A novel score to predict progression in anterior circulation single subcortical infarction patients
Abstract
Objective
Progressive infarction (PI) has a negative effect on functional prognosis. Our study aimed to develop and validate a risk score for predicting PI in patients with anterior circulation single subcortical infarction (ACSSI).
Methods
Between January 2020 and October 2022, we retrospectively enrolled 638 eligible patients with ACSSI. Two-thirds of the eligible patients were randomly allocated to the training cohort (n = 425). Another resampling sample was formed through the bootstrap method and was used as the validation group (n = 425). Multivariate logistic regression analysis was used to identify the independent factors associated with PI. Each factor was then point assigned based on β-coefficient and a risk scoring system was developed. This scoring system was internally validated through 1000-bootstrap resamplings. The C-statistic and Hosmer-Lemeshow test were used to assess model discrimination and calibration.
Results
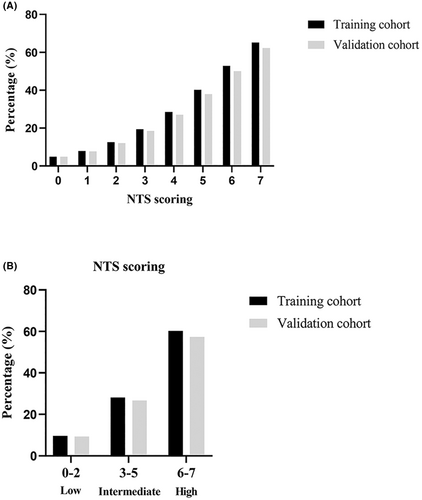
PI occurred in 121 patients, accounting for 19.0% of the total patients. A 7-point NTS score system based on the initial NIHSS score, triglyceride-glucose index, and the number of infarct slices on axial diffusion-weighted imaging was developed. The NTS score showed good discrimination and calibration in the training cohort (C-statistic = 0.686; p value of Hosmer–Lemeshow test = 0.797) and validation cohort (C-statistic = 0.681; p value of Hosmer–Lemeshow test = 0.451). The three risk levels for predicting PI in the training and validation cohorts based on NTS score were as follows: low (0–2, 9.6% vs. 9.3%), intermediate (3–5, 28.2% vs. 26.7%), and high risk (6–7, 60.2% vs. 57.4%).
Interpretation
The NTS score is a valid and convenient risk score for predicting PI in ACSSI patients.
Introduction
Single subcortical infarction (SSI), defined as a single deep infarction in the territory of perforating arteries, usually has a small infarction volume and presents with relatively limited neurological deficits.1, 2 There are two types of SSIs, lacunar infarction (LI) and branch atheromatous disease (BAD), which are more likely to develop early neurological deterioration (END).3 END, a symptomatic diagnosis, was defined as an increase of ≥1 point in motor power or ≥2 points in the total National Institute of Health Stroke Scale (NIHSS) score within 7 days after admission.4 However, multiple mechanisms have been proposed to account for the pathology of END including progressive infarction (PI), recurrent cerebral ischemia, increased intracranial pressure, and secondary parenchymal bleeding.5 Among them, progressive infarction is the most frequent cause.5
Anterior circulation single subcortical infarction (ACSSI), the most common type of SSI, is commonly caused by occlusion of the lenticulostriate artery stemming from the middle cerebral artery (MCA), the Heubner's artery originating from the anterior cerebral artery (ACA) and the anterior choroidal artery (AchA) arising from the internal carotid artery (ICA).6 Posterior circulation single subcortical infarction (PCSSI) usually results from the occlusion of perforating branches, including thalamoperforating branches, paramedian branches, and short circumferential branches.6 Previous studies by others and our group have shown that multiple factors, including inflammatory factors, fast glucose levels, blood pressure variability, hypertriglyceridemia, and some imaging markers, are associated with END in patients with acute ischemic stroke.7-11 However, the predictive factors of END between the ACSSI and PCSSI are inconsistent, indicating that different mechanisms may be responsible for END at different lesion locations.12 In addition, some intuitive imaging markers for predicting END are not universal in the ASCCI or PCSSI.8, 13 For example, our previous research showed that a lesion extending three or more slices could predict PI, but this cannot predict PI in PCSSI because the single infarction in the brainstem rarely extends three slices.8 Thus, the predictors of END or PI differ among locations.
