Study on clinical features and factors related to long-term outcomes of antibody-negative autoimmune encephalitis
Abstract
Objective
To delineate the clinical characteristics of antibody-negative autoimmune encephalitis (AE) and to investigate factors associated with long-term outcomes among antibody-negative AE.
Methods
Patients diagnosed with antibody-negative AE were recruited from January 2016 to December 2022 at the Second Xiangya Hospital of Central South University. The study assessed the long-term outcomes of antibody-negative AE using the modified Rankin scale (mRS) and the Clinical Assessment Scale in Autoimmune Encephalitis (CASE). Predictors influencing long-term outcomes were subsequently analyzed. External validation of RAPID scores (refractory status epilepticus [RSE], age of onset ≥60 years, ANPRA [antibody-negative probable autoimmune encephalitis], infratentorial involvement, and delay of immunotherapy ≥1 month) was performed.
Results
In total, 100 (47 females and 53 males) antibody-negative AE patients were enrolled in this study, with approximately 49 (49%) experiencing unfavorable long-term outcomes (mRS scores ≥3). Antibody-negative AE was subcategorized into ANPRA, autoimmune limbic encephalitis (LE), and acute disseminated encephalomyelitis (ADEM). Psychiatric symptoms were prevalent in LE and ANPRA subtypes, while weakness and gait instability/dystonia were predominant in the ADEM subtype. Higher peak CASE scores (odds ratio [OR] 1.846, 95% confidence interval [CI]: 1.163–2.930, p = 0.009) and initiating immunotherapy within 30 days (OR 0.210, 95% CI: 0.046–0.948, p = 0.042) were correlated with long-term outcomes. Receiver operating characteristic (ROC) analysis returned that the RAPID scores cutoff of 1.5 best discriminated the group with poor long-term outcomes (sensitivity 85.7%, specificity 56.9%).
Interpretation
The ANPRA subtype exhibited poorer long-term outcomes compared to LE and ADEM subtypes, and early immunotherapy was crucial for improving long-term outcomes in antibody-negative AE. The use of RAPID scoring could aid in guiding clinical decision making.
Introduction
Autoimmune encephalitis (AE) linked to autoantibodies targeting neuronal surface, synaptic, or intracellular antigens constitutes a significant and swiftly advancing category of neuroinflammatory disorders.1-3 Patients with AE present with subacute or acute impaired cognition, neuropsychiatric symptoms often accompanied by newly onset seizures, language problems, sleep disorders, or movement disorders.4 For the past decade or so, the diagnosis of AE has relied on neuronal autoantibody testing. However, among the AE patients, antibody-negative AE represented a subtype characterized by the absence of detectable pathogenic autoantibodies, the underlying mechanism of which remains unknown.5, 6 The diagnostic criteria by Graus et al.4 showed that the absence of antibodies did not exclude AE, but the inclusion criteria for antibody-negative AE varied widely among available studies.7-9 Recognition of antibody-negative AE patients is crucial as with prompt diagnosis and early administration of immunotherapy the majority have favorable outcomes.4, 10
According to data from Olmstead County published in 2015, the estimated prevalence was 6.5 per 100,000 persons (with an incidence of 0.4 per 100,000 person-years) for antibody-positive AE and 1.3 per 100,000 persons (with an incidence of 0.1 per 100,000 person-years) for antibody-negative AE.2 Furthermore, there was an observed increase in the incidence of antibody-negative AE from the 1995–2005 interval to the 2006–2016 interval (0.1 [1995–2005] to 0.2 [2006–2015]).2 Currently, antibody-negative AE has emerged as a significant subtype within the spectrum of AE. However, there has been a notable disparity in the volume of studies conducted between antibody-negative AE and antibody-positive AE, particularly regarding distinct disease mechanisms, clinical characteristics, treatment modalities, prognostic factors, and relapse associated with different neuronal autoantibodies in antibody-positive AE.11-13 To further understand antibody-negative AE, the consensus by Graus et al.4 classified antibody-negative AE into three subtypes: antibody-negative but probable AE (ANPRA), definite autoimmune limbic encephalitis (LE), and acute disseminated encephalomyelitis (ADEM).
In 2022, Lee et al.14 evaluated the clinical features, treatment outcomes, and prognosis of subtypes of antibody-negative AE patients based on a large cohort. They developed an early available clinical score for antibody-negative AE. The RAPID score consists of five factors (RSE, age of onset ≥60 years, ANPRA, infratentorial involvement, and delay of immunotherapy for ≥1 month), with each factor scoring 1 point. A score of ≥2 indicates a poorer long-term prognosis.
However, comprehensive studies focusing on antibody-negative AE have not been done, and no studies have focused on the clinical characteristics and prognostic factors of antibody-negative AE in China. Moreover, the RAPID score has not been validated at an external institution. Therefore, the primary aim of this study was to comprehensively delineate the clinical characteristics, ancillary examinations, cerebrospinal fluid (CSF) analyses, immunotherapeutic strategies, and long-term prognosis of antibody-negative AE. External validation of the RAPID score14 was also performed in our cohort.
