A multi-omics study to monitor senescence-associated secretory phenotypes of Alzheimer's disease
Abstract
Objective
Alzheimer's disease (AD) is characterized by the progressive degeneration and damage of neurons in the brain. However, developing an accurate diagnostic assay using blood samples remains a challenge in clinic practice. The aim of this study was to explore senescence-associated secretory phenotypes (SASPs) in peripheral blood using mass spectrometry based multi-omics approach and to establish diagnostic assays for AD.
Methods
This retrospective study included 88 participants, consisting of 29 AD patients and 59 cognitively normal (CN) individuals. Plasma and serum samples were examined using high-resolution mass spectrometry to identify proteomic and metabolomic profiles. Receiver operating characteristic (ROC) analysis was employed to screen biomarkers with diagnostic potential. K-nearest neighbors (KNN) algorithm was utilized to construct a multi-dimensional model for distinguishing AD from CN.
Results
Proteomics analysis revealed upregulation of five plasma proteins in AD, including RNA helicase aquarius (AQR), zinc finger protein 587B (ZNF587B), C-reactive protein (CRP), fibronectin (FN1), and serum amyloid A-1 protein (SAA1), indicating their potential for AD classification. Interestingly, KNN-based three-dimensional model, comprising AQR, ZNF587B, and CRP, demonstrated its high accuracy in AD recognition, with evaluation possibilities of 0.941, 1.000, and 1.000 for the training, testing, and validation datasets, respectively. Besides, metabolomics analysis suggested elevated levels of serum phenylacetylglutamine (PAGIn) in AD.
Interpretation
The multi-omics outcomes highlighted the significance of the SASPs, specifically AQR, ZNF587B, CRP, and PAGIn, in terms of their potential for diagnosing AD and suggested neuronal aging-associated pathophysiology.
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder that primarily affects the brain, one of the main characteristics of AD, is the gradual degeneration and damage of neurons within the brain.1 The limitations of current targeted therapeutic strategies in effectively slowing down cognitive decline in Alzheimer's disease have raised concerns. To address these concerns and improve therapeutic outcomes, it is essential to analyze and understand the molecular and cellular components of the Alzheimer's disease brain at both asymptomatic and symptomatic stages of disease progression.
It is well-known that aging is a significant risk factor for the development of AD. Emerging evidence indicates that the number of cells expressing biomarkers of cellular senescence increases in tissues with aging, which implies that cellular senescence is an important player in organismal aging.2 Senescent cells progressively accumulate in aging tissues, contributing to the development of age-related diseases through their secretion of inflammatory factors.3 Recent evidence suggests that the increased inflammatory response observed in AD could be influenced, at least in part, by an aggravation of cellular senescence triggered by amyloid-β and/or tau pathology. However, the exact reasons for this association are still not fully understood.4 Senescent cells express senescence-associated secretory phenotypes (SASPs), leading to the increased secretion of various proteins and metabolites, including inflammatory cytokines, chemokines, growth factors, matrix metalloproteinases, and lipids into the surrounding extracellular fluid.5 Thus, monitoring SASP factors shall offer a promising and feasible approach to evaluate the senescence status of aging or dementia cohort. Recent research indicates that experts consider proteomic and metabolic approaches essential for developing senescence-related biomarkers for future therapeutic interventions aimed at targeting senescent cells.6
Over the past few decades, there have been numerous attempts to understand the mechanisms of aging through omics approaches. High-resolution mass spectrometry (HRMS) has provided opportunities to quantify aging beyond using a limited number of well-studied and selective biomarkers.7, 8 This approach can reveal age-related changes at early stages when traditional biomarkers are not yet detectable.9 Recent research studies highlight the potential of proteomics and metabolomics in AD.10, 11 Proteomics involves protein digestion followed by mass spectrometry analysis to identify various proteins based on peptide sequences.10, 12 Mass spectrometry-based proteomics in AD provides insights into protein networks related to amyloid and tau pathways, as well as other key processes like RNA splicing, lipid metabolism, and synaptic function.13 Metabolomics aims to identify and quantify small-molecule metabolites in samples, revealing disruptions in sphingolipid metabolism in early stages of AD.14 Nevertheless, the utilization of single omics approaches yields merely a fragmented comprehension of the biological system, thereby susceptible to underlying biases in interpretation. Consequently, the integration of proteomics and metabolomics data provides a more comprehensive understanding of neuronal biology, potentially leading to the discovery of novel biomarkers for AD diagnosis.
Blood biomarkers play a crucial role in not only categorizing patients but also identifying diseases and monitoring disease progression. They significantly contribute to the decision-making process regarding effective treatment strategies. Our previous metabolomics study with plasma samples from AD patients revealed a significant upregulation of phenylacetylglutamine (PAGIn), a gut–microbiota-derived metabolite. We also observed a positive correlation between PAGIn and the Aβ42/Aβ40 ratio, suggesting its involvement in one or more pathophysiological mechanisms underlying AD.15 Ongoing research aims to identify the senescence-associated secretory molecules that shall disclose the underlying biological processes of AD through a comprehensive omics approach. Moreover, the analysis of blood samples is an extensively utilized diagnostic procedure across multiple medical disciplines. This approach could enable deep insights into characterizing the blood proteome and metabolome and shall greatly benefit biological research.