Although the ACSSI is the most common type of PI, no reliable scoring system is currently available in clinical practice. The establishment of a predictive model for PI in patients with ACSSI would be helpful for identifying vulnerable patients and developing preventive measures. Therefore, the current study aimed to construct and validate a risk score for PI in patients with ACSSI.
Methods
Study participants
This single-center retrospective study included patients who were admitted to the Stroke Unit of the First Affiliated Hospital of Nanchang University within 48 h after symptom onset between January 2020 and October 2022. The inclusion criteria were as follows: (1) completed the first diffusion-weighted imaging (DWI) within 48 h of onset; (2) diagnosed as ACSSI on DWI consistent with the clinical deficits; (3) PI should be confirmed by DWI or computerized tomography (CT). The exclusion criteria were as follows: (1) received intravenous thrombolysis or endovascular therapy; (2) suspected of cardiogenic embolism, arterial to arterial embolism, or other determined etiologies (moyamoya disease, arterial dissection, vasculitis, and so on); (3) neurological deficits worsening occurring before the first DWI; and (4) lacked complete imaging, laboratory, or follow-up data. This study was approved by the Ethics Committee of First Affiliated Hospital of Nanchang University.
Data collection
Demographic characteristics (age and sex), history of disease (hypertension, diabetes, and stroke), clinical assessment (initial NIHSS score), and laboratory data within 24 h of admission [white blood cell (WBC), red blood cell (RBC), hemoglobin (HGB), platelet (PLB), absolute value and percentage of lymphocytes, absolute value and percentage of monocytes, absolute value and percentage of neutrophils, blood urea nitrogen (BUN), creatinine, uric acid, fasting glucose, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), homocysteine, fibrinogen, and d-dimer] were recorded. Two markers of insulin resistance, the triglyceride-glucose index (TyG index) and TG/HDL-c ratio, were calculated using the following formula: TyG index = Ln [Triglyceride (mg/dL) × Fasting glucose (mg/dL)/2], or TyG index = Ln [Triglyceride (mmol/L) × 88.6 × Fasting glucose (mmol/L) × 18.02 /2]; TG/HDL-c ratio = TG (mg/dL)/HDL-c(mg/dL), respectively. The neutrophil–lymphocyte ratio (NLR), lymphocyte–monocyte ratio (LMR), and platelet–lymphocyte ratio (PLR) were also calculated.
Imaging procedures and assessment
All patients underwent magnetic resonance imaging (MRI) on a 3.0 Tesla scanner (MAGNETOM Trio, Siemens, Erlangen, Germany). The protocol included T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), DWI (TR/TE of 3100/91 ms; field of view 230 × 230 mm2; 19 slices with a slice thickness of 5 mm; voxel size = 1.2 × 1.2 × 5 mm3; 2b values of 0 and 1000 s/mm2; scan time of 1.16 min), and three-dimensional time-of-flight MRA (TR/TE of 22/3.86 ms; Field of view 235 × 235 mm2; voxel size = 0.9 × 0.6 × 0.6 mm3; 2b values of 0 and 1000 s/mm2; scan time of 3.12 min). All the images were analyzed by two trained neurologists who were blinded to the patients' information. Leukoaraiosis was evaluated by a 4-point score as proposed by Fazekas et al.14 We collected the number of axial infarct slices and the maximal diameter of the infarcts on axial DWI.