Materials and Methods
Study population
A total of 457 individuals who were initially suspected AE at the Second Xiangya Hospital of Central South University from 1 January 2016 to 1 December 2022. Sixteen cases were excluded because of incomplete medical records. Based on the diagnostic criteria4 patients were excluded and included in order of possible, probable, and definite AE, patients diagnosed with infectious and other etiologies (n = 74), and patients who did not meet the diagnostic criteria for possible AE (n = 50) were excluded sequentially. The remaining 307 patients were tested for antibodies, and antibody-positive patients (n = 187) were excluded. Those patients with less than 12 months of follow-up (n = 4) and lost to follow-up (n = 6) were excluded among the antibody-negative patients (n = 120). Those patients who did not meet the diagnostic criteria for autoantibody-negative but probable AE (n = 10) were excluded. Finally, 100 antibody-negative AE subjects were enrolled in this study (Fig. 1).
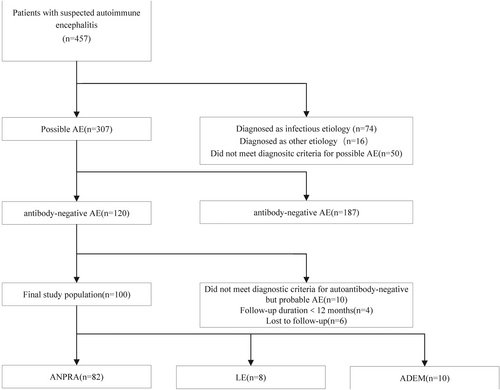
Inclusion criteria and exclusion criteria
This study included all consecutive patients who met the following criteria: (1) patients aged ≥16 years; (2) initially admitted with the following symptoms: acute or subacute onset (rapid progression of less than 3 months) of working memory deficits (short-term memory loss), altered mental status, psychiatric symptoms, seizure, speech dysfunction, dyskinesia, gait instability, or weakness; (3) at least one of the following criteria: seizures not explained by a previously known seizure disorder, CSF pleocytosis (white blood cell count of more than five cells per mm3), CSF-specific oligoclonal bands or elevated CSF IgG index, CSF elevated protein, magnetic resonance imaging (MRI) abnormalities suggestive of AE; (4) negative results of tests of an autoantibody panel of AE: none of autoimmune antibody was identified in the serum and CSF sampling; (5) reasonable exclusion of alternative causes; and (6) follow-up time ≥12 months. The altered mental status was defined as a decreased or altered level of consciousness, lethargy, or personality change.4
The exclusion criteria were as follows: (1) encephalitis of infectious origin (bacteria and major viruses) with laboratory-confirmed central nervous system infection, such as Herpesviridae, Enterovirus, respiratory virus, John Cunningham (JC) virus, measles, Japanese B virus, Mycobacterium, fungus, mycoplasma, Toxoplasma, Cryptococcus, and other infectious etiologies; (2) patients previously diagnosed with toxic/metabolic encephalopathy, brain tumors, vitamin deficiency, alcohol-related encephalopathy, seizures, or other neurological disorders before the onset of AE; (3) primary psychiatric diseases; (4) Hashimoto's encephalopathy and Bickerstaff encephalitis; and (5) patients with incomplete clinical data. All enrolled patients were evaluated by two experienced neurologists according to the above criteria and procedures before inclusion.
Criteria for subgroups
Based on the diagnostic criteria in 2016,4 100 antibody-negative AE patients were categorized into three subgroups: antibody-negative but probable AE (ANPRA) (n = 82), autoimmune limbic encephalitis (LE) (n = 8), and acute disseminated encephalomyelitis (ADEM) (n = 10). After the above criteria have been fulfilled, the diagnosis for definite LE could be made when the presence of bilateral brain abnormalities on T2-weighted fluid-attenuated inversion recovery (T2/FLAIR) MRI is highly restricted to the medial temporal lobes. Diagnosis for definite ADEM can be made when all five of the following criteria have been met: (1) a first multifocal, clinical CNS event of presumed inflammatory demyelinating cause; (2) encephalopathy that cannot be explained by fever; (3) abnormal brain MRI: (a) diffuse, poorly demarcated, large (>1–2 cm) lesions predominantly involving the cerebral white matter, (b) T1-hypointense lesions in the white matter in rare cases, (c) deep gray matter abnormalities (e.g., thalamus or basal ganglia) can be present; and (4) reasonable exclusion of alternative causes. The remaining patients were classified as definite ANPRA.
Written informed consent for participation and publication was obtained from involved patients or their legal guardians. This study was approved by the institutional review board of the Second Xiangya Hospital of Central South University (2023- Z0919-02).
Clinical data and laboratory parameters
The following clinical data of antibody-negative AE patients were collected at admission and subsequently analyzed: (1) demographic data, including the age of onset and sex; (2) clinical profiles: a. mRS scores (0–6) and CASE scores (0–27) were obtained at three time points: at admission, at peak, and at the last follow-up; b. RSE; c. the intensive care unit (ICU) admission; (3) prodromal symptoms and onset of symptoms; and (4) the presence of clinical manifestations in the patient based on the nine items of the CASE score was evaluated. RSE was defined as persistent status epilepticus despite using an appropriate dose of ≥2 intravenous antiepileptic drugs, including benzodiazepine.14
Review of brain MRI
The brain MRIs of the patients were obtained using a 3.0-Tesla or 1.5-Tesla MAGNETOM Verio scanner (Siemens, Germany), at the Second Xiangya Hospital of Central South University. The following sequences were acquired: T1-weighted images (T1WI), T2-weighted images (T2WI), fluid-attenuated inversion recovery (FLAIR), diffusion-weighted images (DWI), and gadolinium-enhanced images. The lesions were observed in the cortex, medial temporal, subcortex/white matter, striatum/capsule, infra-tentorium, spine, and parenchymal enhancement. A blinded review of the patient's baseline MRI was conducted independently by two trained neuroradiologists (BHH and YWD).