As the field of molecular science has witnessed a paradigm shift with the advent of machine learning algorithms, facilitating data-driven scientific exploration. The ongoing research focuses on extracting meaningful MS features and discriminating between samples based on these features.16 Therefore, it is necessary to utilize a machine learning-based approach to assess the stability and predictive value of the resulting list of biomarkers. In a recent study, a machine learning classification algorithm, specifically the K-nearest neighbor (KNN) algorithm, has been implemented to classify and detect the main four early stages of AD: non-dementia or normal, very mild dementia, mild dementia, and moderate dementia. This study leveraged the Kaggle dataset and achieved promising results in terms of accuracy and discrimination sensitivity.17
We performed a comprehensive analysis of the proteome and metabolome using blood samples from individuals with AD and cognitively normal (CN). Machine learning-based KNN algorithm was introduced to extract the MS features with significance, enhance the accuracy of model in a multi-dimensional fashion and also promote novel strategies for AD diagnosis. This analysis not only motivated us to visit the multi-omics phenotype associated with AD and explore the aging related SASP molecules that may elucidate the underlying pathogeneses of AD.
Materials and Methods
Participants recruitment and blood sample collection
Human plasma and serum specimens were collected under the auspices of Shanghai Baoshan Luodian Hospital. The study was approved by the local research ethics committee (Approval document number: No. LDYY-KY-2020-04). Informed consent was obtained from all research participants, either through written consent or verbal agreement, ensuring their understanding and voluntary participation in the study. Basic demographic data were documented along with Alzheimer's Disease Assessment Scale-Cognitive (ADAS-Cog) Subscale. Scores on the ADAS-Cog scale span from 0 to 75, where a score equal to or exceeding 18 signifies recognition impairment, qualifying individuals for inclusion in the Alzheimer's disease (AD) group. Conversely, a score below 18 signifies normal recognition ability and qualifies participants for inclusion in the cognitively normal (CN) group. Based on the wills of the control volunteers, some were willing to provide either plasma or serum samples. Then, collections of control plasma and control serum were performed separately. Consequently, two separate enrollment sessions were conducted: plasma samples were collected from the CN1 group, while serum samples were obtained from the CN2 group.
Collected samples were frozen and stored at −80°C in aliquots of polyethylene tubes until use. As a quality control measure, samples from each participant were pooled to create quality control samples. These samples were utilized to evaluate downstream sample preparation and MS measurements' stability, ensuring accurate and reliable results. Overall, this study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, promoting the ethical conduct of research and safeguarding the welfare of the participants.
Plasma protein extraction and digestion
Protein quantification used the Bicinchoninic Acid (BCA) assay (Beyotime, P0012). To preserve the integrity of low-abundance proteins, we chose not to deplete high-abundance proteins during sample preparation. Each sample had 100 μg of plasma protein diluted with 8 M urea and 0.1% FA to a final volume of 90 μL. The pH was adjusted to approximately 8.0 with 10 μL of 1 M triethylammonium bicarbonate (TEAB) buffer (Sigma, T7408). To reduce protein disulfide bonds, 2 μL of 0.5 M tris(2-carboxyethyl) phosphine (TCEP) reagent (Thermo, 77720) was added and incubated at 500 rpm and 37°C for 1 h. After cooling, 4 μL of 1 M chloroacetamide (CAA) was mixed for 40 min. Proteins were precipitated with 700 μL of pre-cooled acetone at −20°C for at least 4 h, then dissolved and washed with pre-cooled 90% acetone twice. After evaporating residual acetone, proteins were reconstituted with 100 μL of 100 mM TEAB buffer. Trypsin (Promega, V5113) was added and incubated for 16 h at 37°C. The resulting peptide mixture was desalted, dried, and redissolved in 0.1% FA. Final concentration of 500 ng/μL was achieved.
Plasma proteomics analysis
Peptide concentration was determined with a Nanodrop spectrophotometer (Thermo Fisher Scientific). For each sample, 1 μg of peptide was injected. The LC–MS instrumentation used was an EASY-nLC 1200 coupled to a Q Exactive 480 Orbitrap mass spectrometer (Thermo Fisher Scientific, USA). Purified peptides were separated on nano HPLC-columns (Acclaim PepMapTM RSLC, 75 μm × 25 cm, nanoViper, Thermo Scientific, USA) and eluted using a gradient of buffer A and buffer B over 60 min. MS data acquisition followed the Top10 data-dependent MS/MS scan method. Target values for full-scan MS spectra were set at 3 × 106 in the range of 400-1650 m/z. Fragmentation of precursor ions was achieved through higher energy C-trap dissociation. MS/MS scans were performed at a resolution of 15,000 at m/z 200. A control step was conducted using a 500 ng Hela Protein Digest standard from ThermoScientific to assess measurement quality.
Database search of proteins
Proteomics MS data were acquired and processed using the Xcalibur data visualization platform and Proteome Discover 2.5, both from Thermo Scientific. To identify peptides, extensive searches were performed against the protein database of Homo sapiens (sp_canonical, TAXID = 9606) obtained from the UniProt FASTA database. A stringent false discovery rate (FDR) of 0.01 was applied at both the protein and peptide levels to ensure rigorous quality control. Peptides with a minimum length of 6 amino acids were considered, and FDR estimation was performed using a decoy database. Enzymatic specificity was defined, with trypsin selected as the protease. Search parameters allowed for up to two missed cleavages and had specified precursor and fragment mass deviations. These parameters ensured accurate and reliable peptide and protein identification in the proteomics analysis.