Definition
ACSSI was defined as a single subcortical infarct supplied from the lenticulostriate artery, Heubner's artery or AchA.6 PI was defined as an increase of ≥1 point in motor power or ≥2 points in the total NIHSS score within 7 days after admission, and extension of the original infarction was further confirmed by DWI or CT.8
Statistical analysis
Continuous variables are presented as the mean (± SD), or median [interquartile range (IQR)] dependent on the distribution, and comparisons between groups adopted Student's t-test or Mann–Whitney U-test. Categorical variables were presented as frequency (percentages) and the between-group comparisons were used by chi-squared test or Fisher's exact test.
Internal validation was performed by combining bootstrap repeated sampling and random splitting method. Two-thirds of the eligible patients were randomly allocated to the training cohort (n = 425). Subsequently, another resampling sample using the bootstrap method with a 1:1 ratio to the training group was formed into the validation group (n = 425). Furthermore, 1000-bootstrap resamples through the same resampling method were used to internally validate the stability of the model. Calibration was assessed by the Hosmer–Lemeshow test to determine goodness of fit. Discrimination was measured by the C statistic to predict accuracy.
Univariate and multivariate logistic regression analyses were used to determine the independent predictors of PI in the training cohort. Several variables (initial NIHSS score and TyG index) were categorized based on cutoff values using receiver operating characteristic (ROC) curve analysis. Factors that retained significance in the multivariable logistic regression analysis were included in the final scoring system for predicting PI. Notably, we compared the predictive ability of the TyG index and TG/HDL-c ratio using the area under the curve (AUC), and the marker with the stronger predictive ability was included in the scoring system. The NTS score system (NIHSS score, TyG index, and number of infarct slices) was subsequently generated and a score was assigned for each risk factor based on the Framingham Study.15 In our study, the TyG index was divided into four intervals including <8.350, 8.350–8.800, 8.800–9.300, and ≥9.300. Three risk categories for PI were determined by the scores: low (0–2 score), intermediate (3–5 score), and high risk (6–7 score).
All variables with a p-value <0.05 were considered statistically significant. All the statistical analyses were performed using SPSS (version 26.0) and R software package (version 4.2.1).
Results
Patient characteristics
From January 2020 and October 2022, a total of 1035 patients who met the inclusion criteria were recruited. After an exclusion of 175 patients who received intravenous thrombolysis or endovascular therapy, 15 patients who were suspected of cardiogenic embolism, arterial to arterial embolism, and other determined etiologies, 98 patients who experienced neurological deficits worsening before the first DWI, 109 patients who lacked complete imaging, laboratory, or follow-up data. Overall, 638 ACSSI patients were enrolled in the current analysis. The mean age of the total patients was 62.77 ± 11.79 years, and 69.4% were men. A total of 121 (19.0%) patients experienced PI. Two-thirds of the total patients were randomly sampled to form the training cohort (n = 425) and validation cohort (n = 425).
The baseline characteristics between the training group and validation group were shown in Table 1. There were no significant differences in demographic information, clinical characteristics, admission laboratory data, and imaging data between patients in the two cohorts.