Antibody testing
All patients received antibody testing in both serum and CSF, which included assessments for neuronal cell-surface antibodies (N-methyl-D-aspartate receptor [NMDAR], leucine-rich glioma inactivated-1 [LGI-1], contactin-associated protein-2 [CASPR2], α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor [AMPAR], γ-aminobutyric acid type A receptor [GABAAR], γ-aminobutyric acid type B receptor [GABABR], dipeptidyl-peptidase-like protein-6 [DPPX], glycine receptor [GlyR]), IgLON family member 5 (IgLON5), and intracellular antibodies (such as glutamic acid decarboxylase 65-kDa isoform [GAD65], SOX1, Hu, Yo, Ri, Ma1, Ma2, CV2, and amphiphysin).
The antibody testing was conducted in the Guangzhou KingMed Center for Clinical Laboratory. The initial screening of serum and/or CSF samples was performed by immunohistochemistry on rat brain sections. Then, the neuronal cell-surface antibodies, including NMDAR, LGI-1, CASPR2, AMPAR, GABAAR, GABABR, DPPX, GlyR, and IgLON5, were tested using indirect immunofluorescence assay (IIFT) fixed by commercial transfected HEK293 assays in, and immunoblotting was applied to detect intracellular antibodies, including GAD65, SOX1, Hu, Yo, Ri, Ma1, Ma2, CV2, and amphiphysin. Positivity was determined by repeat testing (≥2 times per sample). Antibody negativity was defined as the absence of autoimmune antibodies detected in both serum and CSF tests. All patients' serum and CSF samples were tested during this period (after admission but before the commencement of immunotherapy).
Treatment profiles and follow-up outcomes
The following information was obtained through the electronic medical record: whether immunotherapy was administered after admission and whether immunotherapy was initiated within 30 days. In all patients undergoing immunotherapy, corticosteroids, intravenous immunoglobulin (IVIG), and plasmas exchange as first-line immunotherapy,13 rituximab and cyclophosphamide as second-line immunotherapy.15, 16 For first-line immunotherapy, separate or combined use of high-dose methylprednisolone (1000 mg/day, for 3-5 days), intravenous immunoglobulin (2 g/kg over 2–5 days), or therapeutic plasma exchange (1 session every other day for 5–7 cycles) was initiated in our hospital. For second-line immunotherapy, rituximab (375 mg/m2 weekly IV infusion for 4 weeks) or cyclophosphamide (750 mg/m2 monthly for 3–6 months). Early and timely treatment was considered as starting immunotherapy within 30 days of the onset of symptoms.
The follow-up was realized through subsequent outpatient visits. In this study, the long-term outcomes were defined as at least 12 months after the onset of symptoms. Based on mRS scores measured at the last follow-up, patients were divided into groups with favorable (mRS score <3) and poor (mRS score ≥3) long-term outcomes. Two experienced neurologists (QY and TXY) assessed mRS scores based on patients' follow-up results and reached an agreement through discussion when disagreements existed.
The CASE scores were used to assess the functional status of patients. Sub-items of each key item were scored 0–3, and the sum of the nine key items has a maximum score of 27.17, 18 All of the above data were obtained retrospectively from electronic medical records by two experts (BHH and QY) in AE.
The RAPID score and external validation
The RAPID score includes five items (assigning each factor 1 point)—RSE, age of onset≥60 years, ANPRA, infratentorial involvement, and delay of immunotherapy for ≥1 month.14 Two neurologists (TXY and YWD) retrospectively assessed the RAPID scores based on baseline information.
To assess the performance of the RAPID score, the area under the receiver operating characteristic curves (AUCs) and its 95% confidence interval (CI) were calculated. An AUC of 1.0 indicates a perfect prediction, while an AUC of 0.5 suggests a prediction no better than random chance.
Statistical analysis
Categorical variables are presented as numbers and percentages. Parametric continuous variables are shown as the mean ± standard deviation; nonparametric variables are described as the median and interquartile range (IQR) values. Here, t-tests, Mann–Whitney U-tests, or Kruskal–Wallis test for parametric variables, the chi-squared test, or Fisher's exact test for categorical variables were used for the intergroup comparisons, as appropriate. In the univariate analysis of poor long-term outcomes, variables with P values <0.05 were subjected to a multivariate logistic regression analysis. The Statistical Package for the Social Sciences (version 25.0, International Business Machines Corporation, Armonk, NY) was used for all statistical analyses, and p values <0.05 were considered statistically significant.
