Effects of sulfatide on peripheral nerves in metachromatic leukodystrophy
Target journal: ACTN
Abstract
Objective
To evaluate the longitudinal correlations between sulfatide/lysosulfatide levels and central and peripheral nervous system function in children with metachromatic leukodystrophy (MLD) and to explore the impact of intravenous recombinant human arylsulfatase A (rhASA) treatment on myelin turnover.
Methods
A Phase 1/2 study of intravenous rhASA investigated cerebrospinal fluid (CSF) and sural nerve sulfatide levels, 88-item Gross Motor Function Measure (GMFM-88) total score, sensory and motor nerve conduction, brain N-acetylaspartate (NAA) levels, and sural nerve histology in 13 children with MLD. Myelinated and unmyelinated nerves from an untreated MLD mouse model were also analyzed.
Results
CSF sulfatide levels correlated with neither Z-scores for GMFM-88 nor brain NAA levels; however, CSF sulfatide levels correlated negatively with Z-scores of nerve conduction parameters, number of large (≥7 μm) myelinated fibers, and myelin/fiber diameter slope, and positively with nerve g-ratios and cortical latencies of somatosensory-evoked potentials. Quantity of endoneural litter positively correlated with sural nerve sulfatide/lysosulfatide levels. CSF sulfatide levels decreased with continuous high-dose treatment; this change correlated with improved nerve conduction. At 26 weeks after treatment, nerve g-ratio decreased by 2%, and inclusion bodies per Schwann cell unit increased by 55%. In mice, abnormal sulfatide storage was observed in non-myelinating Schwann cells in Remak bundles of sciatic nerves but not in unmyelinated urethral nerves.
Interpretation
Lower sulfatide levels in the CSF and peripheral nerves correlate with better peripheral nerve function in children with MLD; intravenous rhASA treatment may reduce CSF sulfatide levels and enhance sulfatide/lysosulfatide processing and remyelination in peripheral nerves.
Introduction
Metachromatic leukodystrophy (MLD) is a rare lysosomal storage disease affecting the central nervous system (CNS) and the peripheral nervous system (PNS).1, 2 It is characterized by deficient activity of arylsulfatase A (ASA), which functions to break down sulfatide, a structural component of myelin.3, 4 For the nervous system to function optimally, myelin undergoes continual degradation and replenishment.5 Deficient ASA activity causes accumulation of sulfatide and lysosulfatide in oligodendrocytes and Schwann cells during this turnover.1, 2 Progressive demyelination and axonal loss follows, resulting in cognitive and motor dysfunction.1, 2
Three clinical forms of MLD have been classified by age of onset: late-infantile (<30 months old), juvenile (2.5–16 years old), and adult (>16 years old).1, 2 Although genotype–phenotype correlations are not fully established, late-infantile MLD is generally associated with homozygosity or compound heterozygosity for alleles that cause very limited or absent ASA activity.2, 6, 7 Late-infantile MLD is the most severe subtype: patients typically experience complete loss of locomotion before age 4 years.8, 9 Although CNS deterioration dominates the clinical picture in late-infantile MLD, PNS symptomatology also contributes to morbidity.10
The association between severity of ASA deficiency and rapidity of disease progression suggests that the level of accumulated (lyso)sulfatide reflects the extent of demyelination. In exploratory cross-sectional analyses of baseline clinical trial data, sural nerve and cerebrospinal fluid (CSF) sulfatide levels were strongly correlated with motor and sensory nerve dysfunction in patients with MLD.11 Increased sulfatide levels alongside demyelination in the PNS have also been observed in MLD mouse models.12 Furthermore, lysosulfatide has cytotoxic properties in cell cultures.13
Several histological abnormalities may be observed in MLD, including reduced large myelinated fiber density, thinning of myelin sheaths, axonal atrophy, increased nerve g-ratios, higher demyelination load, and nerve enlargement.11, 14-17 However, not all of these are associated with peripheral nerve conduction abnormalities.16 Further investigation is required to understand how each relates to CNS and PNS (lyso)sulfatide levels in patients with MLD.
