Comparison of stress hyperglycemia ratio and glycemic gap on acute ICH in-hospital outcomes
Abstract
Objective
To compare the effect of different indicators on stress-induced hyperglycemia for predicting in-hospital outcomes of acute intracerebral hemorrhage.
Methods
Using data from the Chinese Stroke Center Alliance database, which is a national, multicenter, prospective, and consecutive program. Stress-induced hyperglycemia was described as glycemic gap (GG, defined as fasting blood glucose [FBG] minus estimated average blood glucose) and stress hyperglycemia ratio (SHR, defined as FBG-to-estimated average blood glucose ratio [SHR 1] or FBG-to-HbA1c ratio [SHR 2]). The primary outcome was in-hospital mortality, and the second outcome was hematoma expansion.
Results
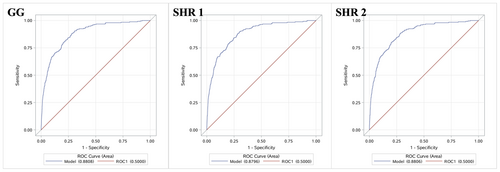
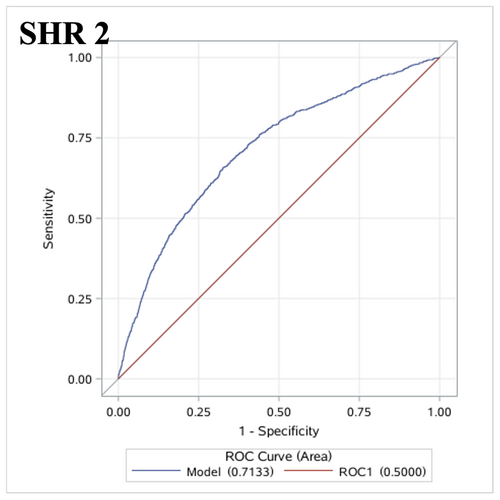
A total of 71,333 patients with acute intracerebral hemorrhage were included. In multivariate analyses, the highest levels of GG (OR 1.68, 95% CI 1.12–2.51), SHR 1 (OR 1.73, 95% CI 1.15–2.60), and SHR 2 (OR 2.07, 95% CI 1.33–3.23) were associated with in-hospital death (all the p trends <0.01). Only the highest level of SHR 2 (OR 1.24 [1.02–1.51], p trend >0.05) was related to hematoma expansion. No association between GG or SHR 1 and hematoma expansion was observed. The areas under the ROC curve of GG, SHR 1, and SHR 2 for in-hospital mortality were 0.8808 (95% CI 0.8603–0.9014), 0.8796 (95% CI 0.8589–0.9002), and 0.8806 (95% CI 0.8600–0.9012). The areas under the ROC curve of SHR 2 for hematoma expansion were 0.7133 (95% CI 0.6964–0.7302).
Interpretation
SHR (FBG-to-HbA1c ratio) was associated with both in-hospital death and hematoma expansion in intracerebral hemorrhage, and might serve as an accessory indicator for the in-hospital prognosis of intracerebral hemorrhage.
Introduction
Spontaneous intracerebral hemorrhage (ICH) is the second common subtype of stroke, with high rates of mortality and poor prognosis.1 In acute ICH, hyperglycemia is a common finding, and many studies demonstrate its importance in outcomes. Hyperglycemia may be caused by poorly controlled diabetes or caused by acute medical conditions.2 Stress-induced hyperglycemia is the transient status with relative elevated glucose level as a result of neuroendocrine response to stress.3 Stress-induced hyperglycemia is a wide term with no clear meaning. Stress-induced hyperglycemia could be defined as a rise in random plasma glucose or fasting blood glucose (FBG).3 On the other hand, glycemic control status at the time of disease development is not taken into account when using FBG to measure stress-induced hyperglycemia,4, 5 absolute glucose levels can not accurately reflect the influence of stress-induced hyperglycemia on outcomes. Sudden increases in blood glucose beyond the patient's regular background glycemia are more strongly connected with outcomes than absolute FBG and are more likely to indicate stress-induced hyperglycemia.6, 7 As a result, the stress hyperglycemia ratio (SHR) and the glycemic gap (GG) have been proposed to represent stress-induced hyperglycemia.7 These two biomarkers distinguish between interpatient variance in normal premorbid background glycemia related to glycosylated hemoglobin and the acute relative increase in glucose related to physiological stress, which accurately reflects stress-induced hyperglycemia.6, 7
Previous studies have demonstrated the SHR and GG were associated with poor outcomes in ICH,8, 9 but it remains unclearly which of them has the better performance on predicting unfavorable outcomes. Thus, in this study, we aimed to compare the differences in SHR and GG projected values on acute ICH in-hospital outcomes.