AD diagnostic model based on plasma proteomics
ROC curves can be used to evaluate the diagnostic or predictive capabilities of screening tests or differential proteins for Alzheimer's disease. In transfer learning, the proteomics mass spectrometry dataset was partitioned into training, testing, and validation sets following Shen's method for small sample sizes. The training group comprised 52% of the data, the testing group 17%, and the validation group 31%.18 To enhance accuracy, the KNN algorithm was utilized with a logarithmic transformation applied to normalize variables.19 KNN analysis was performed using the SPSS 18.0 Modeler platform (SPSS Software Inc., USA) and a minimum k of 3 and maximum of 5. Distances were computed with Euclidean measurement, utilizing a 10-fold cross-validation technique. Probabilities for targeted biomarkers represented the constructed models.
Serum metabolomics analysis and relative response calculation
The metabolomic analysis followed established protocols as previously reported.15 Serum metabolites were extracted and analyzed using a UPLC-MS system (Dionex Ultimate 3000, Q-Exactive Plus, Thermo Scientific). Thermo Compound Discover 3.1 (Thermo Scientific, USA) was utilized for MS feature extraction and compound identification. 2-Chloro-L-phenylalanine served as the internal standard for MS stability evaluation. Semiquantification was conducted by determining the relative responses (Rel. Res.) of the designated compounds. Additionally, the calculated Rel. Res. values were used to examine the significant differences between the AD and CN groups.
Statistical analysis and graphics
The statistical analysis involved the use of Mann–Whitney U-tests to compare the quantitative variables between the AD and CN groups. The up or down-regulation of 28 plasma protein biomarkers was visualized using boxplots. The cross-sectional correlation between molecules and MS intensities was assessed using Spearman's correlation analysis. Logistic regression models were used to explore the cross-sectional association between molecules with statistical significance. Graphical representations were performed using GraphPad Prism 9 (GraphPad Software Inc., USA). KNN analysis was performed using IBM SPSS Statistics Modeler 18.0 (SPSS Software Inc., USA), while nonlinear surface fitting models were constructed using Origin 2021 (OriginLab Corporation, USA).
Results
Demographic characteristics of AD patients and cognitively controls
The assessment scores of ADAS-Cog Subscale demonstrated that the average evaluation scores were 72.7 ± 5.6 for the AD group (N = 29), 6.5 ± 6.5 for the CN1 group (N = 29), and 6.2 ± 6.3 for the CN2 group (N = 30). The average age for the AD group was 70.5 ± 5.1 years, for the CN1 group was 70.7 ± 5.1 years, and for the CN2 group was 70.7 ± 5.1 years. Additionally, among the three study groups, 62% of females were in the AD group, 59% of females were in the CN1 group, and 60% of females were in the CN2 group. The average duration of education was 4.2 ± 2.4 years in the AD group, 5.6 ± 3.6 years in the CN1 group, and 5.7 ± 3.4 years in the CN2 group. Statistical analysis indicated no significant differences in age distribution and duration of education between each pair of groups.
In a previous study, metabolomics analysis was performed on plasma samples from the AD and CN1 groups, while ELISA was used to measure the levels of Aβ42, Aβ40, and T-tau in plasma to distinguish between the groups.15 In this work, a proteomics analysis was performed on plasma samples of the AD and CN1 groups, and a metabolomics analysis was conducted on serum samples of the AD and CN2 groups. Detailed information and values were summarized in Table 1.
| Characteristics | AD | CN 1 | CN 2 |
|---|---|---|---|
| Number (N) | 29 | 29 | 30 |
| ADAS-Cog score (Mean ± SD) | 72.7 ± 5.6 | 6.5 ± 6.5 | 6.2 ± 6.3 |
| Age (y) | 70.5 ± 5.1 | 70.7 ± 5.1 | 70.7 ± 5.1 |
| Education (y) | 4.2 ± 2.4 | 5.6 ± 3.6 | 5.7 ± 3.4 |
| Gender (female/male, percentage) | 18/11, 62% | 17/12, 59% | 18/12, 60% |
| Aβ 40 (pg/mL)1 | 333.91 ± 94.16 | 303.07 ± 114.86 | n.a. |
| Aβ 42 (pg/mL)1 | 9.42 ± 11.43 | 3.73 ± 2.72 | n.a. |
| T-tau (pg/mL)1 | 1199.26 ± 959.95 | 2217.14 ± 975.05 | n.a. |
| Collected Specimen | Plasma and serum | Plasma | Serum |
| Omics experiment | Metabolomics | n.a. | Metabolomics |
| Proteomics | Proteomics | n.a. |
- n.a., not applicable.
- 1 The levels of Aβ40, Aβ42, and T-tau in plasma samples were determined using ELISA, as reported in a previous study.15
Plasma proteome profiling by LC–MS/MS
The raw files containing proteomics data were collected once the MS acquisition was completed. Proteome Discoverer 2.5 software (PD 2.5, Thermo Scientific, USA) was utilized to process peptide signals, annotate unique peptides to their corresponding proteins, and provide relative responses for each identified protein. Data-dependent analysis (DDA) enabled the identification and annotation of 516 non-depleted plasma proteins through database searching. An overview of the proteomics profile of Alzheimer's disease (AD) was depicted in Figure 1A, showcasing the average intensities of log-transformed plasma proteins (N = 516). In this analysis, the most abundant protein in plasma was albumin (ALB), which had a log value of MS abundance of 11.87. Conversely, the protein with the lowest log value of MS abundance was Talin-1 (TLN 1), which had a value of 5.27.