Autologous hematopoietic stem cell gene therapy with atidarsagene autotemcel (Libmeldy, Orchard Therapeutics) has been approved in some regions for the treatment of patients with presymptomatic/early-symptomatic juvenile MLD or presymptomatic late-infantile MLD.18, 19 However, an unmet therapeutic need remains for symptomatic patients. Studies in MLD mouse models demonstrated that treatment with intravenous enzyme replacement therapy reduced sulfatide storage in peripheral nerves and improved nerve conduction velocities (NCVs).20 Although the clinical trial of intravenous recombinant human ASA (rhASA; HGT-1111, previously known as Metazym) was discontinued owing to lack of efficacy, CSF sulfatide levels were reduced and peripheral nerve function, assessed by electrophysiology and morphometry, remained relatively stable, suggesting that treatment may partially protect against disease progression in the PNS.21
This study sought to explore further the possible correlations between (lyso)sulfatide levels and CNS and PNS function in children with MLD by analyzing longitudinal data from the intravenous rhASA clinical trials.21 Additionally, the potential impact of intravenous rhASA on myelin turnover in the PNS was assessed using ultrastructural nerve images from patients in the trials and an untreated mouse model.
Methods
Intravenous rhASA Phase 1/2 clinical trial and extension
Study population and design
Data were obtained from 13 patients enrolled in a Phase 1/2 study (NCT00418561; NCT00633139) and long-term extension (NCT00681811) designed to assess the safety and efficacy of intravenous rhASA in children with MLD. Full study design, inclusion criteria, and safety and efficacy outcomes of this single-center, open-label, non-randomized, dose-escalation study have been described previously.21 Briefly, patients with a confirmed diagnosis of MLD and aged 12 months–6 years at screening were included in the trial. Patients received intravenous rhASA (50, 100, or 200 U/kg body weight) every other week (EOW) for 52 weeks (Fig. 1A). Patients who completed the initial Phase 1/2 trial were enrolled into the extension study. This study was discontinued owing to lack of efficacy after 24 months. Assessments of CSF sulfatide levels, 88-item Gross Motor Function Measure (GMFM-88) total score, sensory and motor nerve conduction in the median, fibular, and sural nerves, brain N-acetylaspartate (NAA) levels, sural nerve sulfatide/lysosulfatide levels, and nerve morphometry were performed at frequencies indicated in Figure 1B.
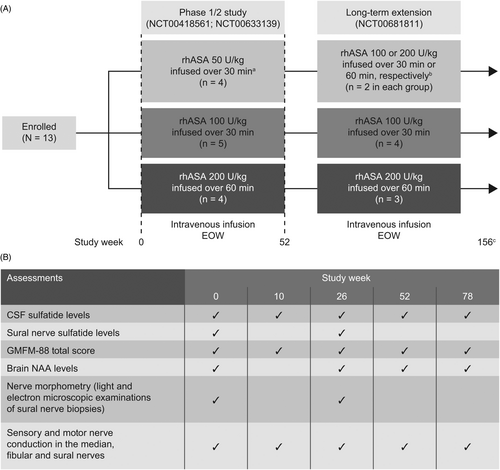
All study-related documents (including protocols, protocol amendments, and informed consent forms) were reviewed and approved by the Ethics Committees for Copenhagen and Frederiksberg Municipalities, Denmark. The studies were conducted in accordance with the International Conference on Harmonisation of Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki. Local regulations in Denmark were also adhered to. Each patient's legal guardian(s) provided informed consent before study-related activities were performed.
Measurement of sulfatide and lysosulfatide levels
Right sural nerve biopsies were examined in 12 children at baseline11 and contralateral left sural nerve biopsies were examined in 11 children at 26 weeks. Sulfatide and lysosulfatide in the sural nerves (ng/mg dry weight) were measured using high-pressure liquid chromatography–mass spectrometry as described previously.11 CSF sulfatide levels (nmol/L) were measured using thin-layer chromatography and immunostaining with the Sulph I anti-sulfatide antibody following a previously described methodology.22, 23
Motor function evaluation
Motor function in children with MLD was assessed using GMFM-88 total score as described previously.24 Z-scores were estimated based on normative data from a control population of children without known motor dysfunction.25 An exponential regression equation for the normative population was estimated at ages 20–80 months as: ; individual Z-scores were calculated using the resulting age-specific means and an estimated standard deviation (SD) of 5.
Evaluation of brain NAA levels
Sequence-dependent NAA/Cr ratios in the centrum semiovale of patients with MLD were measured via magnetic resonance spectroscopy imaging as described previously.21, 26 Z-scores were estimated using normative data from an adult control population based on a mean ± SD of 3.49 ± 0.39.