Materials and Methods
Study design and participants
The Chinese Stroke Center Alliance study is a national, multicenter, prospective, and consecutive cohort which enrolled acute stroke/transient ischemic attach patients between 2015 and 2019 from 1,476 hospitals across China. All of the Chinese secondary and tertiary grade hospitals were eligible to participate and report clinical data on all stroke patients. The Chinese Stroke Center Alliance study were announced by the Chinese Stroke Association and got with the guidance of the National Center of Neurological Diseases Care Management. Clinical Research Center for Neurological Disease, Beijing Tiantan Hospital, Capital Medical University is mandate to coordinate, extract, and analyze the data. Details of the study's design and implementation have been described.10 The inclusion criteria of the Chinese Stroke Center Alliance study were as follows: (1) age ≥18 years, (2) with a primary diagnosis of acute stroke/TIA confirmed by brain CT or MRI, (3) onset within 7 days, and (4) admitted to wards or through the emergency department. In the present study, we included patients diagnosed as acute ICH and excluded those without fasting blood glucose, HbA1c, or complete information of in-hospital death. Ultimately, 71,333 patients were included in the current analyses (Fig. 1).
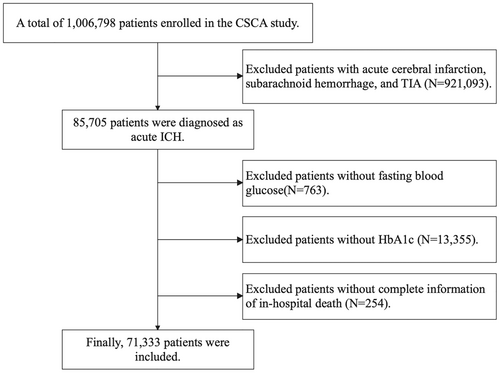
The study was approved by the Ethics Committee of Beijing Tiantan Hospital, and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Individual consent was waived by the committee because the data were anonymized.
Baseline characteristics
Trained registrars of each subcenter conducted data collection via a web-based patient data collection and management tool (Medicine Innovation Research Center, Beijing, China). The baseline information of all participants was uploaded and saved in the web-based tool. Before statistical analysis, we removed all identifiers on each data to protect the privacy and confidentiality of recruited patients. Information on age, sex, smoking, drinking, medical and medication history (such as hypertension, diabetes mellitus, dyslipidemia, and prior stroke), and clinical data (including systolic blood pressure [SBP], diastolic blood pressure [DBP], National Institutes of Health Stroke Score [NIHSS], Glasgow Coma Scale [GCS] on admission, and body mass index [BMI], premodified Rankin Scale [mRS]) was collected. Blood samples were collected after an 8–12 h fast within the first 24 h after admission at each subcenter.10 Laboratory data included fasting blood glucose (FBG), glycated hemoglobin (HbA1c), and other blood biochemical variables were examined.
Outcome assessment
The primary outcome of this study was in-hospital death, while the second outcome was hematoma expansion (HE). In-hospital death was defined as all-cause death during hospitalization. HE was defined according to radiographic criteria: (1) absolute increase of hematoma >6 mL from baseline or (2) relative increase in intraparenchymal hematoma in subsequent CT scanning >33%.11 CT scans were performed on admission and at short intervals during the initial hospitalization period. Two neurologists calculated the hematoma volume on CT scans by the ABC/2 formula.12
Stress-induced hyperglycemia assessment
Blood samples from the antecubital vein following an 8–12 h fast within the first 24 h after admission were obtained and examined for FBG and HbA1c. The procedure was standardized across all patients. We used GG and SHR to indicate stress-induced hyperglycemia. GG and SHR use HbA1c to determine patient-specific background glucose. GG is calculated as FBG minus estimated average blood glucose (eAG).13 eAG reflects glycemic control status from the previous 3 months and is not affected by short-term variability associated to stress-induced hyperglycemia, which is calculated by 13 There are two ways to calculate SHR: SHR 1 is defined as FBG divided by eAG,14 and SHR 2 is defined as FBG divided by HbA1c.15
Statistics
SAS software (version 9.4, SAS Institute, Cary, NC, USA) was used to perform statistical analyses. Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range [IQR]) and compared by ANOVA or Kruskal–Wallis test. Categorical variables were presented as frequencies (percentages) and compared using the chi-square test. Logistic regression models were used to calculate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for outcomes. We fitted two models to systematically adjust for the potential confounding factors. We chose the variables with statistical differences (p < 0.05) between groups in Tables 1 or 2 as confounders, referring to previous studies. Model 1 was adjusted for age, sex, BMI, SBP on admission, DBP on admission, pre-mRS, hypertension, diabetes mellitus, dyslipidemia, history of stroke, antiplatelet agents, anticoagulant agents, antidiabetic agents, smoking, alcohol, low density lipoprotein cholesterol, and surgical intervention. Model 2 included an adjustment for all the covariates in Model 1 plus NIHSS and GCS on admission. Receiver operating characteristic (ROC) curves were conducted to determine the predict value of GG, SHR 1 and SHR 2 via area under the ROC curve (AUC). All statistical analyses were two-sided, and difference with a p value <0.05 was considered statistically significant.