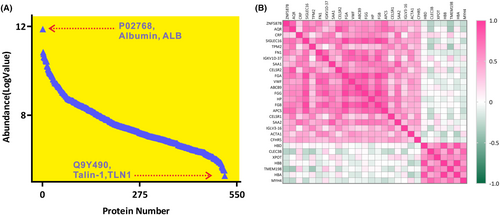
Data process and differentially expressed proteins analysis
All proteomics data underwent normalization using QC samples as part of the quantitation workflow in PD 2.5, resulting in the generation of normalized MS intensities for subsequent analysis (Table S1). Out of the total annotated proteins, 334 proteins were successfully quantified with missing values below 20%, meeting the criteria for differential analysis. Subsequently, 198 proteins were selected based on the sample/control abundance ratio cutoff, with 136 proteins having ratios above 1.2 and 62 proteins having ratios below 0.8, signaling their inclusion for further analysis. The Mann–Whitney U-test identified 28 candidate proteins that demonstrated significant differential expression between CN1 and AD (Table 2). Correlation heatmaps of plasma proteins illustrated the regulatory trends of the screened molecules, as depicted in Figure 1B. Boxplots revealed that 21 proteins were significantly upregulated (p < 0.05), while 7 proteins demonstrated significant downregulation (p < 0.05) in individuals with AD (Fig. S1).
| No | UniProt accession ID | Protein name | Gene | Abundance ratio: (AD) /(CN) | Abundance ratio adjusted p-value: (sample)/(control) | Subcellular location1 |
|---|---|---|---|---|---|---|
| 1 | E7ETH6 | Zinc finger protein 587B | ZNF587B | 8.432 | 1.17194E-07 | Nucleus |
| 2 | O60306 | RNA helicase aquarius | AQR | 4.308 | 0.001005392 | Nucleus |
| 3 | P02741 | C-reactive protein | CRP | 3.541 | 0.006993768 | Secreted |
| 4 | A6NMB1 | Sialic acid-binding Ig-like lectin 16 | SIGLEC16 | 3.181 | 0.017279823 | Membrane |
| 5 | P07951 | Tropomyosin beta chain | TPM2 | 2.981 | 0.029521031 | Cytoplasm, cytoskeleton |
| 6 | P02751 | Fibronectin | FN1 | 2.931 | 0.033538641 | Secreted |
| 7 | P0DSN7 | Probable nonfunctional immunoglobulin kappa variable 1D-37 | IGKV1D-37 | 2.881 | 0.038515406 | Cell membrane, secreted |
| 8 | P0DJI8 | Serum amyloid A-1 protein | SAA1 | 2.861 | 0.040472261 | Secreted |
| 9 | Q9HCU4 | Cadherin EGF LAG seven-pass G-type receptor 2 | CELSR2 | 2.743 | 0.053606708 | Cell membrane |
| 10 | P02671 | Fibrinogen alpha chain | FGA | 2.374 | 0.121653905 | Secreted |
| 11 | P04275 | von Willebrand factor | VWF | 2.353 | 0.128040944 | Secreted |
| 12 | Q9NP78 | ABC-type oligopeptide transporter ABCB9 | ABCB9 | 2.353 | 0.128040944 | Secreted |
| 13 | P02679 | Fibrinogen gamma chain | FGG | 2.347 | 0.129695671 | Secreted |
| 14 | P00738 | Haptoglobin | HP | 2.321 | 0.139085688 | Secreted |
| 15 | P02675 | Fibrinogen beta chain | FGB | 2.273 | 0.155309958 | Secreted |
| 16 | P02743 | Serum amyloid P-component | APCS | 2.257 | 0.161858393 | Secreted |
| 17 | Q9NYQ6 | Cadherin EGF LAG seven-pass G-type receptor 1 | CELSR1 | 2.17 | 0.200901633 | Cell membrane |
| 18 | P0DJI9 | Serum amyloid A-2 protein | SAA2 | 2.164 | 0.203510016 | Secreted |
| 19 | A0A075B6K0 | Immunoglobulin lambda variable 3–16 | IGLV3-16 | 2.148 | 0.211228324 | Cell membrane, secreted |
| 20 | P68133 | Actin, alpha skeletal muscle | ACTA1 | 2.077 | 0.241212237 | Cytoplasm, cytoskeleton |
| 21 | Q9BXR6 | Complement factor H-related protein 5 | CFHR5 | 2.023 | 0.265142733 | Secreted |
| 22 | P02042 | Hemoglobin subunit delta | HBD | 0.546 | 0.237743501 | Blood microparticle |
| 23 | P05452 | Tetranectin | CLEC3B | 0.51 | 0.163347401 | Secreted |
| 24 | O43592 | Exportin-T | XPOT | 0.46 | 0.09761194 | Nucleus, Cytoplasm |
| 25 | P68871 | Hemoglobin subunit beta | HBB | 0.444 | 0.080222037 | Blood microparticle |
| 26 | Q66K66 | Transmembrane protein 198 | TMEM198 | 0.421 | 0.05588057 | Membrane |
| 27 | P69905 | Hemoglobin subunit alpha | HBA1; HBA2 | 0.365 | 0.018902331 | Blood microparticle |
| 28 | Q9Y623 | Myosin-4 | MYH4 | 0.333 | 0.008978847 | Cytoplasm |
- 1 The subcellular location information was cited from UniProt (https://www.uniprot.org/).