Electrophysiological assessments
Nerve conduction studies were performed in the right median nerve (motor and sensory fibers), the right fibular nerve (motor fibers), and the right and left sural nerves (sensory fibers; left only at follow-up) in patients as described previously.11, 21 The compound muscle action potentials (CMAP) were obtained in the abductor pollicis brevis and the extensor digitorum brevis. Z-scores of the amplitudes of the CMAP and sensory nerve action potential (SNAP), the distal motor latencies, the motor NCVs (MNCVs), and the sensory NCVs (SNCVs) were estimated for each nerve using normative data from children in the control group of the same ages without neurological disease.27, 28 The means of the amplitude and conduction velocity Z-scores were calculated and used in the analysis owing to a strong observed correlation between these Z-scores.
Bilateral median and tibial nerve somatosensory-evoked potentials (SSEPs) were measured using surface electrodes as described previously.11
Nerve morphometry
Light microscopy micrographs at 40× and 100× magnification of sural nerve biopsies taken from the children recruited into the clinical trial were examined, and measures of fiber diameter and density were assessed as described previously.11
For ultrastructural studies, 70 nm cross-sections of the nerves were examined via electron microscopy, and myelin thickness and nerve g-ratios (the ratio of the inner axonal diameter to the total outer diameter) were calculated as described previously.11 To investigate the morphology of the Remak bundles and associated Schwann cells, 20 electron micrographs per nerve were acquired using a Zeiss Libra transmission electron microscope at 8000–10,000× magnification. The electron micrographs were obtained from fields of views representing the entire cross-sectional area of the nerve. The process used to sample the nerves ensured that all fascicles were represented. The amount of endoneural litter and fragmented basal lamina (indicating fiber degeneration) per image was counted, and the mean across the 20 images was calculated for each nerve. Inclusion bodies per Schwann cell unit in Remak bundles and denervated Schwann cell processes (indicative of unmyelinated axon loss) were also counted in each image.
MLD mouse model
Nerve morphometry
Nerve pathology was examined in sciatic and urethral nerves from a male mouse model of aggravated MLD. This mouse model (transgenic[tg]/ASA[−/−]) possesses an ASA deficiency and overexpression of the sulfatide-synthesizing enzyme galactose-3-O-sulfotransferase-1 in myelinating cells.12 Mice (two aggravated MLD mice and three control tg/ASA[+/−] mice; 16–20 months old) were fixed in a solution of 4% paraformaldehyde with 2% glutaraldehyde in 1× phosphate-buffered saline. Sciatic nerves and penises were dissected out and post-fixed in the same solution. The dissected samples were processed for electron microcopy analysis by immersion in osmium tetroxide followed by embedding in Epon (reagents from Electron Microscopy Sciences, PA, USA). The sciatic nerves and penises were sectioned at 70 nm and stained with citrate/uranyl acetate. Electron micrographs were acquired using a Zeiss Libra transmission electron microscope at 8000–10,000× magnification.
Statistical analyses
All pathology analyses were done blinded to the clinical and electrophysiology picture and sulfatide measurements. Simple linear regressions were used to investigate the relationships between CSF sulfatide levels and measures of motor function, brain NAA levels, electrophysiological assessments, and nerve morphometry as described above. Simple linear regressions were also used to investigate the relationship between sural nerve lysosulfatide levels and endoneural litter and inclusions in the Remak bundles. The differences in nerve g-ratio and inclusion bodies between baseline and Week 26 were calculated using paired-samples t-tests. P values for GMFM-88 and NAA changes over time were obtained using the Kruskal-Wallis non-parametric test. All reported correlations were confirmed using Spearman rho (R); correlations were considered weak for absolute values of ≤0.3, moderate for absolute values of >0.3–0.5, and strong for absolute values of >0.5–1.0.
To evaluate the effect of intravenous rhASA treatment over the 78-week study period, the change in CSF sulfatide levels was assessed by calculating the ratio between the levels at individual time points and baseline (for one patient, the baseline measurement was lost and the 10-week measurement was used as baseline instead) and log-transformed.