Results
Demographic data
Table 1 summarizes various demographic information, clinical profiles, results of auxiliary examination, treatment, and follow-up. Among the 100 (47 females and 53 males) antibody-negative AE patients, the median age at disease onset was 38.50 years (IQR 23.00–52.75), with 13 patients being over the age of 60. These 100 antibody-negative AE cases were categorized into three distinct subgroups, with ANPRA representing the majority (82/100, 82%).
| Total (n = 100) | ANPRA (n = 82) | LE (n = 8) | ADEM (n = 10) | p | |
|---|---|---|---|---|---|
| Age in years at onset, median (IQR) | 38.50 (23.00–51.75) | 38.00 (21.75–50.25) | 55.50 (47.50–67.75) | 35.00 (22.50–54.75) | 0.029* |
| Age of onset ≥60 | 13 (13.0%) | 10 (12.2%) | 3 (37.5%) | 0 (0.0%) | 0.053 |
| Sex (%) | 0.312 | ||||
| Female | 47 (47%) | 39 (47.6%) | 2 (25.0%) | 6 (60.0%) | |
| Male | 53 (53%) | 43 (52.4%) | 6 (75.0%) | 4 (40.0%) | |
| Clinical profiles | |||||
| Initial CASE scores, median (IQR) | 6.00 (4.00–8.75) | 6.00 (4.00–9.00) | 7.00 (5.25–7.00) | 5.00 (3.75–6.50) | 0.396 |
| CASE at discharge, median (IQR) | 2.00 (1.00–3.75) | 2.00 (0.00–4.00) | 2.00 (1.00–3.75) | 2.00 (1.00–3.75) | 0.781 |
| Peak CASE scores, median (IQR) | 5.00 (3.00–7.00) | 6.00 (3.00–8.00) | 4.00 (3.25–6.50) | 2.50 (1.00–4.00) | 0.011* |
| Initial mRS scores, median (IQR) | 3.00 (3.00–4.00) | 3.00 (3.00–4.00) | 3.50 (3.00–4.00) | 4.00 (3.00–4.00) | 0.038* |
| mRS at discharge, median (IQR) | 2.00 (1.00–3.00) | 1.50 (0.00–3.00) | 2.00 (1.00–2.00) | 2.00 (2.00–3.00) | 0.183 |
| Peak mRS scores, median (IQR) | 3.00 (3.00–3.00) | 3.00 (3.00–3.00) | 3.00 (2.00–3.75) | 2.00 (1.00–2.25) | 0.004* |
| RAPID score ≥2 (%) | 64 (64%) | 60 (73.2%) | 4 (50%) | 0 (0%) | <0.001** |
| RSE (%) | 16 (16.0%) | 14 (17.1%) | 2 (25.0%) | 0 (0.0%) | 0.137 |
| ICU admission rate (%) | 10 (10.0%) | 9 (11.0%) | 0 (0.0%) | 1 (10.0%) | 0.413 |
| Prodromal symptoms (%) | 51 (51.0%) | 41 (50.0%) | 3 (37.5%) | 7 (70.0%) | 0.348 |
| Onset of symptoms | |||||
| Epileptic seizures (%) | 34 (34.0%) | 30 (36.6%) | 4 (50.0%) | 0 (0.0%) | 0.009* |
| Psychiatric symptoms (%) | 58 (58.0%) | 52 (63.4%) | 6 (75.0%) | 0 (0.0%) | <0.001** |
| Memory problems (%) | 5 (5.0%) | 4 (4.9%) | 1 (12.5%) | 0 (0.0%) | 0.425 |
| Gait instability and ataxia (%) | 17 (17.0%) | 7 (8.5%) | 0 (0.0%) | 10 (100.0%) | <0.001** |
| Symptom profiles | |||||
| Seizure (%) | 44 (44.0%) | 38 (46.3%) | 6 (75.0%) | 0 (0.0%) | <0.001* |
| Memory dysfunction (%) | 33 (33.0%) | 28 (34.1%) | 4 (50.0%) | 1 (10.0%) | 0.138 |
| Psychiatric symptoms (%) | 59 (59.0%) | 52 (63.4%) | 7 (87.5%) | 0 (0.0%) | <0.001** |
| Impaired consciousness (%) | 26 (26.0%) | 22 (26.8%) | 0 (0.0%) | 4 (40.0%) | 0.056 |
| Speech dysfunction (%) | 57 (57.0%) | 50 (61.0%) | 5 (62.5%) | 2 (20.0%) | 0.041* |
| Dyskinesia/dystonia (%) | 63 (63.0%) | 50 (61.0%) | 4 (50.0%) | 9 (90.0%) | 0.105 |
| Gait instability and ataxia (%) | 29 (29.0%) | 18 (22.0%) | 1 (12.5%) | 10 (100.0%) | <0.001** |
| Brainstem dysfunction (%) | 5 (5.0%) | 5 (6.1%) | 0 (0.0%) | 0 (0.0%) | 0.360 |
| Weakness (%) | 26 (26.0%) | 16 (19.5%) | 0 (0.0%) | 10 (100.0%) | <0.001** |
| CSF profiles | |||||
| CSF protein level (mg/dL) | 27.00 (19.25–40.00) | 25.00 (16.00–36.10) | 37.00 (22.68–60.25) | 35.00 (23.75–51.00) | 0.080 |
| Elevated CSF protein level (%) | 26 (26.0%) | 17 (20.7%) | 4 (50.0%) | 5 (50.0%) | 0.051 |
| Elevated CSF leucocyte level (%) | 24 (24.0%) | 19 (23.2%) | 2 (25.0%) | 3 (30.0%) | 0.894 |