Diagnostic power of single plasma protein
Twenty-eight proteins out of 516 in a table were identified based on AD/CN average abundance ratios, with those having ratios above 2 and below 0.6 selected as being differential. Subsequently, 10 proteins meeting the criteria of an adjusted p-value <0.05 were considered statistically significant. These proteins included: zinc finger protein 587B (ZNF587B), RNA helicase aquarius (AQR), C-reactive protein (CRP), sialic acid-binding Ig-like lectin 16 (SIGLEC16), tropomyosin beta chain (TPM2), fibronectin (FN1), probable nonfunctional immunoglobulin kappa variable 1D-37 (IGKV1D-37), serum amyloid A-1 protein (SAA1), hemoglobin subunit alpha (HBA1; HBA2), and myosin-4 (MYH4). Based on their subcellular localization, we focused on nucleus proteins for their roles in gene regulation and selected secretory proteins for their involvement in various physiological processes. Finally, ZNF587B, AQR, CRP, FN1, and SAA1 were chosen for ROC analysis (Table 3). To assess their discriminatory ability, the cohort, consisting of individuals with AD and CN1 groups, was randomly divided into three datasets: the training dataset, which comprised 28 individuals (15 AD and 13 CN), the testing dataset, which included 9 individuals, and the validation dataset, which comprised 17 individuals. The proteins FN1, SAA1, CRP, ZNF587B, and AQR exhibited varying levels of performance, as indicated by their respective receiver operating characteristic (ROC) values, also known as the area under the curve (AUC). The highest AUC values were observed for FN1 with a score of 0.933, followed by SAA1 with 0.918, ZNF587B with 0.846, AQR with 0.810, and CRP with the AUC value of 0.692 (Fig. 2A).
| Protein name/gene name | UniProt accession No. | Amino acids number | Molecular weight [kDa] | PSM1 | Training ROC-AUC2 | Youden J Max3 | Cutoff value (MS intensity) |
|---|---|---|---|---|---|---|---|
| Fibronectin/FN1 | P02751 | 2477 | 272.2 | 4435 | 0.933 | 0.867 | 5,298,769,777 |
| Serum amyloid A-1 protein/SAA1 | P0DJI8 | 122 | 13.5 | 333 | 0.918 | 0.8 | 50,429,916.51 |
| C-reactive protein/CRP | P02741 | 224 | 25 | 72 | 0.69 | 0.333 | 77,010,959.7 |
| Zinc finger protein 587B/ZNF587B | E7ETH6 | 402 | 45.5 | 106 | 0.846 | 0.713 | 144,108,794.6 |
| RNA helicase aquarius/AQR | O60306 | 1485 | 171.2 | 67 | 0.81 | 0.6 | 114,232,250.7 |
- 1 PSMs: peptide-spectrum matchs, the number of PSMs is the total number of identified peptide spectra matched for the protein.
- 2 Training ROC-AUC was calculated based on training dataset.
- 3 The maximum value of Youden's J statistic corresponds to the optimal cutoff point on the ROC curve.
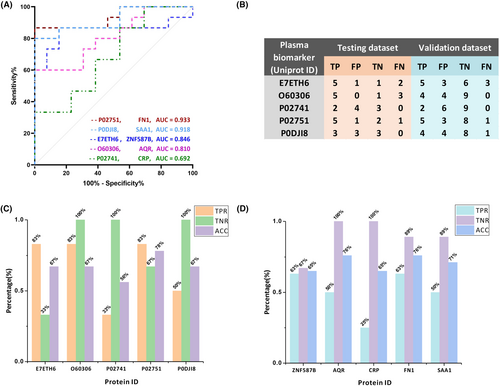
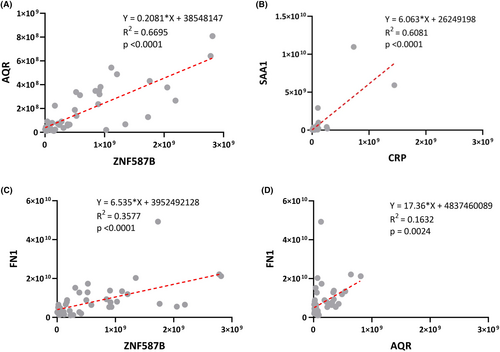
In accordance with the training model results, the evaluation of five protein biomarkers was conducted individually for testing dataset and validation dataset (Fig. 2B). The evaluation results of this analysis were represented using three metrics: true-positive rate (TPR), true-negative rate (TNR), and accuracy (ACC). In the case of testing dataset, the TPR values for ZNF587B, AQR, and FN1 were 83%, while SAA1 had a TPR of 50%, and CRP had a TPR of 33%. The TNR values were 100% for AQR, CRP, and SAA1, 67% for FN1, and 33% for ZNF587B. The corresponding ACC values were 67% for ZNF587B, AQR, and SAA1, 56% for CRP, and 78% for FN1 (Fig. 2C). In validation dataset, the TPR values were 63% for ZNF587B and FN1, 50% for SAA1 and AQR, and 25% for CRP. The TNR values were 100% for AQR and CRP, 89% for FN1 and SAA1, and 67% for ZNF587B. The ACC values were 76% for AQR and FN1, 71% for SAA1, and 65% for CRP (Fig. 2D). In addition, the linear correlation plots revealed strong correlations between ZNF587B and AQR (R2 = 0.6695, p < 0.0001). Besides, significant correlations were observed between SAA1 and CRP (R2 = 0.6081, p < 0.0001), ZNF587B and FN1 (R2 = 0.3577, p < 0.0001), as well as between AQR and FN1 (R2 = 0.1632, p = 0.0024) (Fig. 3).