Results
Motor function and brain NAA findings
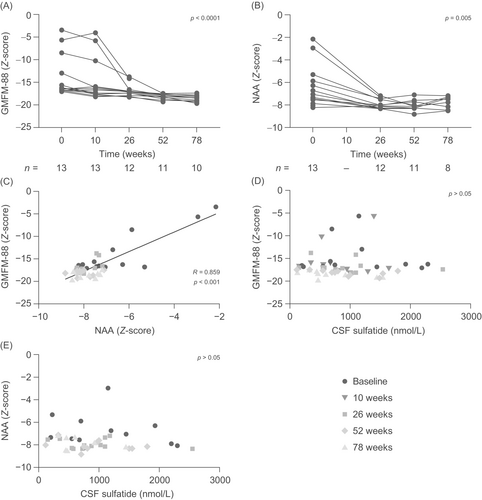
In total, 13 children with MLD (eight girls and five boys; age range: 24.0–59.0 months at baseline) were enrolled in the initial clinical study of intravenous rhASA; of these, 11 children continued into the extension study (Fig. 1A). Full characteristics of the study population have been described previously.11, 21 Motor function, as assessed by GMFM-88 total scores, strongly declined over the course of the 78-week study period (P < 0.0001; Fig. 2A), and was substantially impaired compared with a control population of children of a similar age, with a Z-score below −15 for all patients with data available at Weeks 52 and 78. Brain NAA levels also showed a clear decrease over the study period (P = 0.005; Fig. 2B) and were significantly lower than those in the control population; Z-scores fell to below −7 in all patients with data available at Weeks 52 and 78. GMFM-88 Z-scores showed a strong positive correlation with NAA Z-scores (R = 0.859, P < 0.001; Fig. 2C); however, neither GMFM-88 scores nor brain NAA levels were correlated with sulfatide levels measured in the CSF (both P > 0.05; Fig. 2D, E).
Relationship between sulfatide levels and peripheral nerve electrophysiology
Overall, the Z-scores for the combined amplitudes of CMAPs and SNAPs and the Z-scores for the combined MNCVs and SNCVs remained relatively stable over the course of the study period, as reported previously.21 Strong negative correlations were observed between both these parameters and CSF sulfatide levels (R = −0.638, P < 0.0001 and R = −0.642, P < 0.0001, respectively; Fig. 3A, B). The cortical latencies of the SSEPs in the median and tibial nerves did not change significantly over the 78-week observation period, but they did show strong positive correlations with CSF sulfatide levels (R = 0.511, P = 0.0005 and R = 0.516, P = 0.0011, respectively; Fig. 3C, D). There were also moderate negative correlations between the sulfatide level in the sural nerves and the nerve conduction parameters (combined Z-scores for CMAP and SNAP amplitudes: R = −0.454, P = 0.0297; combined Z-scores for MNCVs and SNCVs: R = −0.465, P = 0.0254). The amplitudes and the conduction velocities of motor and sensory responses both strongly correlated with the number of large (≥7 μm) fibers (R = 0.844 and 0.897, respectively; both P < 0.0001) (data not shown).
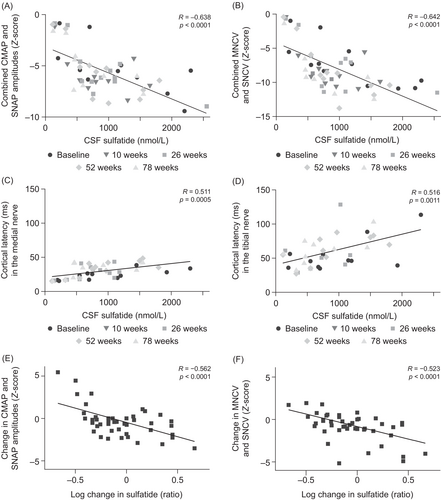
However, in an analysis in individual patients, the log change in CSF sulfatide levels and treatment dose over the 78-week period were strongly negatively correlated (R = −0.544, P < 0.0001). This reduction in sulfatide levels strongly correlated with an improvement in nerve conduction parameters (combined Z-scores for CMAP and SNAP amplitudes: R = −0.562, P < 0.0001; combined Z-scores for MNCVs and SNCVs: R = −0.523, P < 0.0001; Fig. 3E, F). The change in sulfatide levels, however, did not significantly correlate with GMFM-88 or NAA Z-scores.
Overall, these findings suggest that lower sulfatide levels in the CSF and peripheral nerves correlate with better peripheral nerve function.
