease burden, we could not include MRI data because of the lack of a systematic acquisition of these data in the I-MS&RD. Furthermore, in comparison with RCTs, in this real-world study we are unable to account for potential confounders that could have affected results.
Despite all these considerations, our study used the large and validated database of the I-MS&RD to compare the impact of two of HE DMTs on PIRA and RAW events, so far not available in MS literature.
In conclusion, our results confirm that both OCR and NTZ effectively eliminate RAW events in RRMS patients. In the short term, the number and risk of PIRA, EDSS 4.0 irreversible and EDSS 6.0 events were not significantly different between the two treated groups. A longer follow-up and a larger cohort will be essential to confirm data on the effects of NTZ and OCR especially on disability progression outcomes.
Acknowledgments
None.
Funding Information
The present work received no specific funding.
Conflict of Interest
The authors report no conflicts of interest with respect to the contents of the current study, but note that the patients in the study were treated with a number of disease-modifying drugs and that authors have received advisory board, membership, speakers honoraria, travel support, research grants, consulting fees, or clinical trial support from the manufacturers of those drugs, including Actelion, Allergan, Almirall, Alexion, Bayer Schering, Biogen, Celgene, Excemed, Genzyme, Forward Pharma, Ipsen, Medday, Merck, Mylan, Novartis, Sanofi, Roche, Teva, and their local affiliates.
Author Contributions
PI, TG, GL, and MT: conception and design of the study, acquisition and analysis of data, drafting the manuscript and figures; DP, EP, MI, MF, FP, FG, SR, PC, GDL, PG, PB, AG, SM, ADS, MV, RQ, DS, RC; VTR, EC, VBM, AGM, DVB, MF, and MPA: data acquisition and drafting a significant portion of the manuscript.
Open Research
Data Availability Statement
Anonymized data will be shared on reasonable request from a qualified investigator.