K-Nearest Neighbors based diagnostic model for AD recognition
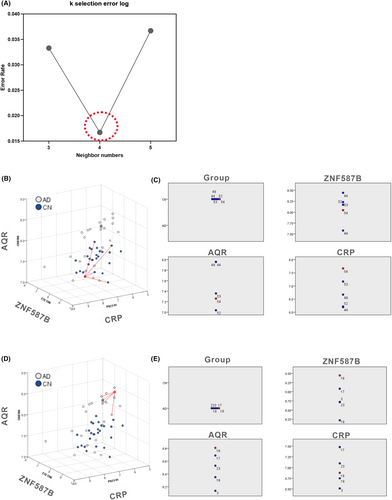
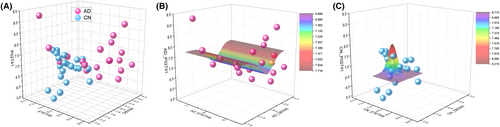
According to the KNN analysis, ZNF587B, AQR, and CRP were identified as the key components of the three-dimensional model. To provide a concrete representation of the model, 3D models were created based on the X, Y, and Z coordinates of each sample. Specifically, X represents ZNF587B (UniProt ID: E7ETH6), Y represents CRP (P02741), and Z represents AQR (O60306). The focal case in either the AD or CN1 group, along with its four nearest neighbors, was chosen based on the values of ZNF587B, AQR, and CRP within the coordinates, as depicted in Figure 4B,D. The selected focal case in the feature space is displayed on the peers chart together with their four nearest neighbors in AD and CN1 group, respectively (Fig. 4C,E). The KNN model in AD diagnosis achieved AUC values of 0.941, 1.000, and 1.000 for the training, testing, and validation datasets, respectively (Table 4), indicating an excellent separation ability in the 3D diagnosis model.
| Group | Training dataset | Testing dataset | Validation dataset |
|---|---|---|---|
| Characteristics | |||
| Model accuracy | |||
| True | 30 (96.77%) | 8 (100%) | 15 (100%) |
| False | 1 (3.23%) | 0 (0%) | 0 (0%) |
| Performance measure | |||
| AUC | 0.941 | 1.000 | 1.000 |
| Gini1 | 0.882 | 1.000 | 1.000 |
- 1 Gini is a metric that used to evaluate KNN classification models. The Gini coefficient ranges from 0 to 1, with 0 representing perfect equality (no discrimination) and 1 representing perfect inequality (perfect discrimination).
Furthermore, to illustrate the projection profiles of the AD and CN1 groups, the model was built with three features of ZNF587B, AQR, and CRP as depicted in Figure 5A. The nonlinear fit model generated a distinct wave-shaped 3D model to represent the AD group, as shown in Figure 5B. The equation for this model was calculated as follows: z = z0 + A*exp(−0.5*((x − xc)/w1)2 − 0.5*((y − yc)/w2)2), z0 = 7.71447 ± 0.37699, A = −0.81556 ± 1.60552E7, xc = 1.53554E24 ± 2.60916E39, w1 = 1.01879E28 ± 1.57859E43, yc = 8.13763 ± 0.17634, w2 = 0.25117 ± 0.23375. Besides, the nonlinear fit resulted in a prominent hat-shaped 3D model to represent the CN group, as depicted in Figure 5C. The equation for this model was calculated as z = z0 + A * exp(−0.5 * ((x − xc)/w1)2 − 0.5 * ((y − yc)/w2)2), z0 = 6.71895 ± 0.19783, A = 1.49591 ± 3.59014, xc = 7.86469 ± 0.14001, w1 = 0.22029 ± 0.19711, yc = 7.36064 ± 0.18199, w2 = 0.09631 ± 0.22961.
Serum metabolome profiling by LC–MS/MS
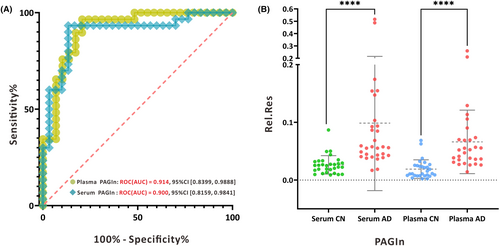
Serum metabolomics analysis was conducted on an independent group comprising 29 AD patients and 30 CN2 individuals. Mass spectrometry data were processed using Thermo Compound Discover 3.1 (CD 3.1, Thermo Scientific, USA), and all compound information is presented in Table S2. Data cleaning and semiquantitation were performed according to a protocol established in previous research.15 A total of 286 metabolites were screened, with 38 endogenous metabolites showing stable mass spectrometry intensities with coefficients of variation (CV%) below 30%, and these were selected for further analysis. Subsequently, receiver operating characteristic (ROC) analysis was conducted to identify potential metabolic features, as detailed in Table S3. Notably, serum phenylacetylglutamine (PAGln) exhibited an area under the ROC curve (ROC-AUC) of 0.900, indicating excellent performance in distinguishing individuals with AD from CN individuals. This finding was consistent with our earlier results where plasma PAGln demonstrated an ROC-AUC of 0.914. These outcomes suggest that both serum and plasma PAGIn exhibit strong sensitivity and specificity in discriminating AD from CN individuals (Fig. 6A). Furthermore, serum PAGIn levels were significantly higher in AD patients compared to controls (p < 0.001), consistently demonstrating an upregulation trend in PAGln compared to previous semiquantitative results in plasma (Fig. 6B).