Relationship between (lyso)sulfatide levels and peripheral nerve morphometry
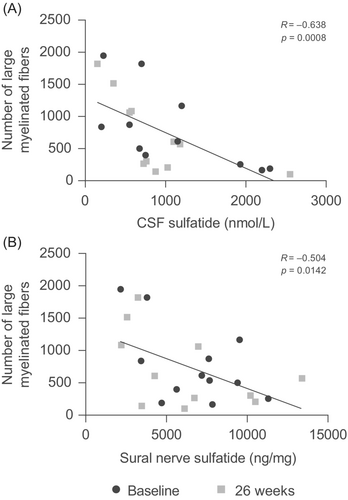
As reported previously, there was no significant change in the number of sural nerve fibers from baseline to 26 weeks.21 Over this time period, however, there was a strong negative correlation between CSF sulfatide levels and the number of large myelinated fibers (R = −0.638, P = 0.0008; Fig. 4A). Similarly, the number of large myelinated fibers showed a strong negative correlation with sulfatide levels in the sural nerves (R = −0.504, P = 0.0142; Fig. 4B). Axon diameters remained normal in all patients and were not correlated with sulfatide levels or electrophysiological measures.
Ultrastructural examination allowed calculation of the g-ratio (the ratio of the inner axonal diameter to the total outer diameter) in sural nerves. Over the course of the 26-week period, CSF sulfatide levels showed a strong positive correlation with nerve g-ratios (R = 0.608, P = 0.0027; Fig. 5A). Consistently, a moderate negative correlation was observed between CSF sulfatide levels and the slope of myelin thickness versus fiber diameter (myelin/fiber diameter slope) (R = −0.457, P = 0.0323; Fig. 5B). Endoneural litter, a marker of nerve fiber degeneration, was also quantified in the sural nerves. There was a moderate positive correlation between the amount of endoneural litter per section and sural nerve sulfatide levels (R = 0.475, P = 0.0219; Fig. 5C) and a strong positive correlation with sural nerve lysosulfatide levels (R = 0.511, P = 0.0150; Fig. 5D). No relationship was observed between nerve g-ratios and the number of inclusion bodies over the 26-week period.
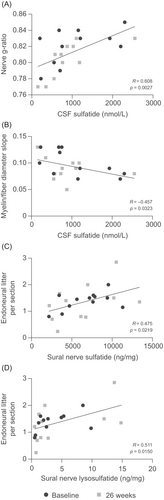
Effect of intravenous rhASA treatment on peripheral nerve morphometry
Overall, there was a 2% decrease in nerve g-ratio from baseline to 26 weeks of intravenous rhASA treatment (P = 0.0342; Fig. 6A). Combined with the observed stability in axon diameters (no change from baseline to 26 weeks; P = 0.280), this suggests an overall increase in myelin sheath diameter after treatment. There was also a 55% increase in the number of inclusion bodies per Schwann cell unit during the observation period (P = 0.0394; Fig. 6B).
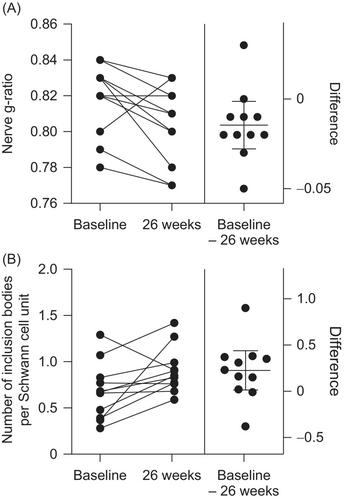
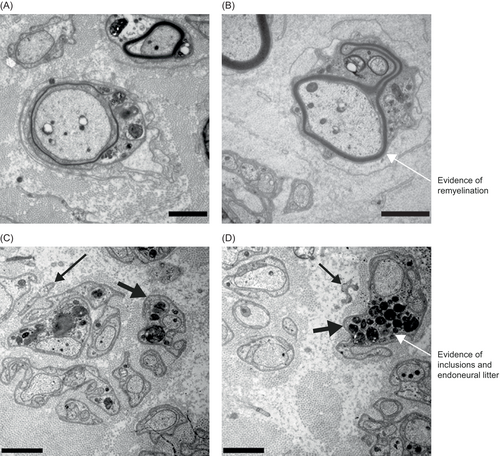
Figure 7 provides an illustrative example of pre- (Fig. 7A) and posttreatment (Fig. 7B–D) ultrastructural nerve findings from one patient. Potential remyelination can be observed after treatment (Fig. 7B), along with increased inclusion bodies in Remak bundles (Fig. 7C), suggesting a potential clearance of endoneural litter (Fig. 7D).
