Brain imaging signatures in amyotrophic lateral sclerosis: Correlation with peripheral motor degeneration
Abstract
Objective
This study aimed to explore the clinical significance of brain imaging signatures in the context of clinical neurological deficits in association with upper and lower motor neuron degeneration in amyotrophic lateral sclerosis (ALS).
Methods
We performed brain MRI examinations to quantitatively evaluate (1) gray matter volume and (2) white matter tract fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD). Image-derived indices were correlated with (1) global neurological deficits of MRC muscle strength sum score, revised amyotrophic lateral sclerosis functional rating scale (ALSFRS-R), and forced vital capacity (FVC), and (2) focal scores of University of Pennsylvania Upper motor neuron score (Penn score) and the summation of compound muscle action potential Z scores (CMAP Z sum score).
Results
There were 39 ALS patients and 32 control subjects matched for age and gender. Compared to controls, ALS patients had a lower gray matter volume in the precentral gyrus of the primary motor cortex, which was correlated with FA of corticofugal tracts. The gray matter volume of the precentral gyrus was correlated with FVC, MRC sum score, and CMAP Z sum score, while the FA of the corticospinal tract was linearly associated with CMAP Z sum score and Penn score on multivariate linear regression model.
Interpretation
This study indicated that clinical assessment of muscle strength and routine measurements on nerve conduction studies provided surrogate markers of brain structural changes for ALS. Furthermore, these findings suggested parallel involvement of both upper and lower motor neurons in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a relentless neurodegenerative disorder with multiple mechanisms affecting the upper motor neurons in the primary motor cortex of the precentral gyrus and the lower motor neurons in the ventral horn of the spinal cord innervating skeletal muscles.1 The degeneration of the upper motor neurons in the primary motor cortex results in reduced gray matter volume of the precentral gyrus2 and subcortical gray matter structures.2 The degree of gray matter atrophy paralleled the reduction of motor neuron density in the primary motor cortex.3 Gray matter volume of the primary motor cortex was correlated with upper motor neuron signs of ALS.4, 5 Since the previous studies mainly evaluated the overall functions of upper motor neuron, there remained an important issue unanswered, that is, the relationship between the degree of gray matter atrophy and lower motor neuron degeneration. In addition to the gray matter degeneration, the degeneration of subcortical white matter was evident in ALS.4, 6 The degeneration of the white matter was commonly accessed by diffusion tensor imaging, with the fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD) being the most commonly used parameters. Axonal degeneration and demyelination of the white matter tracts underly this phenomenon of changes in FA, AD, RD, and MD.2 The change in white matter integrity mainly reflects the clinical manifestations of upper motor neurons.7 However, the relationship between the structural integrity of the white mater tracts and the other aspects of ALS, such as lower motor neuron degeneration and white matter tracts integrity, along with the possible contribution of the former to the latter, had not been systemically examined.8 Furthermore, despite the fact that ALS predominantly affects motor neurons in the brain and spinal cord pathways, pathology studies have indicated the involvement of additional brain areas and tracts9 paralleling the spread of TDP-43.10, 11 Such observations raised the intriguing issues of (1) neuroimaging patterns in ALS in addition to the degeneration of primary motor cortex and corticospinal tract and (2) its relationship with the clinical deficits of upper and lower motor neurons degeneration.
Currently, clinical examinations are used to evaluate the upper and motor neuron dysfunction in ALS. However, clinical evaluation of these parameters may be interfered by various factors, while image study may reveal a more accurate description of neurodegeneration in these patients. To address the above issues, we examined the structural MRI in ALS to access the gray matter volume and white matter tract integrity via parameters as derived from T1-weighted images and diffusion tensor images, respectively, and analyzed the relationship of imaging signatures with the parameters reflecting the degeneration of upper and lower motor neurons.
Methods
Subjects and study design
The study enrolled patients with ALS who visited or were referred to the Department of Neurology of National Taiwan University Hospital, Taipei, Taiwan from January 2016 to December 2021. This study enrolled patients that fulfilled the diagnosis criteria of definite or probable ALS according to the revised Awaji Criteria.12 The exclusion criteria were significant sensory complaints, presence of other peripheral or central nervous disorders, and trauma history involving the peripheral nerves, spinal cord and brain. All the patients were regularly followed–up, and received medical treatments and supportive care such as non-invasive ventilation and parenteral nutritional support depending on the neurological deficits of the patients.
The enrolled patients underwent detailed evaluations by the study protocol (Fig. 1). In addition to clinical history and neurological examinations, the general conditions were assessed with the revised amyotrophic lateral sclerosis functional rating scale (ALSFRS-R)13 and King's clinical staging system for ALS (King's stage).14 Muscle strength was measured according to the Medical Research Council (MRC) grading.15 The upper motor neuron signs were graded using a modified version of the University of Pennsylvania upper motor neuron score (Penn score),16 by which we scored the right and left upper/lower extremities separately and added the score of either side to form a “left” or “right” upper motor neuron sign score to correlate with unilateral structure parameters. All patients received brain MRI for the structure of motor-related gray and white matters, and nerve conduction study and electromyography for the electrophysiology of motor neurons following the established protocols detailed in the following paragraphs. In addition, we recruited 32 controls (13 men, p = 0.028; age: 63.09 ± 9.48 years, p = 0.130) for comparison of image parameters in the present study. The controls fulfilled the following criteria: (1) absence of neurological symptoms and absent past history of neurological disorders, (2) normal findings on neurological examinations and standardized neuropsychiatric tests, (3) no significant renal or hepatic dysfunction, and (4) exclusion of significant abnormalities in the neurological images as interpreted by radiologists.