Results
A total of 71,333 patients with acute ICH from CSCA were included in the present analysis (Fig. 1), 1494 (2.1%) of whom experienced in-hospital death, and 5875 (8.3%) had HE. The mean age was 63.0 ± 12.8 years, and 44,537 (62.4%) patients were male.
Characteristics of participants
The overall characteristics of all patients are shown in Table 1. According to the outcomes during hospitalization, the patients were divided into groups as nonsurvivors and survivors, or with HE and without HE. In comparison with the survivors, the nonsurvivors were more likely to be older and male, to have higher systolic and diastolic blood pressure, a higher NIHSS score on admission and pre-mRS score, a lower GCS score on admission, to have diabetes mellitus, dyslipidemia, and history of stroke, as well as to be taking antiplatelet, anticoagulant, and antidiabetic agents, and to smoke and drink alcohol, have received more surgical intervention, and have higher plasma concentrations of HbA1c, FBG, GG, SHR 1, and SHR 2 (shown in Table 1). Except for a lower prevalence of diabetes and smoking, a higher prevalence of hypertension, and no significant differences on distribution of sex, the differences between patients with and without HE are similar to those between nonsurvivors and survivors (shown in Table 2).
| Variables | All (n = 71,333) | Survivors (n = 69,839 [97.9%]) | Nonsurvivors (n = 1494 [2.1%]) | p value |
|---|---|---|---|---|
| Age, years | 63.0 ± 12.8 | 62.9 ± 12.8 | 66.9 ± 14.5 | <0.001 |
| Male, n (%) | 44,537 (62.4) | 43,550 (62.4) | 987 (66.1) | 0.003 |
| BMI, kg/m2 | 23.5 (21.6–25.4) | 23.5 (21.6–25.4) | 23.4 (21.4–25.6) | 0.250 |
| SBP on admission, mmHg | 162 (145–180) | 161 (145–180) | 173 (150–200) | <0.001 |
| DBP on admission, mmHg | 94 (83–105) | 94 (83–105) | 98 (84–110) | <0.001 |
| NIHSS on admission | 6 (2–12) | 5 (2–11) | 21 (11–34) | <0.001 |
| GCS on admission | 13 (8–15) | 14 (9–15) | 5 (4–8) | <0.001 |
| Pre-mRS | 1 (1–3) | 1 (1–3) | 2 (1–5) | <0.001 |
| Medical history, n (%) | ||||
| Hypertension | 51,782 (72.6) | 50,679 (72.6) | 1103 (73.8) | 0.279 |
| Diabetes mellitus | 7261 (10.2) | 7003 (10.0) | 258 (17.3) | <0.001 |
| Dyslipidemia | 5023 (7.0) | 4893 (7.0) | 130 (8.7) | 0.011 |
| History of stroke | 20,613 (28.9) | 20,062 (28.7) | 551 (36.9) | <0.001 |
| Medication History, n (%) | ||||
| Antiplatelet agents | 5109 (7.2) | 4938 (7.1) | 171 (11.4) | <0.001 |
| Anticoagulant agents | 1384 (1.9) | 1317 (1.9) | 67 (4.5) | <0.001 |
| Antidiabetic agents | 5312 (7.4) | 5128 (7.3) | 184 (12.3) | <0.001 |
| Smoking, n (%) | ||||
| Never smoke | 45,391 (63.6) | 44,484 (63.7) | 907 (60.7) | <0.001 |
| Used to smoke, quit now | 9578 (13.4) | 9357 (13.4) | 221 (14.8) | |
| Current smoking | 13,824 (19.4) | 13,557 (19.4) | 267 (17.9) | |
| Unclear | 2540 (3.6) | 2441 (3.5) | 99 (6.6) | |
| Alcohol, n (%) | 17,429 (24.4) | 17,039 (24.4) | 390 (26.1) | <0.001 |
| Surgical intervention, n (%) | 7211 (10.7) | 6859 (10.4) | 352 (25.1) | <0.001 |
| Laboratory test | ||||