Findings from tg/ASA(−/−) mice
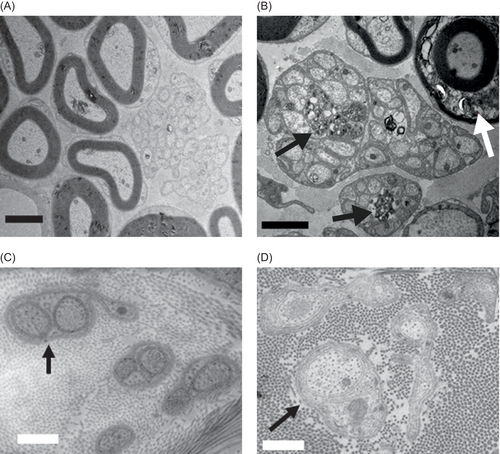
To investigate whether the accumulation of abnormal sulfatide in Schwann cells of Remak bundles may reflect phagocytosis of metachromatic inclusions from myelin-forming Schwann cells, we compared electron micrographs of Remak bundles in the sciatic nerves (with mixed myelinated and unmyelinated fibers) and the urethral nerves (unmyelinated fibers) in an aggravated MLD mouse model.
Remak bundles in the urethral nerves of tg/ASA(−/−) mice did not contain abnormal sulfatide storage material and were indistinguishable from those of controls (Fig. 8). In contrast, non-myelinating Schwann cells in Remak bundles of the sciatic nerve of tg/ASA(−/−) mice contained abnormal sulfatide storage material. Together, these findings suggest that non-myelinating Schwann cells of Remak bundles do not accumulate sulfatide storage material produced endogenously but instead accumulate sulfatide from an exogenous source by phagocytosis.
Discussion
The present analyses of longitudinal data from a Phase 1/2 trial and extension study of intravenous rhASA in children with MLD suggest that lower sulfatide levels in both the CSF and peripheral nerves correlate with improved peripheral nerve function. Furthermore, sulfatide levels may affect myelination processes in peripheral nerves, and continued treatment with intravenous rhASA may be associated with lowering sulfatide levels in the CSF and phagocytic removal of debris and remyelination; thus, peripheral nerve function may be partially recoverable in MLD.
The longitudinal correlation of sulfatide levels with peripheral nerve pathology, but not motor dysfunction, supports previous cross-sectional findings from the baseline analysis of this patient population.11 These findings, together with the longitudinal correlation between GMFM-88 and NAA Z-scores, are consistent with initial indications from the overall primary trial results, specifically that CNS pathology, and not PNS pathology, is probably the primary contributor to motor dysfunction in this patient population with MLD.21 In some patients with late-infantile MLD, rapidly progressive peripheral neuropathy precedes CNS symptoms and can be associated with motor and sensory deficits10; however, the multiple analyses of data from this clinical trial and extension study suggest that CNS pathology dominates motor dysfunction in the majority of patients. Similar discordance between CNS and PNS in function and pathology have been reported previously.16, 29 For example, an analysis of patients who underwent hematopoietic stem cell transplantation (HSCT) demonstrated that the severity of neuropathy as measured by NCV did not correlate with functional outcome after HSCT.29
There was a negative correlation between sulfatide levels and the number of large myelinated fibers, consistent with the cross-sectional analysis of baseline data.11 The present analysis also showed a positive longitudinal correlation between CSF sulfatide levels and nerve g-ratios and a negative longitudinal correlation between CSF sulfatide levels and the myelin/fiber diameter slope, suggesting an effect of sulfatide levels on myelination processes in peripheral nerves. This negative effect on myelin formation in Schwann cells may occur through several mechanisms. For example, sulfatide accumulation could alter the cerebroside–sulfatide ratio and cause insufficiencies in the metabolic processes underlying myelin formation.10, 30 Indeed, ultrastructural studies have suggested that the abnormal myelin patterning in the Schwann cells of patients with MLD resembles an aborted attempt at normal myelin production.31 It is also possible that sulfatide exerts an inhibitory effect on axonal outgrowth and regeneration.32, 33 Overexpression of a sulfatide-synthesizing enzyme has also been shown to lead to demyelination of neuronal axons in mouse models of MLD.12 ASA-deficient mice also demonstrated a delay in myelination without any significant reduction of oligodendrocyte numbers, suggesting that the accumulation of sulfatide may inhibit the signaling processes necessary for normal myelin formation in these cells.34 However, it is currently unknown whether this interpretation can extend to our findings in Schwann cells.