Discussion
Alzheimer's disease is the predominant cause of progressive dementia. Global burden of disease (GBD) 2019 reported that the number of people with dementia would increase to approximately 152.8 million cases in 2050.20 Currently, there is a lack of effective treatments or preventive measures available for this debilitating neurodegenerative disorder.21 Recent advances in multi-omics approaches will facilitate the discovery of significant SASP molecules that may provide a better understanding of the underlying cellular and molecular mechanisms and the pathogenesis of AD. Consequently, these advances might enable the discovery of biomarkers suitable for improving the accuracy of AD diagnosis as well as novel therapeutics and preventive strategies.
In this work, we analyzed both proteomics and metabolomics features of AD patient's blood sample utilizing advanced mass spectrometers equipped with high-resolution orbitrap mass analyzer system. Accurate identification of specific blood biomolecules has the potential to enhance the detection of cognitive decline features in future clinical diagnoses.
For proteomics analysis, plasma samples from AD patients (N = 29) and CN1 controls (N = 29) were subjected to analysis using nanoLC coupled to an Orbitrap Exploris 480 mass spectrometer. To maintain the integrity of low-abundance proteins, we opted against depleting high-abundance proteins during sample preparation. A total of 516 plasma proteins were identified through a combination of tandem mass spectrometry and a protein sequence database search. This approach represents a significant advancement of accurate protein identification in proteomic mass spectrometry, enhancing the potential for the discovery of proteomic markers in AD diagnostics.13, 22, 23 Traditional evaluation by ROC was used to screen those markers with potential power for AD discrimination.24, 25 Following the supervised learning workflow,26 we randomly divided the whole dataset into three groups for biomarkers training, testing, and validation.18, 27 The proteins AQR, ZNF587B, CRP, SAA1, and FN1 demonstrated promising performance in training dataset. However, their performance was not consistent in testing dataset and validation dataset. This incongruity could be attributed to the limited number of samples available in the dataset as well as the non-normal distribution of MS intensities, which can exhibit either positive skewness or negative skewness. To enhance the model's fitness for diagnostic analysis, we applied a logarithmic transformation to normalize the skewed MS variables. Consequently, a machine learning-based approach was employed to develop a model that can improve the accuracy and stability of AD prediction. The KNN algorithm was chosen for analyzing the proteomics data due to its suitability for high-dimensional data analysis.28, 29 Notably, based on the KNN algorithm, we identified AQR, ZNF587B, and CRP as the three optimal features from a pool of 198 screened proteins.
Linear regression analysis showed a statistically significant relationship between AQR and ZNF587B, indicating an underlying pathophysiology in AD. AQR is a gene encoding intron-binding protein, which is believed to be involved in RNA splicing. RNA helicases play a crucial role in unwinding RNA duplexes, facilitating interactions between RNAs and proteins. They have been linked to aging, lifespan regulation, and neurodegenerative diseases like amyotrophic lateral sclerosis and Alzheimer's disease.30 A recent study showed that AQR expression was significantly upregulated by 85.92% ± 3.75% in aged cells.31 Zinc finger proteins (ZNFs) are a diverse and abundant protein group with important biological functions. Changes in ZNFs have been associated with diseases such as neurodegeneration, skin disorders, and diabetes.32 Interestingly, gene expression analysis on peripheral blood specimens from major depressive disorder (MDD) patients and healthy controls revealed an upregulation of ZNF587 in individuals with MDD, in line with publicly available microarray data.33 Recent research has revealed the expression of ZNF417/587 in specific brain regions during development and adulthood is vital for regulating transposable elements in neurons derived from embryonic stem cells and brain organoids. This regulation indirectly impacts neuronal differentiation, neurotransmission profiles, and safeguards against the activation of neurotoxic retroviral proteins and an interferon-like response.34
Besides, inflammation has long been thought to play a vital role in the pathophysiology of AD in older adults, whereby inflammation is related to further neurodegeneration and cognitive decline.35-37 C-reactive protein, complement proteins, and serum amyloid A protein (SAA) are the principal acute phase proteins, mainly generated in the liver and released into the systemic circulation in response to inflammation.38, 39 CRP has the potential to affect blood brain barrier permeability and interacts with the central nervous system (CNS) as demonstrated by a strong correlation between CRP levels in plasma and cerebrospinal fluid (r > 0.85).40 Besides, CRP serves as an extensively investigated biomarker of inflammation and possesses the capability to activate the classical complement system, thereby inducing cell lysis and phagocytosis of cellular fragments.41, 42 An expanding body of evidence suggests that CRP, a well-established marker of chronic inflammation, plays a pivotal role in the pathogenesis of AD.43
For metabolomics analysis, serum samples from AD patients (N = 29) and CN2 (N = 30) controls were processed following standard protocols, employing vanquish HPLC coupled with Q Exactive Plus mass spectrometer for metabolites profiling. A comprehensive database search led to the identification of 286 metabolites with confirmed compound names. Notably, the metabolite PAGIn exhibited the best performance, with an ROC-AUC of 0.90, consistently aligning with previously reported findings.