| CSF leucocyte level (cells/μL) | 0.00 (1.00–4.00) | 0.00 (0.00–4.00) | 2.50 (0.00–7.00) | 2.00 (0.00–20.00) | 0.406 |
| MRI profiles | |||||
| Any abnormality in MRI (%) | 56 (56.0%) | 38 (46.3%) | 8 (100.0%) | 10 (100.0%) | <0.001** |
| Cortex (%) | 17 (17.0%) | 11 (13.4%) | 1 (12.5%) | 5 (50.0%) | 0.036* |
| Medial temporal (%) | 13 (13.0%) | 6 (7.3%) | 7 (87.5%) | 0 (0.0%) | <0.001** |
| Subcortex/white matter (%) | 31 (31.0%) | 22 (26.8%) | 3 (37.5%) | 6 (60.0%) | 0.111 |
| Striatum/capsule (%) | 7 (7.0%) | 7 (8.5%) | 0 (0.0%) | 0 (0.0%) | 0.236 |
| Thalamus (%) | 3 (3.0%) | 3 (3.7%) | 0 (0.0%) | 0 (0.0%) | 0.546 |
| Infra-tentorium (%) | 7 (7.0%) | 1 (1.2%) | 1 (12.5%) | 5 (50.0%) | <0.001** |
| Spine (%) | 7 (7.0%) | 0 (0.0%) | 0 (0.0%) | 7 (70.0%) | <0.001** |
| Parenchymal enhancement (%) | 2 (2.0%) | 2 (2.4%) | 0 (0.0%) | 0 (0.0%) | 0.669 |
| Treatment profiles | |||||
| Immunotherapy (%) | 62 (62.0%) | 47 (57.3%) | 5 (62.5%) | 10 (100.0%) | 0.006* |
| Immunotherapy within 30 days (%) | 41 (41.0%) | 27 (32.9%) | 5 (62.5%) | 9 (90.0%) | 0.001* |
| Only first-line treatment (%) | 62 (62.0%) | 47 (57.3%) | 5 (62.5%) | 10 (100.0%) | 0.006* |
| Second-line treatment (%) | 1 (1.0%) | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) | 0.819 |
| Follow-up outcomes | |||||
| Interval between last follow-up and disease onset, months | 52.50 (33.00–71.00) | 55.00 (33.00–71.00) | 38.00 (27.00–70.75) | 54.00 (33.00–75.00) | 0.740 |
| CASE scores at the last follow-up, median (IQR) | 3.00 (1.00–6.00) | 4.00 (2.00–6.25) | 1.50 (1.00–3.50) | 2.00 (1.00–4.00) | 0.046* |
| mRS scores at the last follow-up, median (IQR) | 2.00 (1.00–3.00) | 3.00 (1.00–3.00) | 1.50 (1.00–2.00) | 1.00 (0.00–2.25) | 0.038* |
| mRS scores ≥3 at the last follow-up (%) | 49 (49%) | 46 (56.1%) | 1 (12.5%) | 2 (20%) | 0.006* |
| mRS scores<3 at the last follow-up (%) | 51 (51%) | 36 (43.9%) | 7 (87.5%) | 8 (80%) | 0.022* |
- ADEM, acute disseminated encephalomyelitis; ANPRA, antibody-negative probable AE; CASE, Clinical Assessment Scale in Autoimmune Encephalitis; CSF, cerebrospinal fluid; ICU, intensive care unit; IQR, interquartile range; LE, autoimmune limbic encephalitis; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; RSE, Refractory status epilepticus.
- * p < 0.05;
- ** p < 0.001.
Clinical characteristics
The ICU admission rate was 10%, with 16 (16/100, 16%) individuals experiencing RSE. A total of 51 (51/100, 51%) patients reported prodromal symptoms, such as dizziness, headache, or fever, preceding the clinical onset of AE. The onset of symptoms displayed variation among antibody-negative AE patients, with psychiatric manifestations being the most frequently observed (58/100, 58%).
Clinical symptoms exhibited by patients with antibody-negative AE were collected according to the nine entries of the CASE score. The most common clinical manifestation in all patients was dyskinesia/dystonia (63/100, 63%), followed by psychiatric symptoms (59/100, 59%), speech dysfunction (57/100, 57%), seizure (44/100, 44%), memory dysfunction (33/100, 33%), impaired consciousness (26/100, 26%), weakness (26/100, 26%), gait instability and ataxia (29/100, 29%), and brainstem dysfunction (5/100, 5%).
For the total antibody-negative AE patients (n = 100), the median CASE score at admission, at discharge, and at peak was 6.00 (IQR 4.00–8.75), 2.00 (IQR 1.00–3.75), and 8.00 (IQR 3.00–7.00), respectively (Fig. 2A). The median initial mRS score was 3.00 (IQR 3.00–4.00), the median mRS score at discharge was 2.00 (IQR 1.00–3.00), and the median peak mRS score was 3.00 (IQR 3.00–3.00) (Fig. 2B).