The positive correlation between sural nerve sulfatide and lysosulfatide levels and endoneural litter per Schwann cell unit (indicating fiber degeneration) in the present longitudinal analysis supports our hypothesis of the potential cytotoxic contribution of these compounds to nerve degeneration; however, the exact mechanisms behind these effects remain unknown. Lysosulfatide has shown cytotoxic properties in cell cultures,13 whereas sulfatide is a natural component of brain myelin.35 The lipid bilayer has demonstrated instability in patients with MLD.36 It is possible that sulfatide and lysosulfatide exert their cytotoxic effects on myelin through contrasting mechanisms, and future work is required to explore these in detail.
Other processes involved in MLD pathogenesis may also have contributed to the correlations observed in this study. For instance, there is evidence to suggest a neuroinflammatory component in MLD pathogenesis and that complement activation may amplify myelin damage.10, 37-39 Future work is necessary to investigate the mechanisms behind these correlations directly.
In a previous analysis, there were no significant changes in the numbers of large or small fibers in peripheral nerves over 26 weeks of treatment with intravenous rhASA, suggesting a potential protective effect of treatment against disease progression in the PNS.21 Here, we observed a correlation between a reduction in CSF sulfatide levels and continued high-dose treatment; this reduction correlated with improved peripheral nerve conduction. Furthermore, using ultrastructural examination, we demonstrated a reduction in sural nerve g-ratio (with constant nerve diameter), suggesting an overall increase in myelin sheath diameter, and an increase in inclusion bodies within Remak bundles after the 26-week treatment with intravenous rhASA. Non-myelinating Schwann cells and macrophages act to clear myelin debris from the PNS40-42; therefore, the change in amount of inclusion bodies in Remak bundles and denervated Schwann cell processes before and after treatment suggests enhanced turnover of sulfatide and lysosulfatide levels in treated patients with MLD. This hypothesis is further supported by the comparison of sulfatide storage material present in Remak bundles in the urethral and sciatic nerves of untreated tg/ASA(−/−) mice. Nerve fibers in the urethra are unmyelinated, given that the motor innervation is predominantly autonomic, whereas the sciatic nerve consists of more typically myelinated somatic motor and sensory axons. The lack of abnormal sulfatide storage in Remak bundles observed in the urethral nerve and the abnormal amounts of storage in the Remak bundles of the sciatic nerve suggest that sulfatide inclusions do not accumulate in Remak bundles endogenously but arise from phagocytic removal of myelin debris from myelin-forming Schwann cells. These findings align with a previous study of nerve cell injury, which suggested that Schwann cells contribute to both the initial endogenous breakdown of myelin and the phagocytic phase,43 which is typically driven by macrophages.44 Further investigation into the role of macrophages in phagocytic removal of myelin debris before and after treatment would also benefit our understanding. Phagocytic removal of debris by Remak bundles may be transferring toxicity to the Schwann cells in Remak bundles, perhaps offering a rebalance of sulfatide levels, and allowing for remyelination, which supports the potential therapeutic effects of intravenous rhASA in the PNS. Further, lower nerve g-ratios in the absence of changes in nerve diameter are suggestive of remyelination processes and may indicate a treatment effect in these peripheral nerves. Visual analysis of electron micrographs provided qualitative support for this finding, with examples of improved remyelination in these patients. Evidence of remyelination following treatment has also been observed in patients with MLD who underwent HSCT.45
Although this work offers several insights into the pathogenesis of MLD in the PNS, limitations must be considered. The results here are correlational, and further work is necessary to establish causal relationships and to understand the underlying mechanisms. The potential evidence of remyelination observed via electron microscopy in both mice and patient data, although supportive of the overall hypothesis, is a static and illustrative observation that would benefit from further corroboration. However, it is reassuring that the analyses from the electron microcopy of tg/ASA(−/−) mice supported our hypothesis regarding phagocytic clearance of myelin debris. Aspects of the trial design itself, such as the relatively short period of observation, may have made it difficult to observe changes in PNS function and may also have contributed to the relative lack of treatment-related changes. Given that the population of this study consisted of symptomatic patients with MLD, it is likely that some floor effects were present, with CNS and PNS function being already substantially impaired in some patients at baseline. Residual nerve function is also challenging to measure because the data display a low signal-to-noise ratio.