| Characteristics | Training cohort (n = 425) | Validation cohort (n = 425) | p Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), median (IQR) | 65.0 (55.0, 71.5) | 64.0 (55.0, 72.0) | 0.946 |
| Male, n (%) | 299 (70.4%) | 290 (68.2%) | 0.552 |
| Clinical data | |||
| Hypertension, n (%) | 380 (89.4%) | 384 (90.4%) | 0.733 |
| Diabetes, n (%) | 123 (28.9%) | 133 (31.3%) | 0.501 |
| History of stroke, n (%) | 0.876 | ||
| None | 370 (87.1%) | 365 (85.9%) | |
| Ischemic | 47 (11.1%) | 51 (12.0%) | |
| Hemorrhagic | 8 (1.9%) | 9 (2.1%) | |
| Initial NIHSS score, median (IQR) | 3 (2, 5) | 3 (2, 5) | 0.677 |
| Admission laboratory data | |||
| WBC (10*9/L), median (IQR) | 6.47 (5.19, 7.58) | 6.41 (5.12, 7.57) | 0.848 |
| RBC (g/L), median (IQR) | 4.45 (4.16, 4.82) | 4.45 (4.15, 4.81) | 0.727 |
| HGB (g/L), median (IQR) | 135 (126, 145) | 135 (126, 145) | 0.931 |
| PLT (10*9/L), median (IQR) | 202.0 (171.0, 235.5) | 201.0 (167.3, 244.0) | 1.000 |
| NLR, median (IQR) | 2.68 (1.93, 3.73) | 2.54 (1.87, 3.52) | 0.117 |
| LMR, median (IQR) | 3.48 (2.79, 4.36) | 3.62 (2.88, 4.52) | 0.162 |
| PLR, median (IQR) | 129.56 (100.24, 172.20) | 125.94 (98.42, 167.02) | 0.208 |
| BUN (mmol/L), median (IQR) | 4.74 (3.73, 5.60) | 4.70 (3.80, 5.60) | 0.955 |
| Creatinine (μmol/L), median (IQR) | 75.30 (61.10, 88.05) | 74.25 (60.13, 86.83) | 0.527 |
| Uric acid (mmol/L), median (IQR) | 341.00 (287.45, 410.90) | 341.00 (280.43, 403.75) | 0.643 |
| Fasting glucose (mg/dL), median (IQR) | 99.11 (86.59, 127.49) | 101.81 (86.68, 128.66) | 0.740 |
| TC (mg/dL), median (IQR) | 184.84 (153.91, 208.82) | 185.04 (156.61, 207.85) | 0.887 |
| TG (mg/dL), median (IQR) | 122.27 (88.16, 178.09) | 120.50 (89.71, 173.44) | 0.677 |
| HDL-c (mg/dL), median (IQR) | 43.70 (36.35, 51.63) | 44.08 (36.45, 51.43) | 0.564 |
| LDL-c (mg/dL), median (IQR) | 110.98 (88.17, 133.80) | 112.92 (88.94, 130.51) | 0.987 |
| Homocysteine (μmol/L), median (IQR) | 13.50 (11.00, 15.90) | 13.60 (11.09, 16.00) | 0.580 |
| Fibrinogen (g/L), median (IQR) | 2.90 (2.51, 3.37) | 2.89 (2.53, 3.29) | 0.508 |
| d-dimer (mg/L), median (IQR) | 0.25 (0.15, 0.47) | 0.26 (0.16, 0.47) | 0.705 |
| TyG index, median (IQR) | 8.81 (8.35, 9.32) | 8.81 (8.37, 9.23) | 0.825 |
| TG/HDL-c ratio, median (IQR) | 2.96 (1.84, 4.50) | 2.90 (1.84, 4.35) | 0.568 |
| Imaging date | |||
| Diameter of infarcts (mm), median (IQR) | 14.39 (10.43, 19.08) | 15.05 (10.43, 19.77) | 0.732 |
| Diameter of infarcts ≥10 mm, n (%) | 340 (80.0%) | 333 (78.4%) | 0.612 |
| Axial slices of infarcts, median (IQR) | 3 (2, 4) | 3 (2, 4) | 0.755 |
| Axial slices of infarcts ≥3, n (%) | 243 (57.2%) | 245 (57.6%) | 0.945 |
| Leukoaraiosis | 0.722 | ||
| 0 | 11 (2.6%) | 13 (3.1%) | |
| 1 | 250 (58.8%) | 240 (56.5%) | |
| 2 | 107 (25.2%) | 120 (28.2%) | |
| 3 | 57 (13.4%) | 52 (12.2%) |
- BUN, blood urea nitrogen; HDL-c, high-density lipoprotein cholesterol; HGB, hemoglobin; IQR, interquartile range; LDL-c, low-density lipoprotein cholesterol; LMR, lymphocyte–monocyte ratio; NIHSS, National Institute of Health Stroke Scale; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; PLT, platelet; RBC, red blood cell; TC, total cholesterol; TG, triglyceride; TyG index, triglyceride-glucose index; WBC, white blood cell.