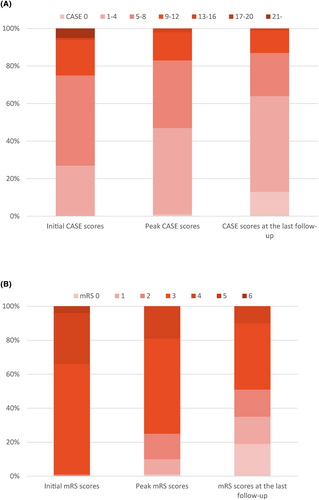
Auxiliary examination
CSF and serum samples of all patients were collected at admission before the onset of treatment. The median CSF protein level (mg/dL) was 27 (IQR 19.25–40.00) and 26 (26/100, 26%) had elevated CSF protein level (≥40 mg/dL). The median CSF leucocyte level (cells/μL) was 0.00 (IQR 1.00–4.00) and 24 (24/100, 24%) had elevated CSF leucocyte level (≥5 cells/μL). There was no significant difference in the CSF profiles among the three subtypes.
Among all patients who received MRI, abnormal MRI findings suggestive of these patients were noted in 56 (56/100, 56%) patients. The most common lesion was subcortex/white matter (31/100, 31%), followed by cortex (17/100, 17%), medial temporal lobe (MTL, 13/100, 13%), striatum/capsule (7/100, 7%), infra-tentorium (7/100, 7%), spine (7/100, 7%), thalamus (3/100, 3%), and parenchymal enhancement (2/100, 2%). Brain MRI findings of representative patients in each disease subtype have been shown in Fig. 3.
Sixty-two (62/100, 62%) patients underwent immunotherapy, while 41 (41/100, 41%) of them within 30 days of disease onset. Merely 1 (1/100, 1%) of patients underwent second-line immunotherapy. The median CASE score and mRS score at the last follow-up were 3.00 (IQR 1.00–6.00) and 2.00 (IQR 1.00–3.00), respectively. The frequency of poor long-term outcomes (mRS scores ≥3) was 49%.
Subgroups
Comparisons of clinical, laboratory, brain MRI, treatment, and outcome profiles among the three AE subtypes are summarized in Table 1. Among 100 antibody-negative AE patients, 82 (82/100, 82%) were classified as ANPRA, 8 (8/100, 8%) as LE, and 10 (10/100, 10%) as ADEM subtype. There were significant differences in the symptom profiles among the three subtypes. Within the ANPRA subtype, 36.3% (30/82) presented with epileptic seizures as the initial symptomatic manifestation, representing the largest subgroup in antibody-negative AE with seizure onset. Psychiatric symptoms were the initial presentation in 52 cases (52/82, 63.4%) of the ANPRA subtype, constituting the most prevalent symptom in both ANPRA (52/82, 63.4%) and LE (7/8, 87.5%) subtypes. Within the ADEM subtype, primary symptoms included gait instability and ataxia (10/10, 100%) along with weakness (10/10, 100%), with no reported cases of psychiatric symptoms (0/10, 0%). In addition, MRI revealed abnormalities in all cases in the LE and ADEM subtypes. The ANPRA subtype exhibited higher CASE scores both at peak and at the last follow-up. Moreover, it was associated with elevated mRS scores at the last follow-up and a greater frequency (56.1%) of poor long-term outcomes. There was a significant difference in the disease severity among the three subtypes. The median follow-up time for 100 antibody-negative AE patients was 52.50 (IQR 33.00–71.00) months.
Factors associated with the long-term outcomes of antibody-negative AE
In univariate analysis, the patients with poor long-term outcomes were associated with age of onset; initial CASE scores; peak CASE scores; peak mRS scores; RSE; disease subtypes; CSF leucocyte level; immunotherapy within 30 days; immunotherapy, compared to the patients with favorable long-term outcomes (Supplemental Table S1).
At the last follow-up, the most common symptom in all patients (n = 100) was psychiatric symptoms (48/100, 48%), and the least common symptom was brainstem dysfunction (1/100, 1%). In patients with poor long-term outcomes (n = 49), the most common symptoms were psychiatric symptoms (33/49, 67.3%) and dyskinesia/dystonia (33/49, 67.3%) (Supplemental Fig. S1A). Among the three subtypes, psychiatric symptoms (45/82, 54.9%) were most common in the ANPRA subtype, memory dysfunction (4/8, 50%) in the LE subtype, and dyskinesia/dystonia (8/10, 80%) and weakness (8/10, 80%) in the ADEM subtype (Supplemental Fig. S1B and Supplemental Table S2).