| LDL-C, mmol/L | 2.6 (2.1–3.3) | 2.6 (2.1–3.3) | 2.6 (1.9–3.4) | 0.127 |
| HbA1c, (%) | 5.6 (5.1–6.1) | 5.6 (5.1–6.1) | 5.8 (5.0–6.7) | <0.001 |
| FBG, mmol/L | 5.9 (5.1–7.1) | 5.9 (5.1–7.1) | 7.6 (6.0–10.2) | <0.001 |
| GGa, mmol/L | −0.3 (−1.3–0.8) | −0.4 (−1.3–0.8) | 0.7 (−0.8–3.0) | <0.001 |
| SHR 1b | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 1.1 (0.9–1.5) | <0.001 |
| SHR 2c | 1.1 (0.9–1.3) | 0.9 (0.8–1.1) | 0.8 (0.6–1.0) | <0.001 |
- BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; GCS, Glasgow Coma Scale; GG, glycemic gap; HbA1c, glycated hemoglobin; LDL-C, low density lipoprotein cholesterol; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; SBP, systolic blood pressure; SHR, stress hyperglycemia ratio.
- a GG is defined as fasting blood glucose minus estimated average blood glucose.
- b SHR 1 is defined as fasting blood glucose divided by estimated average glucose.
- c SHR 2 is defined as fasting blood glucose divided by HbA1c.
| Variables | Without hematoma enlargement (n = 65,330 [91.7%]) | With hematoma enlargement (n = 5875 [8.3%]) | p value |
|---|---|---|---|
| Age, years | 63.0 ± 12.8 | 63.4 ± 12.8 | 0.021 |
| Male, n (%) | 40,730 (62.3) | 3717 (63.3) | 0.162 |
| BMI, kg/m2 | 23.5 (21.6–25.4) | 23.6 (21.5–25.5) | 0.569 |
| SBP on admission, mmHg | 161 (145–180) | 164 (145–185) | <0.001 |
| DBP on admission, mmHg | 94 (83–105) | 95 (84–106) | <0.001 |
| NIHSS on admission | 5 (2–11) | 7 (3–15) | <0.001 |
| GCS on admission | 14 (9–15) | 10 (6–14) | <0.001 |
| Pre-mRS | 1 (1–3) | 2 (1–4) | <0.001 |
| Medical history, n (%) | |||
| Hypertension | 47,242 (72.3) | 4453 (75.8) | <0.001 |
| Diabetes mellitus | 707 (12.0) | 6533 (10.0) | <0.001 |
| Dyslipidemia | 4331 (6.6) | 675 (11.5) | <0.001 |
| History of stroke | 17,736 (27.1) | 2830 (48.2) | <0.001 |
| Medication History, n (%) | |||
| Antiplatelet agents | 4484 (6.9) | 610 (10.4) | <0.001 |
| Anticoagulant agents | 1195 (1.8) | 185 (3.1) | <0.001 |
| Antidiabetic agents | 4749 (7.3) | 551 (9.4) | <0.001 |
| Smoking, n (%) | |||
| Never smoke | 41,523 (63.6) | 3796 (64.6) | 0.002 |
| Used to smoke, quit now | 8736 (13.4) | 826 (14.1) | |
| Current smoking | 12,761 (19.5) | 1030 (17.5) | |
| Unclear | 2310 (3.5) | 223 (3.8) | |
| Alcohol, n (%) | 15,724 (24.1) | 1662 (28.3) | <0.001 |
| Surgical intervention, n (%) | 5987 (9.6) | 1224 (23.4) | <0.001 |
| Laboratory test | |||
| LDL-C, mmol/L | 2.6 (2.1–3.3) | 2.6 (2.0–3.3) | 0.693 |
| HbA1c, (%) | 5.6 (5.1–6.1) | 5.6 (5.1–6.2) | <0.001 |
| FBG, mmol/L | 5.9 (5.1–7.1) | 6.2 (5.3–7.8) | <0.001 |
| GGa, mmol/L | −0.4 (−1.3–0.8) | −0.1 (−1.2–1.2) | <0.001 |
| SHR 1b | 0.9 (0.8–1.1) | 1.0 (0.8–1.2) | <0.001 |
| SHR 2c | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | <0.001 |
- BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; GCS, Glasgow Coma Scale; GG, glycemic gap; HbA1c, glycated hemoglobin; LDL-C, low density lipoprotein cholesterol; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; SBP, systolic blood pressure; SHR, stress hyperglycemia ratio.