Predictors of PI
In univariate analysis, the potential predictors of PI in the training cohort were listed in Table 2. Several factors, including initial NIHSS score, TC level, TG level, TyG index, TG/HDL-c ratio, infarct diameter, and axial slice number of infarcts, were greater in the PI group than in the non-PI group (p < 0.05). Additionally, patients with PI more frequently had infarct diameters ≥10 mm (88.2% vs. 77.9%, p = 0.034) and axial slices of infarcts ≥3 (74.1% vs. 52.9%, p < 0.001) in the training cohort. Furthermore, the AUC of the TyG index (0.591, 95% CI 0.527–0.655) was superior to that of the TG/HDL-c ratio (0.570, 95% CI 0.504–0.637) for predicting PI in ACSSI patients.
| Variable | PI (n = 85) | Non-PI (n = 340) | p Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), median (IQR) | 65.0 (55.0, 71.0) | 65 (54.0, 72.0) | 0.929 |
| Male, n (%) | 55 (64.7%) | 244 (71.8%) | 0.202 |
| Clinical data | |||
| Hypertension, n (%) | 78 (91.8%) | 302 (88.8%) | 0.555 |
| Diabetes, n (%) | 25 (29.4%) | 98 (28.8%) | 0.915 |
| History of stroke, n (%) | 0.611 | ||
| None | 76 (89.4%) | 294 (86.5%) | |
| Ischemic | 7 (8.2%) | 40 (11.8%) | |
| Hemorrhagic | 2 (2.4%) | 6 (1.8%) | |
| Initial NIHSS score, median (IQR) | 4 (2, 6) | 3 (2, 5) | 0.006* |
| Admission laboratory data | |||
| WBC (10*9/L), median (IQR) | 6.47 (5.11, 7.56) | 6.46 (5.21, 7.58) | 0.698 |
| RBC (g/L), mean ± SD | 4.44 ± 0.45 | 4.49 ± 0.53 | 0.399 |
| HGB (g/L), median (IQR) | 136.0 (126.0, 146.0) | 135.0 (126.0, 145.0) | 0.824 |
| PLT (10*9/L), median (IQR) | 193.0 (158.0, 233.0) | 202.5 (172.0, 236.0) | 0.158 |
| NLR, median (IQR) | 2.63 (1.96, 3.75) | 2.69 (1.92, 3.72) | 0.817 |
| LMR, median (IQR) | 3.71 (3.06, 4.76) | 3.44 (2.76, 4.31) | 0.07 |
| PLR, median (IQR) | 129.26 (98.13, 187.72) | 129.93 (100.49, 172.03) | 0.512 |
| BUN (mmol/L), median (IQR) | 4.70 (3.71, 5.45) | 4.76 (3.80, 5.60) | 0.366 |
| Creatinine (μmol/L), median (IQR) | 74.90 (58.10, 86.05) | 75.35 (62.15, 88.90) | 0.427 |
| Uric acid (mmol/L), median (IQR) | 356.00 (287.35, 422.90) | 338.00 (287.23, 405.73) | 0.141 |
| Fasting glucose (mg/dL), median (IQR) | 102.35 (89.92, 127.22) | 98.30 (86.01, 127.72) | 0.242 |
| TC (mg/dL), median (IQR) | 191.42 (157.39, 226.22) | 181.17 (153.52, 205.63) | 0.016* |
| TG (mg/dL), median (IQR) | 149.73 (104.99, 193.59) | 118.72 (86.83, 175.87) | 0.013* |
| HDL-c (mg/dL), median (IQR) | 44.47 (35.19, 52.01) | 43.70 (36.35, 51.72) | 0.903 |
| LDL-c (mg/dL), median (IQR) | 114.08 (93.00, 145.02) | 108.86 (87.11, 130.51) | 0.058 |