Logistic regression analysis of risk factors for poor long-term in patients with antibody-negative AE
In subsequent logistic regression analysis revealed a significant positive correlation between peak CASE scores (OR 1.846, 95% CI 1.163 to 2.930, p = 0.009) and poor long-term outcomes. Conversely, immunotherapy initiated within 30 days (OR 0.210, 95% CI 0.046 to 0.948, p = 0.042) exhibited a significant negative association with poor long-term outcomes (Table 2).
| B (95% CI) | p | |
|---|---|---|
| Age of onset | 0.967 (0.932–1.002) | 0.068 |
| Initial CASE scores | 0.815 (0.619–1.073) | 0.145 |
| Peak CASE scores | 1.846 (1.163–2.930) | 0.009* |
| Peak mRS scores | 1.470 (0.466–4.642) | 0.511 |
| RSE | 7.018 (0.593–83.021) | 0.122 |
| Subtypes | ||
| ANPRA | 0.323 | |
| LE | 0.117 (0.005–2.516) | 0.170 |
| ADEM | 2.268 (0.191–26.905) | 0.516 |
| CSF leucocyte level | 0.984 (0.942–1.028) | 0.468 |
| Immunotherapy | 0.759 (0.178–3.238) | 0.709 |
| Immunotherapy within 30 days | 0.210 (0.046–0.948) | 0.042* |
- * p < 0.05.
Validation and explanation of RAPID scores
The AUCs of the RAPID scores are shown in Fig. 4. The RAPID scores proved to be a significant predictor of poor long-term antibody-negative AE in our cohort (AUC: 0.74, 95% CI: 0.64–0.84, p < 0.0001). ROC analysis returned that the RAPID scores cutoff of 1.5 best discriminated the group with poor long-term outcomes (sensitivity 85.7%, specificity 56.9%). The association of the RAPID scores with long-term mRS scores in antibody-negative AE is shown in Fig. 5 (Cuzick–Wilcoxon test p = 0.035).
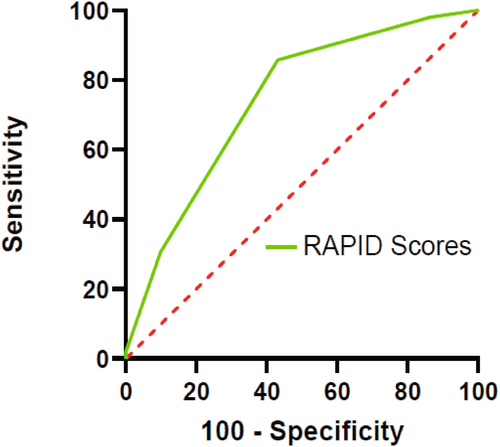

Discussion
This study provided a comprehensive analysis of the clinical features, immunotherapeutic approaches, and prognosis of antibody-negative AE within a sizable cohort. The significant insights gained from this research offered crucial information regarding the long-term outcomes and primary clinical presentations associated with antibody-negative AE. In our study, the age of onset was in the middle-aged range, which contrasts with earlier findings on AE.19, 20 However, some studies showed that the age of onset in their study cohorts is younger.14, 21, 22 The difference in age distribution may be due to the selection of different age groups or to a different pathogenesis than that of antibody-positive AE.
In comparison with a previous study of antibody-negative AE,14 patients with antibody-negative AE in our study more frequently had dyskinesia/dystonia (63% vs 25.2%). This may be attributed to that more patients with ADEM subtypes were enrolled in our study than in previous studies14 (10% vs 4.8%). Most of whom had dyskinesia/dystonia. Within the ANPRA and LE subtypes, psychiatric symptoms remained the most commonly observed, which is consistent with the results of other AE studies.23 In addition, the frequency of psychiatric symptoms was the highest among the symptoms still present in the patient at the last follow-up visit. Moreover, various studies consistently highlighted memory issues as the most frequently reported symptom, irrespective of serostatus.14, 24 Compared to two previous studies, patients with antibody-negative AE in our study less frequently had RSE (16% in our study vs 29.9% in the study by Berger et al.14, 24 and 93.2% in the study by Lee et al.14). These disparities could be attributed to variations in patient demographics and inclusion criteria.
The spectrum of AE symptoms is wide-ranging, often leading to challenges in clinically distinguishing antibody-negative cases from antibody-positive ones due to the absence of distinctive features.25 Compared to a previous study, our cohort exhibited a lower incidence of memory deficits in patients with the LE subtype (50% vs 100%26). In total, 87.5% of patients showed bilateral temporal lobe abnormalities on MRI T2/FLAIR, aligning with previous observations.26 Our findings endorsed previously proposed diagnostic criteria for antibody-negative LE,4 emphasizing that antibody positivity was not obligatory for its diagnosis. Instead, the presence of bilateral temporal lobe hyperintensities remained a highly specific indicator of LE. Limited research has been conducted on antibody-negative ADEM. Following the 2016 diagnostic criteria, our study encompassed cases of ADEM lacking detectable antibodies,4 compared to LE and ANPRA subtypes, with all ADEM patients experiencing initial gait instability, accompanied by weakness. MRI abnormalities were observed in all cases and lesions were located in the cortex, and spine, followed by other lesions in the cortex, subcortex/white matter, and infra-tentorium. These findings aligned with previous research.14 The clinical symptoms of ADEM were varied,27 but in this study gait instability and weakness were more prominent. This may be attributed to the disruptive impact of these symptoms on daily life, prompting patients to seek clinical attention promptly after their onset, even though other symptoms may have also been present but less pronounced.
