Estrogen-induced glial IL-1β mediates extrinsic retinal ganglion cell vulnerability in murine Nf1 optic glioma
Abstract
Optic pathway gliomas (OPGs) arising in children with neurofibromatosis type 1 (NF1) can cause retinal ganglion cell (RGC) dysfunction and vision loss, which occurs more frequently in girls. While our previous studies demonstrated that estrogen was partly responsible for this sexually dimorphic visual impairment, herein we elucidate the underlying mechanism. In contrast to their male counterparts, female Nf1OPG mice have increased expression of glial interleukin-1β (IL-1β), which is neurotoxic to RGCs in vitro. Importantly, both IL-1β neutralization and leuprolide-mediated estrogen suppression decrease IL-1β expression and ameliorate RGC dysfunction, providing preclinical proof-of-concept evidence supporting novel neuroprotective strategies for NF1-OPG-induced vision loss.
Introduction
Vision loss is one of the most significant morbidities associated with brain tumors arising in the neurofibromatosis type 1 (NF1) cancer predisposition syndrome.1, 2 In this respect, 15%–20% of children with NF1 develop low-grade gliomas (pilocytic astrocytomas) of the optic pathway (optic pathway glioma; OPG) that result in axonal injury, retinal ganglion cell (RGC) loss, retinal nerve fiber layer (RNFL) thinning, and subsequent vision loss.1 Similarly, Nf1-OPG preclinical mouse models also exhibit time-dependent axonal injury, RGC loss, and RNFL thinning,3, 4 establishing these avatars as excellent platforms to define the cellular and molecular mechanisms underlying OPG-induced RGC degeneration. Leveraging these resources, prior research suggested both cell-intrinsic and cell-extrinsic causes for RGC death. Impaired cAMP regulation accounts for the RGC-intrinsic vulnerability in Nf1-mutant mice, such that cAMP elevation partly ameliorates RGC loss in vivo.5 However, the mechanism underlying the cell-extrinsic effects remains unelucidated.
Insights into drivers of cell-extrinsic RGC loss derive from studies demonstrating that girls with NF1-OPG more frequently require treatment for vision loss.6-8 Using Nf1OPG mice, only female Nf1OPG mice exhibit significant RGC loss and RNFL thinning, despite the fact that both male and female Nf1OPG mice develop tumors of equal volumes and proliferation rates.6, 9 This sexually dimorphic effect is caused by estrogen, such that surgical or chemical ovariectomy ameliorates Nf1-OPG-induced retinal pathology.9 In the current study, we demonstrate that estrogen regulates glial IL-1β production, which is sufficient to cause Nf1-mutant RGC death in vitro. Moreover, both pharmacologic IL-1β and hypothalamic estrogen inhibition restore RGC numbers and RNFL thickness in female Nf1OPG mice to wild type levels in vivo, revealing potential neurorestorative approaches for attenuating vision loss in children with NF1-OPG.
Materials and Methods
Mice
Experiments were performed under an approved Washington University Institutional Animal Care and Use Committee protocol. Mice had ad libitum access to water and food under 12-h light/dark cycles. Nf1flox/mut; GFAP-Cre (Nf1+/− mice with somatic Nf1 gene loss in neuroglial progenitors; Nf1OPG mice), Nf1+/− (heterozygous germline Nf1 gene inactivation), and wild type (WT) mice were maintained on a C57BL/6 background.10 Surgical ovariectomy was performed in the Washington University Mouse Genetics Core.9
Pharmacological treatments
Four- to six-week-old female Nf1OPG mice were treated with 15 mg/kg leuprolide (Lupron) or saline intraperitoneally every day or with 2.5 mg/kg anti-IL-1β or control IgG antibodies intraperitoneally every other day for 8 weeks.
Immunohistochemistry
Mice were perfused with Ringer's solution followed by 4% paraformaldehyde (PFA). Optic nerves and eyeballs were post-fixed in 4% PFA overnight before processing for paraffin (optic nerves) or OCT (eyes) embedding. Immunohistochemistry and immunofluorescence staining were performed on 5-μm-thick optic nerve sections and 10-μm-thick retinal cryosections, respectively, using appropriate primary and secondary antibodies (Supplementary Table S1).11
Quantitative RT PCR
RNA was isolated from optic nerves using the NucleoSpin RNA Plus kit and cDNA generated using the High-Capacity cDNA Reverse Transcription kit. Real-time quantitative PCR (RT-qPCR) was performed by TaqMan gene expression. ΔΔCT values were calculated using Gapdh as a normalization control.
Primary RGC cultures
RGCs were isolated from postnatal day 4–7 Nf1+/− mice.11 After 5 days in vitro, RGCs were treated with 1 μg/mL IL-1β, 1 μg/mL IL-1α, or saline for 24 h, and lactate dehydrogenase (LDH) release analyzed using the CyQUANT LDH Cytotoxicity Assay kit. Immunocytochemistry was performed on PFA-fixed Nf1+/− RGCs using appropriate primary and secondary antibodies (Table S1).