It is important to note that MLD is a condition defined by CNS symptomatology. As such, peripheral neuropathy may have a limited impact on the overall clinical picture of MLD; any therapeutic effect of intravenous rhASA in maintaining stability of peripheral nerve function is likely to be masked by the dominating gross motor dysfunction of cerebral origin.10, 11, 21 The progressive decline in CNS function with the relative preservation of PNS function suggests that direct intrathecal (rather than intravenous) delivery of rhASA would be required to address the CNS component of MLD. The results from a Phase 1/2 trial of intrathecal rhASA (NCT01510028) suggested a trend towards delayed decline of motor function in patients receiving the highest doses, supporting a potential therapeutic effect of rhASA when delivered to the appropriate target site.24 Nonetheless, the results here also highlight the importance of ensuring that any potential treatment also reduces peripheral sulfatide levels to preserve PNS function in children with MLD.
Together, these longitudinal analyses provide additional insight into the relationships between sulfatide/lysosulfatide levels and PNS function, extending previous observations from the Phase 1/2 trial of intravenous rhASA of relatively stable peripheral nerve function despite a progressive decline in CNS manifestations, while also providing evidence of a possible effect of (lyso)sulfatides on myelination processes in peripheral nerves.11, 21 Further research on this potential cytotoxic effect of (lyso)sulfatide on peripheral myelination is needed to understand the underlying mechanisms and whether treatment with intravenous rhASA may facilitate remyelination. In particular, longitudinal quantitative analysis of relationships between sulfatide levels, inclusion bodies, nerve g-ratios, and endoneural litter will be required to explore the contribution of improved phagocytic removal of debris to the remyelination process.
Acknowledgments
This study was funded by Shire (a Takeda company). Under the direction of the authors and in accordance with the International Committee of Medical Journal Editors Uniform Requirements, Emma Davies PhD of Oxford PharmaGenesis, Oxford, UK provided writing assistance for this manuscript. Editorial assistance in formatting, proofreading, copy editing, and fact-checking was also provided by Oxford PharmaGenesis, and funded by Takeda Development Center Americas, Inc. The sponsor was involved in the study design, but data collection, analysis, and interpretation were made by the authors independently. The authors would like to thank Volkmar Gieselmann for his provision of the MLD mouse models and insightful feedback on the mouse electron microscopy data, and John W. Griffin (Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD, USA), who made a valuable contribution to the ultrastructural analyses and who sadly passed away in 2011.
Author Contributions
Conception or design of the study: M.H.F., C.D., N.B., and C.K. Data acquisition: M.H.F., C.D., N.B., and C.K. Analysis of data: M.H.F., C.D., S.G., M.M., D.A.H.W., C.J.M., I.K.-M., J.L., N.B., and C.K. Drafting the manuscript: M.H.F., C.D., S.G., M.M., D.A.H.W., C.J.M., I.K.-M., J.L., N.B., and C.K.
Conflicts of Interest
M.H.F. has nothing to disclose. C.D. reports personal fees from University Hospital Copenhagen Rigshospitalet during the conduct of the study. S.G. reports an institutional research grant from Shire (a Takeda company) outside of the submitted work. He serves as an adviser for trials in MLD for Clario, Homology Medicines, and Passage Bio, but receives no personal payment related to this role. S.G. is a member of the European Reference Network for Rare Neurological Diseases, project ID 739510, and was partly supported by DFG grant GR 4688/2-1. M.M. has nothing to disclose. D.A.H.W. is a full-time employee of Takeda and a stockholder of Takeda Pharmaceutical Company Limited. C.J.M. is a full-time employee of Takeda and a stockholder of Takeda Pharmaceutical Company Limited. I.K.-M. reports grants from Shire (a Takeda company) during the conduct of the study. J.L. was a full-time employee of Takeda and a stockholder of Takeda Pharmaceutical Company Limited at the time of the study. N.B. was a full-time employee of Takeda and a stockholder of Takeda Pharmaceutical Company Limited at the time of the study. C.K. reports grants and personal fees from Shire (a Takeda company) and grants from Danish Medical Research during the conduct of the study. C.K. has also received royalties for teaching chapters from Gyldenal and FADL publishers.
Open Research
Data Availability Statement
Shire (a Takeda company) does not plan to share data supporting the results reported in this article as, due to the limited number of study sites/participants, there is a reasonable likelihood that individual patients could be reidentified.