Immunotherapy played a pivotal role in the management of AE.5 Our study demonstrated that early initiation of immunotherapy within 30 days was correlated with favorable long-term outcomes in antibody-negative cases, aligning with prior research findings.14 In our study, despite ANPRA not having the highest baseline CASE score, it exhibited the lowest rate of early application of immunotherapy, potentially contributing to a less favorable long-term prognosis. The incidence of favorable long-term outcomes was lower in our antibody-negative AE cohort compared to another study (51% vs 57%, respectively), despite our cohort having less severe baseline CASE scores [6.00 (IQR 4.00–8.75) vs 13 (IQR 9–20)].14 The higher rate of poor long-term prognosis in our study may be because of the smaller number of patients receiving immunotherapy (63% vs 96.6%) and the longer follow-up period (median 52.5 months vs median 24 months) than the study by Lee et al.14 Acute phase disease severity was an important prognostic factor for AE.28 This was consistent with the conclusion reached in our study that higher peak CASE was associated with poorer long-term prognosis. Therefore, the early diagnosis, timely initiation of primary immunotherapy, and precise identification of disease severity might be crucial to improving the outcomes of antibody-negative AE.
Currently, most studies focus on second-line immunotherapy in antibody-positive AE, particularly in NMDAR encephalitis. It is generally believed that second-line immunotherapy should be considered only after the failure of first-line immunotherapy, while the definition of first-line immunotherapy failure varied among studies.16, 29 Nonetheless, some research indicated the necessity of early initiation of second-line immunotherapy to improve prognosis.30 However, the use of second-line immunotherapy or aggressive therapy in antibody-negative AE was relatively limited due to the lack of guidance from positive antibody results, coupled with the cost and side effects of second-line immunotherapy drugs, resulting in low usage rates. In our study, only one individual received second-line immunotherapy. A large prospective study based on antibody-negative AE14 has shown the efficacy of second-line immunotherapy in these patients. In a cohort study on LE,15 43.8% of antibody-negative LE patients showed improvement after receiving second-line immunotherapy. However, research on the efficacy of second-line immunotherapy in antibody-negative AE remains insufficient, indicating the need for more evidence from future evidence-based medicine.
In Lee et al.'s study cohort of 147 patients with a cutoff of 2, the RAPID score demonstrated a sensitivity of 81.3% and specificity of 66.3% in predicting a poor 2-year outcome (mRS ≥3). In our cohort, the AUCs of the RAPID score were 0.74 (95% CI: 0.64–0.84, p < 0.0001) (Fig. 4). ROC analysis returned that the RAPID scores cutoff of 1.5 best discriminated the group with poor long-term outcomes (sensitivity 85.7%, specificity 56.9%), which was consistent with the cutoff derived from the cohort with Lee et al. Of the five factors in the RAPID score, delay of immunotherapy ≥1 month was a recognized factor associated with poor prognosis,31 which underscored the significance of prompt immunotherapy for the long-term outcome of patients. As the inaugural disease severity assessment scale was tailored for antibody-negative AE, the RAPID score addressed a previous research gap in this area. Our study presented the inaugural external validation of the RAPID score, verifying its efficacy in prognosticating long-term outcomes. This implied that in clinical settings, the RAPID score may serve as a valuable and expeditious tool for gauging disease severity in antibody-negative AE patients. This, in turn, could facilitate more targeted and assertive administration of immunotherapy to enhance the long-term prognosis for individuals affected by antibody-negative AE.
Several limitations should be noted. First, this was a retrospective study conducted at a tertiary medical center. The single-center and retrospective properties of the study may have potential selection bias. Besides, AE has been regarded as one of the most expensive neurological diseases, and the expensive price of second-line immunotherapy drugs posed a huge economic burden for individuals, particularly in developing countries. The relatively smaller percentage of second-line therapy in our study might underestimate its actual efficacy. Further prospective studies with more usage of second-line immunotherapy are warranted. Lastly, it was a pity that objective scales for assessing cognitive function and psychiatric symptoms were not applied as part of the standard clinical process of our center. Prospective, multicentric studies involving larger sample sizes should pay more attention to this issue in the future.
Conclusion
In summary, our study shed light on the clinical characteristics and prognostic factors of antibody-negative AE. We found that initiating immunotherapy within 30 days was associated with improved long-term outcomes, while elevated peak CASE scores correlated positively with unfavorable long-term outcomes in antibody-negative AE patients, which highlighted the critical importance of early intervention. Additionally, our findings revealed that the ANPRA subtype exhibited a poorer prognosis compared to other subtypes, underscoring the need for tailored treatment strategies. External validation of the RAPID score in our cohort and demonstration of good performance showed that the use of rapid scoring may be able to help clinically stratify patients for treatment selection and inform outcomes. Further investigations into the underlying mechanisms of antibody-negative AE are warranted, and prospective studies should focus on refining treatment protocols to enhance patient outcomes. Binhong Han and Yuwei Dai are contributed equally to this work.
Author Contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by BHH, YWD, JP, QY, and TXY. The first draft of the manuscript was written by BHH and YWD. The final draft was revised and reviewed by LY. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgements
We thank all study participants and their families for making our research possible. This work was supported by the Natural Science Foundation of China (No. 81971696 to L.Y.) and The Natural Science Foundation of Hunan Province, China (No. 2022JJ30861 to L.Y.).