The study was approved by the Institutional Review Board of National Taiwan University Hospital and conducted in compliance with the declaration of Helsinki. Written informed consent was obtained from all participants before all procedures in the study.
Electrophysiological evaluation
All the enrolled patients received electromyogram and nerve conduction studies using the Nicolet Viking EDX system (Natus Neurology, Middleton, WI, USA) according to standard protocol.17 Muscles in the cranial, cervical, thoracic, and lumbar segments were all sampled, with at least 2 muscles in the cervical and lumbar segments along with at least 1 muscle in the cranial and thoracic segment sampled, as stated in the Awaji criteria.12 Insertional activities, spontaneous activities, and volitional activities were recorded in the sampling of either muscle. Areas with significant previous trauma or surgical interventions were avoided from sampling.
For the compound muscle action potential Z score (CMAP Z sum score), we calculated the number of standard deviations from the mean (Z score)18 based on the reference values in our laboratory of the distal CMAP amplitude in four different nerves on either side,19 namely median, ulnar, peroneal, and tibial nerves. The CMAP Z sum score was the summation of the Z scores from these parameters.
Image studies
MRI on the brain and cervical spinal cord was performed on a 3-Tesla whole-body system (Tim Trio, Siemens, Erlangen, Germany) using an eight-channel phased-array head coil and a four-channel phased-array neck coil for signal reception following our reported imaging protocols,20 including three-dimensional T1-weighted gradient echo (repetition time [TR] = 2530 ms, echo time [TE] = 2.27 ms, inversion time [TI] = 1100 ms), T2-weighted turbo spin echo (TR/TE = 5500/88 ms), fluid-attenuated inversion recovery (TR/TE/TI = 10000/93/2605 ms), and diffusion tensor imaging (TR/TE = 3000/97 ms, b-value = 0 and 800 s/mm2, 20 non-collinear directions of diffusion encoding). The obtained MR images were reviewed by a board-certified neuroradiologist before further quantitative analysis to exclude the presence of structural lesions or other etiologies mimicking ALS.
Preprocessing of gray matter imaging and definition of the target regions of interest
The gray matter volume was calculated from the three-dimensional T1-weighted images by using the Statistical Parametric Mapping software (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and the Computational Anatomy Toolbox 12 (http://www.neuro.uni- jena.de/cat).21 The key steps included tissue segmentation using the Gaussian mixture model22 and spatial normalization using the DARTEL algorithm.23 In the normalized space, regions of interest were defined based on the Neuromorphometrics atlas (http://www.neuromorphometrics.com)24 for the cortical gray matter and the CoBrA atlas25 for subcortical gray matter. The volume was calculated for six areas previously shown to correlate with the motor presentation in ALS: precentral gyrus, superior frontal gyrus, middle frontal gyrus, medial superior frontal gyrus, striatum, and thalamus.1, 4, 26 The total gray matter volume was also calculated. To reduce inter-subject variability, the above volumes were normalized by total intracranial volume (referred to as normalized gray matter volume27 in the context).
Preprocessing of diffusion weighted images for diffusion tensor imaging tractography and definition of target regions of interest
By using DSI Studio (http://dsi-studio.labsolver.org),28 fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD) were voxel-wise calculated and averaged separately within the corticospinal tract, corticostriatal tract, and corticothalamic tract. The tracts were selected from the Human Connectome Project 842 atlas29 and warped to the subject's native space of diffusion tensor images.
Statistics
Numerical variables were expressed as the mean ± SD. For statistical analysis, Student's t test or nonparametric tests were performed for comparison among groups based on whether the data followed a Gaussian distribution or not. Multivariate regression analysis with the covariance of the model R2 and standardized correlation coefficients was used for evaluation of the relationship among image parameters and neurophysiological parameters, with age, gender, and disease duration were used as the covariates. Significance value was defined as p < 0.05. These analyses were based on STATA13 (StataCorp LLC, College Station, TX, USA) and Prism 3.0 (GraphPad Software, San Diego, CA, USA).