| Homocysteine (μmol/L), median (IQR) | 12.60 (10.20, 16.03) | 13.60 (11.20, 15.81) | 0.205 |
| Fibrinogen (g/L), median (IQR) | 2.87 (2.46, 3.48) | 2.90 (2.51, 3.35) | 0.913 |
| d-dimer (mg/L), median (IQR) | 0.24 (0.14, 0.43) | 0.26 (0.15, 0.48) | 0.198 |
| TyG index, median (IQR) | 9.00 (8.56, 9.49) | 8.74 (8.30, 9.22) | 0.009* |
| TG/HDL-c ratio, median (IQR) | 3.34 (2.08, 4.89) | 2.83 (1.76, 4.35) | 0.045* |
| Imaging data | |||
| Diameter of infarcts (mm), median (IQR) | 15.74 (12.19, 21.38) | 14.19 (10.18, 19.03) | 0.048* |
| Diameter of infarcts ≥10 mm, n (%) | 75 (88.2%) | 265 (77.9%) | 0.034* |
| Axial slices of infarcts, median (IQR) | 3 (2, 4) | 3 (2, 4) | 0.001* |
| Axial slices of infarcts ≥3, n (%) | 63 (74.1%) | 180 (52.9%) | < 0.001* |
| Leukoaraiosis | 0.355 | ||
| 0 | 4 (4.71%) | 7 (2.06%) | |
| 1 | 51 (60.0%) | 199 (58.5%) | |
| 2 | 22 (25.9%) | 85 (25.0%) | |
| 3 | 8 (9.41%) | 49 (14.41%) |
- BUN, blood urea nitrogen; HDL-c, high-density lipoprotein cholesterol; HGB, hemoglobin; IQR, interquartile range; LDL-c, low-density lipoprotein cholesterol; LMR, lymphocyte–monocyte ratio; NIHSS, National Institute of Health Stroke Scale; NLR, neutrophil–lymphocyte ratio; PI, progressive infarction; PLR, platelet–lymphocyte ratio; PLT, platelet; RBC, red blood cell; SD, standard deviation; TC, total cholesterol; TG, triglyceride; TyG index, triglyceride-glucose index; WBC, white blood cell.
- * p < 0.05.
As shown in Table 3, after adjusting for confounding factors in the multivariate logistic regression analysis, three variables remained statistically significant: initial NIHSS score ≥4 (OR 1.755, 95% CI 1.057–2.914; p = 0.030), TyG index ≥8.475 (OR 2.560, 95% CI 1.291–5.077; p = 0.007), and axial slices of infarcts ≥3 (OR 2.160, 95% CI 1.224–3.813; p = 0.008).
| Covariate | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Initial NIHSS score ≥4 | 1.944 (1.200–3.150) | 0.007* | 1.755 (1.057–2.914) | 0.03* |
| TyG index ≥8.475 | 2.748 (1.509–5.004) | 0.001* | 2.560 (1.291–5.077) | 0.007* |
| Axial slices of infarcts ≥3 | 2.545 (1.498–4.325) | 0.001* | 2.160 (1.224–3.813) | 0.008* |
| TC (mg/dL) | 0.995 (0.989–1.000) | 0.056 | 0.997 (0.991–1.003) | 0.343 |
| TG (mg/dL) | 0.998 (0.995–1.000) | 0.044* | 1.000 (0.997–1.003) | 0.903 |
| Diameter of infarcts ≥10 mm | 2.123 (1.046–4.308) | 0.037* | 0.770 (0.356–1.664) | 0.506 |
- CI, confidence interval; NIHSS, National Institute of Health Stroke Scale; OR, odds ratio; TC, total cholesterol; TG, triglyceride; TyG index, triglyceride-glucose index.
- * p < 0.05.