Statistical analysis
Statistical analysis was performed using Prism 9.0 (GraphPad). Analyses of two groups were performed using a nonparametric Student's t test (Mann–Whitney test), where analyses of three or more groups were performed using a nonparametric one-way ANOVA (Kruskal–Wallis test) with Dunn's multiple comparison correction. Statistical significance was defined as p < 0.05.
Results
Female Nf1OPG mice harbor increased optic nerve glial IL-1β expression
IL-1β is a cytokine previously implicated in central nervous system (CNS) injury.12, 13 To examine Il-1β RNA expression in the setting of murine Nf1-OPG, we performed RT-qPCR on optic nerves from WT, Nf1+/−, and Nf1OPG mice. Whereas WT and Nf1+/− optic nerves had comparable Il-1β expression, Il-1β expression was increased 5.8-fold in Nf1OPG mice (Fig. 1A), which was confirmed at the protein level to be restricted to female Nf1OPG mice (Fig. 1B).
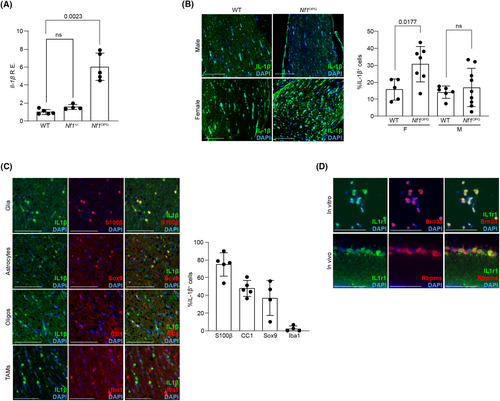
To identify the cell population responsible for IL-1β expression, immunofluorescence co-labeling experiments were performed. The majority of the IL-1β-producing cells express the glial marker S100β, including Sox9+ astrocytes and CC1+ oligodendrocytes. In contrast, tumor-associated monocytes (TAMs, Iba1+ cells) and oligodendrocyte precursor cells (PDGFRα+ cells; not shown) express little IL-1β (Fig. 1C). Consistent with IL-1β as a potential neurotoxic cytokine for RGCs, RGCs express the IL-1 receptor (IL1r1) in vitro and in vivo (Fig. 1D).
IL-1β is sufficient for RGC death in vitro and necessary for RGC death in vivo
To determine whether IL-1β is sufficient to induce Nf1+/− RGC death in vitro, LDH release was measured from Nf1+/− RGCs treated for 24 h with IL-1β, saline, or IL-1α, a closely related cytokine that also activates IL1r1 (Fig. 2A).14 Treatment with IL-1β, but not IL-1α, caused RGC death (two-fold increase), which reflected an increase in RGC apoptosis as measured by cleaved caspase-3 immunofluorescence (Fig. 2B).
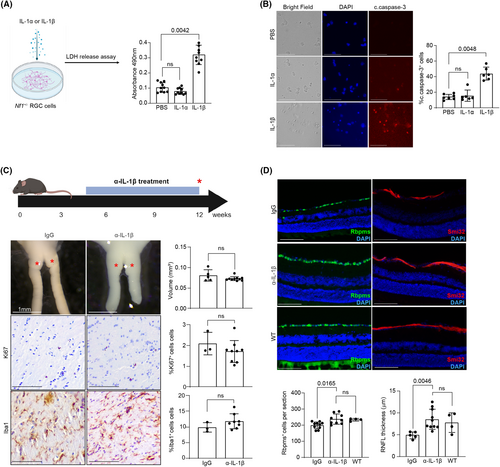
To determine whether IL-1β inhibition could ameliorate RGC death and axonal loss in vivo, female Nf1OPG mice were treated with neutralizing IL-1β (α-IL-1β) or control IgG antibodies for 8 weeks. While IL-1β inhibition had no effect on optic nerve volume, tumor proliferation (%Ki67+ cells), or TAM content (%Iba1+ cells) (Fig. 2C), RGC cell number and RNFL thickness per retinal section increased by ~20% and ~ 74%, respectively, to levels indistinguishable from WT mice (Fig. 2D). These results demonstrate that while IL-1β is not required for tumor growth, it is necessary for Nf1-OPG-induced RGC death and RNFL thinning.