PAGIn, a metabolite derived from the gut microbiota (GM), has been identified as significantly elevated in the plasma of patients with AD, and showed its strong correlation with Aβ42/Aβ40 in our previously work.15 In this study, involving different CN participants, we observed significantly higher serum PAGIn responses in AD patients. It was suggested AD patients exhibit distinct variations in gut microbial taxonomy compared to age-matched controls, including decreased levels of Firmicutes and Actinobacteria, and increased species of Bacteroidetes.44 As GM is known to be involved in the shaping of the immune system during early life. The identification of intestinal dysbiosis in patients with neurological disorders has highlighted the importance of gut–brain communication, and yet the question regarding the identity of the components responsible for this crosstalk remains open. However, the connections between systemic metabolic abnormalities and the pathogenesis of AD are still unclear.
Recent studies also suggested a role for the GM in the regulation of inflammation by influencing differentiation of inflammatory cell types, cytokine production and hematopoiesis.45, 46 A leaky gut and alterations in GM composition can both lead to leakage of endotoxins into the circulation that promotes systemic inflammation and to the development of obesity or related metabolic diseases.47 Besides, extensive research has established connections between various microbial factors and the pathogenesis of AD.48, 49 Furthermore, compelling evidence suggests a correlation between changes in GM composition and AD.50-52 Research conducted with transgenic mouse models devoid of GM has showcased diminished amyloid buildup within the cerebral region, implying the impact of microbial amyloids on neuroinflammation and the levels of β-amyloid peptides via the modulation of reactive gliosis in the brain.53 As we found that GM alterations in AD potentially induce peripheral and central immunological changes via the release of microbial metabolites, we propose that modulating their composition may alter ongoing inflammation and could therefore be a promising future strategy to fight progression of AD.
Our results found AQR, ZNF587B, CRP, and PAGIn have been accumulated in AD patients as well as been reported associated with neurodegenerative diseases, while their relationship with AD has not been thoroughly examined. Cellular senescence, an endogenous mechanism of aging, has recently been linked to dysbiosis, wherein the GM exerts its influence on cellular senescence through the release of microbial metabolites.54 Besides, accumulation of senescent cells in AD has been linked to an overexpression of the SASP, characterized by proinflammatory cytokines, chemokines, growth factors, proteases, metabolites, lipids and extracellular matrix components. It is likely that age-related alterations in the GM facilitate inflammatory processes that may contribute to the neuro-inflammation in AD. However, proving that there is an infective cause to the neuro-inflammation and neurodegeneration seen in patients with AD is logistically and ethically challenging in humans. Thus, the underlying pathophysiology of these AD-associated molecules is worth to be studied in the future.
Nevertheless, this study has several limitations. First, the small sample size of our study represented a major constraint, particularly when employing machine learning techniques for analyzing extensive datasets. Besides, our results should be considered in light of potential confounders such as APOE genotype, gender, and body mass index, which were not taken into consideration. Additionally, our findings warrant further investigations, special at the tissue level, to elucidate the precise molecules changes within specific pathways and network contexts. This shall provide a more comprehensive understanding of the underlying mechanisms.
Conclusions
In this study, we performed a comprehensive multi-omics analysis to investigate the senescence-associated secretory phenotypes (SASPs) associated with Alzheimer's disease (AD). Our results identified three proteins, namely AQR, ZNF587B, and CRP, as well as a metabolite called PAGIn, as SASPs linked to AD. The presence of these markers suggests their potential usefulness as informative diagnostic indicators for AD. Utilizing mass spectrometry-based analysis, we successfully profiled the blood proteome and metabolome of AD patients, allowing for the simultaneous measurement of multiple biomarkers. This approach significantly improved the efficiency of biomarker discovery for accurate diagnosis. Furthermore, we applied a machine learning algorithm, KNN, to identify highly correlated biomarkers and construct an AD diagnostic model. This machine learning approach demonstrated specificity and accuracy in establishing a comprehensive diagnostic framework. Nevertheless, further investigations are crucial to assess the stability and reliability of these SASPs, which have also demonstrated diagnostic potential for AD.
Acknowledgments
We acknowledge the work of all participants in this study. We are grateful for the scientific discussion with colleagues from the Neurology Department and Clinical Research Center of Shanghai Baoshan Luodian Hospital. We would like to thank Xinru Liu and Junjie Chen for the specimen collection. We appreciate Shanghai Yunxiang Medical Technology Co., Ltd. (Shanghai, China) for providing a high-resolution mass spectrometer for metabolomics analysis.
Funding Information
This work was supported by the National Key R&D Program of China (No. 2021YFC2100201).
Author Contributions
Conceptualization: X Dong and L Zhao; investigation: JZ Yang and YG Zhou; methodology and software: JZ Yang and TJ Wang; formal analysis and data curation: JZ Yang; supervision and validation: Q Zhang and S Wu; writing—original draft preparation: JZ Yang, YG Zhou, and TJ Wang; writing—editing: N Li, YF Chao, and SY Gao; Writing—review: X Dong and L Zhao; funding acquisition: X Dong. All authors approved the final version of this manuscript.
Conflict of Interest
All authors declare to have no competing interests with the content of this article.
Consent for Publication
Not applicable.
Open Research
Data Availability Statement
All the data generated and analyzed in this work are included in the manuscript and the supplementary materials.