The NTS score
The NTS score (NIHSS score, TyG index, and number of infarct slices) was developed based on the logistic analysis results in the training cohort. Each of the three independent predictors was point assigned based on the β-coefficient. As a sum of the individual points (0–7 points), the NTS score consisted of the initial NIHSS score (1 point for ≥4), the TyG index (1 point for 8.350–8.800, 2 points for 8.800–9.300, and 4 points for ≥9.300), and the axial slices of infarcts (2 points for ≥3) (Table 4).
| Predictors | Points |
|---|---|
| Initial NIHSS score | |
| <4 | 0 |
| ≥4 | 1 |
| TyG index | |
| <8.350 | 0 |
| 8.350–8.800 | 1 |
| 8.800–9.300 | 2 |
| >9.300 | 4 |
| Axial slices of infarcts | |
| <3 | 0 |
| ≥3 | 2 |
- TyG index = Ln [Triglyceride (mg/dL) × Fasting glucose (mg/dL)/2]. TyG index = Ln [Triglyceride (mmol/L) × 88.6 × Fasting glucose (mmol/L) × 18.02/2].
- NIHSS, National Institute of Health Stroke Scale; NTS, NIHSS score, TyG index, slices of infarcts; TyG index, triglyceride-glucose index.
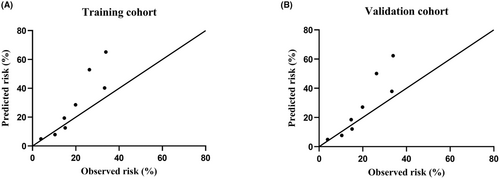
The NTS score demonstrated good discrimination and calibration in the training cohort (C-statistic, 0.686; p value of the Hosmer–Lemeshow test, 0.797). In the validation cohort, the NTS score also exhibited good discrimination with a C statistic of 0.681, and good calibration with a Hosmer–Lemeshow p value of 0.415. A plot of the observed versus predicted risk of PI revealed a high association between observed and predicted risk either in the training cohort or the validation cohort (Fig. 1). Furthermore, after internal validation via 1000-bootstrap resamplings, the mean C-statistic of the risk score was 0.686 (95% CI: 0.622–0.747), indicating good discrimination. In addition, the Hosmer–Lemeshow value for the bootstrapped validation model was 0.814, indicating good calibration.
As shown in Figure 2A, the proportion of patients who developed progressive infarction increased with increasing scores in both the training group and the validation group. In addition, based on the NTS score system, the three risk levels for predicting PI in the training and validation cohorts were as follows: low (0–2, 9.6% vs. 9.3%), intermediate (3–5, 28.2% vs. 26.7%), and high risk (6–7, 60.2% vs. 57.4%) (Fig. 2B).
Discussion
In our study, a novel risk score (NTS score) composed of the initial NIHSS score, TyG index, and axial slices of infarcts was established to predict PI in patients with ASCCI. The NTS score exhibited good discrimination and calibration in the training and internal validation cohorts. Furthermore, patients with ACSSI could be divided into three risk levels based on the 7-point NTS score system, with nearly 60% of high-risk patients experiencing progression. Thus, the NTS score may provide a practical way for clinical physicians to select high-risk patients for more aggressive treatment to prevent PI.
To screen out potential risk factors related to PI, we recorded the demographic information, clinical characteristics, admission laboratory data, and imaging data based on previous studies and pathophysiology. More importantly, the marker with stronger predictive ability from the same pathophysiological mechanism, such as the TyG index or TG/HDL-c ratio, was included in the final scoring system. Previous studies have shown that the TyG index and the TG/HDL-c ratio were two convenient and reliable indicators of insulin resistance.16, 17 In the present study, the TyG index was superior to the TG/HDL-c ratio for predicting PI, and the TyG index was included in the NTS score system. Therefore, our model is informative. Although growing evidence has revealed a higher possibility of PI in BAD compared to LI, there is some controversy over the definition of BAD.18, 19 In our previous study, we tried not to distinguish between BAD and LI to investigate the relationship between imaging markers and PI, and found that a lesion extending ≥3 slices was an independent predictor of PI.8 For simplicity, we selected the slices and diameter of infarcts as variables instead of BAD in the present study. Therefore, the NTS score is applicable and credible.