- a GG is defined as fasting blood glucose minus estimated average blood glucose.
- b SHR 1 is defined as fasting blood glucose divided by estimated average glucose.
- c SHR 2 is defined as fasting blood glucose divided by HbA1c.
Indicators of stress-induced hyperglycemia and in-hospital outcomes
In total, the in-hospital mortality was 2.09% (1494/71,333), and 5875 (8.24%) patients suffered HE. We divided the stress-induced hyperglycemia indicators (GG, SHR 1, and SHR 2) into four groups according to their quartiles. The in-hospital mortality from Q1 to Q4 were 1.5% (268), 1.2% (212), 1.6% (280), and 4.1% (734) for GG; 1.4% (254), 1.2% (205), 1.7% (315), and 4.0% (720) for SHR 1; and 1.2% (203), 1.2% (212), 1.8% (327), and 4.3% (752) for SHR 2.
Tables 3 and 4 showed the association between stress-induced hyperglycemia indicators and in-hospital outcomes. In the unadjusted logistic regression model, the highest level of GG (Q4 [OR 2.74, 95% CI 2.38–3.16]), and the higher levels of SHR 1 (Q3 [OR 1.22, 95% CI 1.03–1.44] and Q4 [OR 2.94, 95% CI 2.55–3.40]) and SHR 2 (Q3 [OR 1.55, 95% CI 1.30–1.85] and Q4 [OR 3.76, 95% CI 3.22–4.40]) were significantly associated with in-hospital death compared to Q1 (all the p and p trend <0.05); and the highest level (Q4) remained significant after adjusting for potential confounding factors, with the ORs for GG, SHR 1, and SHR 2 being 1.68 (95% CI 1.12–2.51), 1.73 (95% CI 1.15–2.60), and 2.07 (95% CI 1.33–3.23) (all the p and p trend <0.01). Similar results were found in univariate logistic regression analyses of the association between stress-induced hyperglycemia indicators and HE. In multivariate logistic regression, only the highest level (Q4) of SHR 2 (OR 1.24 [95% CI 1.02–1.51], p trend >0.05) remained related to HE. No association between GG or SHR 1 and hematoma expansion was observed in this study.
| In-hospital death | |||||||
|---|---|---|---|---|---|---|---|
| n (%) | Crude model | Model 1 | Model 2 | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| GGa, mmol/L | |||||||
| Q1 (<−1.30) | 268 (1.5) | Reference | Reference | Reference | |||
| Q2 (−1.30 to −0.35) | 212 (1.2) | 0.75 (0.63–0.90) | 0.002 | 0.84 (0.70–1.02) | 0.085 | 1.12 (0.70–1.80) | 0.632 |
| Q3 (−0.34–0.79) | 280 (1.6) | 1.02 (0.86–1.21) | 0.807 | 1.09 (0.91–1.31) | 0.349 | 1.22 (0.78–1.90) | 0.383 |
| Q4 (≥0.80) | 734 (4.1) | 2.74 (2.38–3.16) | <0.001 | 2.27 (1.95–2.65) | <0.001 | 1.68 (1.12–2.51) | 0.011 |
| p trend | <0.001 | <0.001 | 0.005 | ||||
| SHR 1b | |||||||
| Q1 (<0.81) | 254 (1.4) | Reference | Reference | Reference | |||
| Q2 (0.81–0.93) | 205 (1.2) | 0.85 (0.71–1.03) | 0.090 | 0.93 (0.76–1.13) | 0.442 | 1.08 (0.67–1.76) | 0.743 |
| Q3 (0.94–1.13) | 315 (1.7) | 1.22 (1.03–1.44) | 0.019 | 1.24 (1.03–1.48) | 0.019 | 1.38 (0.89–2.13) | 0.145 |
| Q4 (≥1.14) | 720 (4.0) | 2.94 (2.55–3.40) | <0.001 | 2.43 (2.07–2.84) | <0.001 | 1.73 (1.15–2.60) | 0.008 |
| p trend | <0.001 | <0.001 | 0.002 | ||||
| SHR 2c | |||||||
| Q1 (<0.92) | 203 (1.2) | Reference | Reference | Reference | |||
| Q2 (0.92–1.05) | 212 (1.2) | 0.99 (0.82–1.20) | 0.928 | 1.05 (0.85–1.29) | 0.664 | 1.25 (0.76–2.08) | 0.381 |
| Q3 (1.06–1.25) | 327 (1.8) | 1.55 (1.30–1.85) | <0.001 | 1.49 (1.23–1.79) | <0.001 | 1.51 (0.95–2.42) | 0.083 |
| Q4 (≥1.26) | 752 (4.3) | 3.76 (3.22–4.40) | <0.001 | 2.91 (2.45–3.45) | <0.001 | 2.07 (1.33–3.23) | 0.001 |
| p trend | <0.001 | <0.001 | <0.001 | ||||
- Model 1: adjusted for age, sex, body mass index, systolic pressure on admission, diastolic pressure on admission, premodified Rankin scale, hypertension, diabetes mellitus, dyslipidemia, history of stroke, antiplatelet agents, anticoagulant agents, antidiabetic agents, smoking, alcohol, low density lipoprotein cholesterol, and surgical intervention. Model 2: adjusted for variables in model 1 + National Institute of Health Stroke Scale and Glasgow Coma Scale on admission.