Inhibition of estrogen-induced IL-1β production rescues Nf1OPG retinal pathology
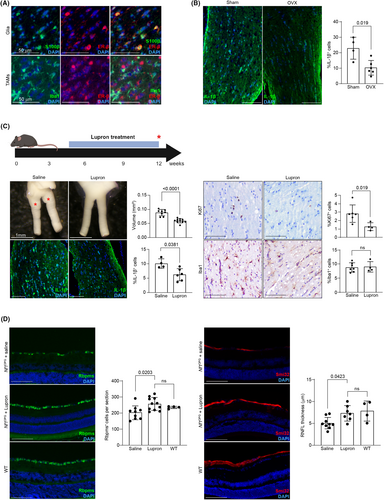
As we previously showed that the sexually dimorphic RGC dysfunction observed in female Nf1OPG mice was partly due to estrogen, rather than a male protective effect,9 we hypothesized that estrogen might be responsible for IL-1β-induced retinal pathology. Consistent with this idea, S100β+ glial cells express estrogen receptor beta (Fig. 3A), such that surgical ovariectomy decreased IL-1β+ cell content in the optic nerves of female Nf1OPG mice (Fig. 3B). Leveraging this new finding, we sought to determine whether pharmacological agents that suppress estrogen levels in children with NF1 and precocious puberty15 could block IL-1β production, RGC death, and RNFL thinning. For these experiments, female Nf1OPG mice were treated with the gonadotropin-releasing hormone (GnRH) agonist, leuprolide (Lupron), for 8 weeks. After treatment, the decrease in optic nerve IL-1β+ cell content was accompanied by a 33% decrease in optic nerve volume and a 55% decrease in tumor proliferation (%Ki67+ cells) (Fig. 3C). Importantly, RGC number and RNFL thickness increased by ~25% and ~45% after Lupron treatment, respectively, to levels indistinguishable from WT controls (Fig. 3D).
Discussion
Approximately 25%–30% of children with NF1-OPG experience some degree of vision loss due to RGC dysfunction. This visual loss can range from minimal to profound, and is not effectively reversed by currently available tumor-focused chemotherapies.7 Herein, we leverage Nf1OPG mice, which form tumors at ages similar to other murine models of pediatric brain tumors16, 17 and recapitulate the sexually dimorphic retinal and optic nerve pathology of children with NF1-OPG, to establish that estrogen-driven glial IL-1β production is responsible for the cell-extrinsic OPG-associated retinal pathology. These findings raise two key points.
First, we demonstrate that Nf1OPG mice express the neurotoxic cytokine IL-1β in a sexually dimorphic manner, which is both necessary and sufficient for RGC death and optic nerve injury in female Nf1OPG mice. The finding that glial cells, rather than TAMs, as previously reported,12 produce IL-1β in female Nf1OPG mice likely reflects the non-specific effects of minocycline.18 In contrast, treatment of female Nf1OPG mice with the more selective monocyte inhibitor (PLX3397) had no effect on IL-1β production (not shown). Intriguingly, IL-1β inhibition offers neuroprotection without affecting tumor maintenance, suggesting that IL-1β acts independently of the cellular circuits governing Nf1-OPG growth.19 Current studies are focused on elucidating the mechanism underlying estrogen-regulated glial IL-1β production.
Second, we demonstrate that estrogen is responsible for glial IL-1β production, such that Lupron, used to suppress precocious puberty in children with NF1,15 restores RGC content and RNFL thickness to WT levels in Nf1OPG mice. Additionally, anti-estrogen therapy also reduces tumor growth, suggesting that estrogen receptor function in other cells is relevant to Nf1-OPG maintenance, independent of IL-1β signaling.4, 9 While further work is required, this finding provides proof-of-concept preclinical support for the use of these agents to attenuate vision loss in children with NF1-OPG, especially for those with prechiasmatic OPGs who also exhibit a female predominance of vision loss.7, 8 Current studies are focused on defining the minimal treatment doses and durations to ameliorate retinal pathology and vision loss in Nf1OPG mice using clinically relevant measures, including ocular coherence tomography and visual optomotor system testing.4, 6
Taken together, these new preclinical discoveries establish that neuroprotective strategies targeting both the NF1 mutation-induced intrinsic (e.g., intracellular cyclic AMP levels6) and estrogen- and IL-1β-mediated extrinsic vulnerabilities might be harnessed to restore or prevent vision loss in children with these common brain tumors.
Acknowledgments
We thank Taylor John-Lewis for technical assistance and Olivia Cobb for assistance with the statistical analysis. This work was partly funded by a Research Training Grant from the National Institute of Neurological Disorders and Stroke (R25-NS090978 to Y.T.), a Research Program Award from the National Institute of Neurological Disorders and Stroke (R35-NS097211 to D.H.G.), and a Gilbert Family Foundation Vision Recovery Initiative grant (to D.H.G). The Washington University Ophthalmology Core facility is supported by funding from the National Eye Institute (P30EY002687).
Author Contributions
Concept and design of the study: Y.T. and D.H.G. Acquisition of data: Y.T., J.C., N.W., and S. B. Analysis of data: Y.T. and J.C. Drafting and editing the paper and figures: Y.T. and D.H.G.
Conflict of Interest
The authors declare no relevant conflicts of interest.
Open Research
Data Availability Statement
No exome, genome, epigenetic, or microarray data were generated as part of this study.