Our study reported that the prevalence of PI in the total cohort was 19.0%, which is similar to the findings of previous studies.20 Multiple risk factors for END have been identified. Consistent with the findings of several previous studies, our study confirmed that PI was independently associated with the initial NIHSS score, the TyG index and infarct slices in ACSSI patients.21-25 Although most studies revealed a higher frequency of a lesion visible ≥3 slices in the END group, one study found no relationship between the two.23-25 This inconsistency can be explained by differences in slice thickness when DWI is used for imaging. In our present study, the slice thickness on DWI was 5 mm, which was consistent with the slice thickness used for positive results.23, 24
The NTS score included comprehensive information on clinical information, laboratory data, and imaging characteristics. It could be readily incorporated into clinical practice by performing a simple score. In the past few years, several prognostic models have been developed for END, but few are universally accepted and applied clinically.26, 27 It is not our intention to show the superiority of the NTS score, but we need to point out the differences in our model. Gong P et al developed a nomogram composed of hypersensitive C-reactive protein levels, age, diabetes mellitus, atrial fibrillation, previous antiplatelet medication, and baseline NIHSS score to predict the risk of END.26 In addition, Xie X et al presented a predictive nomogram for END that included initial NIHSS score, middle cerebral artery stenosis, and carotid stenosis of ≥50% in patients with acute ischemic stroke.27 However, the definition of END in these two studies relied on changes in NIHSS scores. Multiple mechanisms (PI, recurrent cerebral ischemia, increased intracranial pressure, and secondary parenchymal bleeding) can cause END, but the predictive factors for different mechanisms vary.5 In our study, we focused on PI and excluded END caused by other mechanisms by combining the NIHSS score and imaging technology. Moreover, a recent study demonstrated inconsistent contributors for predicting END in ACSSI patients and PCSSI patients.28 Therefore, it is necessary to select patients with different infarctions and develop different predictive models for various infarction mechanisms. Our NTS score focused only on the predictive factors of PI, and purely targeted patients with ACSSI. Therefore, our model had good specificity. To our knowledge, this is the first model for predicting PI in ACSSI patients.
Our study has two limitations that deserve comment. First, this was a single-center retrospective study, although the use of laboratory data from the same reagent kit in a stroke center resulted in improved uniformity. Second, no external data were used to validate the model although we conducted internal validation. Randomly splitting the total samples into a training cohort and a validation cohort is commonly performed, but this method is considered to be statistically inefficient when the development sample is relatively small.29 Therefore, bootstrapping was the preferred method for internal validation in our study. In the future, we plan to conduct a multicenter, prospective study to validate our model.
Conclusions
In summary, we developed a novel clinical score to predict PI in ACSSI patients, and the score features good calibration and discrimination.
Acknowledgements
The authors have nothing to report.
Author Contributions
Concept and design: J.L., S.R., W.S., Y.F., and D.H.; acquisition of data: L.D., S.L., Q.H., X.M., J.Z., K.Z., and H.Z.; analysis or interpretation of data: S.R., W.S., P.H., P.F., and X.L.; drafting of the manuscript: J.L. and S.R.; critical revision of the manuscript for important intellectual content: Y.F. and D.H.; obtained funding: J.L. and D.H.; All authors have read and approved the final manuscript.
Funding Information
This study was supported by the National Natural Science Foundation of China (No. 82101405), the Natural Science Foundation of Jiangxi Province (No. 20212BAB216023), and Double thousand talents program of Jiangxi province (No. jxsq2019101021).
Conflict of Interest
The authors declare that there is no conflict of interest.
Open Research
Data Availability Statement
Data are available upon reasonable request. All data are available from the corresponding author.