- GG, glycemic gap; SHR, stress hyperglycemia ratio.
- a GG is defined as fasting blood glucose minus estimated average blood glucose.
- b SHR 1 is defined as fasting blood glucose divided by estimated average glucose.
- c SHR 2 is defined as fasting blood glucose divided by HbA1c.
| Hematoma enlargement | |||||||
|---|---|---|---|---|---|---|---|
| n (%) | Crude model | Model 1 | Model 2 | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| GGa, mmol/L | |||||||
| Q1 (<−1.30) | 1331 (7.7) | Reference | Reference | Reference | |||
| Q2 (−1.30 to −0.35) | 1310 (7.2) | 0.93 (0.86–1.01) | 0.091 | 0.96 (0.88–1.05) | 0.397 | 0.89 (0.73–1.08) | 0.248 |
| Q3 (−0.34–0.79) | 1398 (7.9) | 1.03 (0.95–1.11) | 0.496 | 0.99 (0.91–1.08) | 0.819 | 0.88 (0.73–1.07) | 0.209 |
| Q4 (≥0.80) | 1836 (10.3) | 1.38 (1.28–1.49) | <0.001 | 1.21 (1.11–1.31) | <0.001 | 1.14 (0.95–1.38) | 0.170 |
| p trend | <0.001 | <0.001 | 0.157 | ||||
| SHR 1b | |||||||
| Q1 (<0.81) | 1337 (7.4) | Reference | Reference | Reference | |||
| Q2 (0.81–0.93) | 1242 (7.3) | 0.98 (0.91–1.06) | 0.638 | 1.01 (0.93–1.10) | 0.806 | 0.98 (0.80–1.19) | 0.812 |
| Q3 (0.94–1.13) | 1496 (8.1) | 1.11 (1.02–1.19) | 0.010 | 1.05 (0.97–1.15) | 0.216 | 0.98 (0.81–1.18) | 0.803 |
| Q4 (≥1.14) | 1800 (10.1) | 1.40 (1.30–1.51) | <0.001 | 1.22 (1.13–1.33) | <0.001 | 1.15 (0.95–1.38) | 0.153 |
| p trend | <0.001 | <0.001 | 0.161 | ||||
| SHR 2c | |||||||
| Q1 (<0.92) | 1237 (7.2) | Reference | Reference | Reference | |||
| Q2 (0.92–1.05) | 1296 (7.1) | 0.99 (0.92–1.08) | 0.869 | 1.01 (0.92–1.10) | 0.910 | 1.05 (0.86–1.28) | 0.628 |
| Q3 (1.06–1.25) | 1497 (8.3) | 1.17 (1.08–1.26) | 0.001 | 1.10 (1.01–1.19) | 0.038 | 0.93 (0.76–1.13) | 0.475 |
| Q4 (≥1.26) | 1845 (10.5) | 1.52 (1.41–1.64) | <0.001 | 1.30 (1.19–1.41) | <0.001 | 1.24 (1.02–1.51) | 0.028 |
| p trend | <0.001 | <0.001 | 0.074 | ||||
- Model 1: adjusted for age, sex, body mass index, systolic pressure on admission, diastolic pressure on admission, premodified Rankin scale, hypertension, diabetes mellitus, dyslipidemia, history of stroke, antiplatelet agents, anticoagulant agents, antidiabetic agents, smoking, alcohol, low density lipoprotein cholesterol, and surgical intervention. Model 2: adjusted for variables in model 1 + National Institute of Health Stroke Scale and Glasgow Coma Scale on admission.
- GG, glycemic gap; SHR, stress hyperglycemia ratio.
- a GG is defined as fasting blood glucose minus estimated average blood glucose.
- b SHR 1 is defined as fasting blood glucose divided by estimated average glucose.
- c SHR 2 is defined as fasting blood glucose divided by HbA1c.
Predictive value of different stress-induced hyperglycemia indicators for in-hospital outcomes
In the ROC analyses, for in-hospital mortality, the AUCs of GG, SHR 1, and SHR 2 were 0.8808 (95% CI 0.8603–0.9014), 0.8796 (95% CI 0.8589–0.9002), and 0.8806 (95% CI 0.8600–0.9012), respectively (Shown in Fig. 2). For HE, the AUC of SHR 2 was 0.7133 (95% CI 0.6964–0.7302) (Shown in Fig. 3).
We got similar results of analyses using FBG (shown in Table S1).
Discussion
In this study, we analyzed the relationship between stress-induced hyperglycemia and in-hospital outcomes among acute ICH patients, and we compared the effect of different stress-induced hyperglycemia indicators on in-hospital death and HE. We found that higher levels of GG, SHR 1, and SHR 2 levels were associated with in-hospital death. SHR 2 and GG were comparable in predicting in-hospital death. Only a higher level of SHR 2 remained associated with HE. Clinical physicians need to be vigilant that the ICH patients with GG ≥0.8 mmol/l, SHR 1 ≥ 1.14, or SHR 2 ≥ 1.26 might be at high risk of death or hematoma enlargement. Our study revealed that HbA1c-based adjusted stress-induced hyperglycemia indicators, including GG and SHR, were useful in assessing the in-hospital prognosis presenting with acute ICH. SHR, defined as fasting blood glucose divided by HbA1c, was related to both in-hospital death and HE in acute ICH patients and might serve as a comprehensive accessory indicator to associate with poor in-hospital prognosis of acute ICH.
Stress-induced hyperglycemia is a transient hyperglycemia secondary to neurohumoral disorders and inflammatory reactions and is a common clinical manifestation in patients with acute ICH.3, 16 Previous studies have investigated the relationship between stress-induced hyperglycemia with different definitions and poor clinical outcomes in acute ICH patients.17 In most studies, stress-induced hyperglycemia was usually measured as an absolutely elevated blood glucose level without considering previous diabetes mellitus-controlled status and basic glucose level.3, 4, 17, 18 Hence, absolute high glucose level cannot reflect the changes of blood glucose in critical situations, especially in the case of poorly glycemic control. Because of the stability of HbA1c over the previous 8–12 weeks, some studies have defined stress-induced hyperglycemia based on HbA1c levels, such as glucose-to-HbA1c ratio, glucose level divided by eAG derived from HbA1c, or glucose level minus eAG. These relative measurements of stress-induced hyperglycemia adjusted for background glycemic status could help to better predict adverse outcomes compared with absolute elevated glucose levels.6, 19, 20 However, due to the difference between eAG and average blood glucose level, some researchers have pointed out that eAG should be used cautiously in clinical practice, and SHR, defined as glucose-to-HbA1c ratio, was more applicable.21 In our study, we assessed all the above relative measurements of stress-induced hyperglycemia and compared the predictive performance for in-hospital outcomes among GG, SHR 1 defined as FBG-to-eAG, and SHR 2 defined as FBG-to-HbA1c in acute ICH patients.
Previous studies have demonstrated that both GG and SHR were predictors for adverse outcomes in patients with ICH.8, 9, 16, 22-24 GG positively correlated with hemorrhage volume and could independently predict in-hospital mortality and poor clinical outcomes at 30 days, 90 days, and 1 year for ICH patients.9, 22, 23 SHR, whether defined by glucose-to-eAG or glucose-to-HbA1c, was associated with poor prognosis of ICH patients.8, 24 However, all the above studies were limited by the lack of comparing the predictive value among different indicators of stress-induced hyperglycemia for adverse outcomes of ICH patients. Consistent with the above study, we found any of GG, SHR defined by glucose-to-eAG, or SHR defined by glucose-to-HbA1c was associated with in-hospital mortality in acute ICH patients. Moreover, SHR defined by glucose-to-HbA1c was related to both in-hospital mortality and HE and might associate with exhaustively unfavorable in-hospital outcomes of acute ICH patients.
Only two studies with a small sample size have compared the AUC of SHR or GG and FBG for in-hospital outcomes among ICH patients; the AUC of SHR (FBG-to-HbA1c ratio) for poor functional outcomes at discharge was higher than FBG24; the AUC of GG for in-hospital death was higher than FBG.22 However, few studies have investigated the predicting value of different SIH indicators. Our study compared the SHR and GG on in-hospital mortality and HE. Besides, we also found similar results using highest fasting blood glucose in the first 24 hours with no significant difference in ROC AUC for FBG versus SHR 1, SHR 2, and GG. Our study had a large-scale population, but further studies are still needed to replicate and confirm the results.
The effects of stress-induced hyperglycemia on poor outcomes in ICH patients are complex and involve multiple potential mechanisms. Stress hyperglycemia after ICH is regarded to be caused by a combination of factors. The elevated intracranial pressure after ICH could directly or indirectly influence the structure and function of the hypothalamus–pituitary–thyroid axis, which led to the increase of hydrocortisone, growth hormone, and glucagon, and the reduction of tri-iodothyronine.25, 26 Catecholamine excess occurs as a component of the stress response after ICH.27 Furthermore, systemic inflammation and oxidative response were strengthened, which produced more cytokines.3, 28 Complex feedforward and feedback mechanisms between these hormones and cytokines resulted in increasing hepatic glucose production, insulin resistance, and decreasing peripheral glucose uptake.3, 29 In addition, insulin resistance could also elevate the serum glucose level.30 All of the above could exert deleterious effects on the blood–brain barrier and parenchymal cells and then exacerbate the brain injury.23 Besides, stress-induced hyperglycemia could interfere with the process of ATP generation by causing calcium influx into mitochondria and downregulate aquaporin-4 in the brain, leading to brain edema, neuronal apoptosis, and the destruction of the brain–blood barrier.31, 32 In addition, stress-induced hyperglycemia damages the integrity of blood vessels near the initial bleeding site and upregulates the expression of nuclear factor-κB and matrix metalloproteinases-9, which may lead to HE.33
The current study compared the effect of three different indicators of stress-induced hyperglycemia on the in-hospital outcomes of acute ICH patients in a large-scale nationwide longitudinal registry sample. However, there are several limitations. First, CSCA program focused on improvement in stroke management quality. Patients admitted into intensive care unit or neurosurgery department may not be enrolled; only recorded all-cause death but not the cause of death or functional assessment (modified Rank Scale); admission ICH volume, ICH location, intraventricular hemorrhage, and some markers of inflammation (such as leukocytosis or neutrophil-lymphocyte ratio) were nonavailable, which needs to be addressed in future registration and studies. Second, this study was observational with only in-hospital data, lack of follow-up information, the predictive value for long-term outcomes cannot be determined. Third, we did not evaluate the glycemic variability during hospitalization. As a large sample study, our findings on CSCA still had some clinical implications.
Conclusion
In conclusion, HbA1c-based adjusted stress-induced hyperglycemia indicators, including GG and SHR, were associated with in-hospital death in acute ICH, besides, SHR (FBG-to-HbA1c ratio) was also associated with HE. GG and SHR (FBG-to-HbA1c ratio) were comparable in predicting in-hospital death. SHR, defined as fasting blood glucose divided by HbA1c, might serve as an accessory indicator for the in-hospital prognosis of ICH. Further studies are needed to replicate current findings.
Acknowledgments
We thank all the CSCA collaborating centers, members, and volunteers for their hard work. We thank all the participants of CSCA study for their contributions. This study was supported by National Science and Technology Major Project (2022ZD0118003), National Natural Science Foundation of China (Grant No. 82371302), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029), Beijing Municipal Committee of Science and Technology (Z201100005620010), Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support (code: 202112), the Ministry of Finance of the People's Republic of China (issued by Finance and Social Security [2015] Document No. 82; [2016] Document No. 50; [2017] Document No. 72; [2018] Document No. 48; [2019] Document No. 77; [2020] Document No. 75; [2021] Document No. 84, [Ministry of Finance]), National Natural Science Foundation of China (81501001), and Beijing Municipal Administration of Hospitals Incubating Program (PX2020022).
Conflict of Interest
Jia Zhang, Qian Zhang, Hongqiu Gu, Qi Zhou, Zixiao Li, and Xingquan Zhao declare that they have no conflict of interest.
Author Contributions
Jia Zhang and Qian Zhang researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. Hongqiu Gu and Qi Zhou researched data and reviewed the manuscript. Zixiao Li and Xingquan Zhao reviewed the manuscript and contributed to discussion. All authors approved the final version of the manuscript. Zixiao Li and Xingquan Zhao are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Open Research
Data Availability Statement
The derived data generated in this research will be shared on reasonable request to the corresponding author.