Results
Clinical profiles of patients
There were 39 ALS patients (26 man, aged 56.4 ± 13.2 years) enrolled in this study. All ALS patients fulfilled the Awaji diagnostic criteria for categories of definite and probable ALS at the time of diagnosis.12 Among the patients, 32 had spinal-onset and 7 had bulbar-onset ALS. The age of disease onset was 56.4 ± 13.2 (range 30–83) years old. The patients were evaluated for the diagnosis of ALS at 23.6 ± 24.2 (range 1–108) months after the initial presentation of symptoms. At the time of MRI examination, based on the clinical manifestations including weakness, muscle wasting, spasticity, dysphagia, or dysarthria, 6, 22, and 8 patients were classified as King's stage 1, 2, and 3, respectively, and 3 patients with gastrostomy were classified as stage 4.14 In these ALS patients, 37 patients (94.9%) had involvement of at least 2 regions of the nervous system according to the revised El Escorial guideline.30 Two types of scales were applied for assessments: (1) generalized functional disability and (2) focal parameters. The former includes (1) summation of muscle strength based on Medical Research Council grade (MRC sum score); (2) forced vital capacity (FVC), presented as percentage of predicted forced vital capacity estimated by age, sex, and height; and (3) ALSFRS-R.13 The latter consisted of (1) CMAP Z sum score and (2) Penn score.16 All these parameters were analyzed with the imaging markers (Fig. 1). The clinical profiles of the patients are summarized in Table 1. A lower MRC sum score (32.66 ± 4.58 and 37.50 ± 2.03, p < 0.01) and CMAP Z sum score (−6.59 ± 4.73 and − 3.51 ± 4.13, p = 0.02) were noted in the spinal-onset group as compared to the bulbar-onset group, while other clinical profiles are similar without significant differences. For comparison, 32 controls (13 man, aged 63.1 ± 9.5 years) participated in this study with similar distributions of age (p = 0.21) and gender (p = 0.10).
| Patients | Controls | ||||||
|---|---|---|---|---|---|---|---|
| Total | Spinal onset | Bulbar onset | p | Total | |||
| Number | 39 | 32 | 7 | 32 | |||
| Gender (male) | 26 | 20 | 6 | 0.24 | 13 | ||
| Onset age | 56.43 ± 13.23 | 56.47 ± 11.77 | 56.29 ± 11.57 | 0.9726 | |||
| Time of study (age) | 59.00 ± 12.18 | 59.03 ± 12.16 | 58.86 ± 12.26 | 0.9753 | 63.09 ± 9.48 | ||
| Disease duration | 23.56 ± 24.22 | 24.97 ± 26.16 | 17.14 ± 9.45 | 0.2093 | |||
| King's clinical stage | |||||||
| 1 | 6 | 5 | 1 | 0.5034 | Underlying conditions | Hypertension | 10 |
| 2 | 22 | 18 | 4 | Diabetes mellitus | 5 | ||
| 3 | 8 | 6 | 2 | Dyslipidemia | 3 | ||
| 4 | 3 | 3 | 0 | ||||
| Number of segment involved | |||||||
| 1 | 2 | 1 | 1 | 0.6220 | |||
| 2 | 15 | 12 | 3 | ||||
| 3 | 19 | 16 | 3 | ||||
| 4 | 3 | 3 | 0 | ||||
| Site of initial presentation | |||||||
| Bulbar | 7 | 0 | 7 | ||||
| Upper limbs | 22 | 22 | 0 | ||||
| Lower limbs | 7 | 7 | 0 | ||||
| Trunk | 3 | 3 | 0 | ||||
| MRC sum score | 33.55 ± 4.58 | 32.66 ± 4.58 | 37.50 ± 2.03 | <0.01* | |||
| FVC (%) | 81.27 ± 23.25 | 82.68 ± 24.20 | 75.61 ± 17.87 | 0.43 | |||
| ALSFRS-R | 34.00 ± 8.68 | 33.58 ± 8.53 | 38.00 ± 4.00 | 0.45 | |||
| CMAP Z sum score | −6.19 ± 4.63 | −6.78 ± 4.53 | −3.51 ± 4.13 | 0.02* | |||
| Penn score | 1.85 ± 2.28 | 1.66 ± 2.13 | 2.71 ± 2.71 | 0.20 | |||
- ALSFRS-R, revised amyotrophic lateral sclerosis functional rating scale; CMAP, compound muscle action potential; FA, fractional anisotropy; FVC, forced vital capacity, predicted percentage; MRC sum score, Medical Research Council muscle strength sum score; Penn Score, University of Pennsylvania upper motor neuron score.
- * p < 0.05.
Structural changes in ALS: gray matter volume and white matter integrity
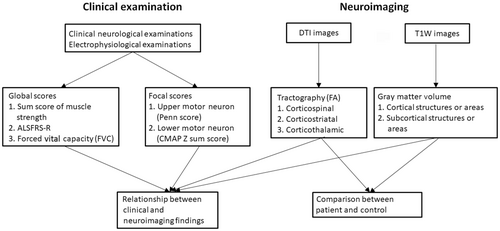
To understand the effect of ALS on the upper motor neurons in the brain, we analyzed (1) the normalized volume of motor-associated gray matters and (2) region-based fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD) of white matter tract integrity. The normalized volume of the precentral gyrus was significantly lower in ALS patients compared to that of controls (0.697% ± 0.074% vs. 0.742% ± 0.081%, p = 0.0295, Fig. 2A1). There was no difference in the gray matter volume of other cortices (superior frontal gyrus, medial superior frontal gyrus, and middle frontal gyrus) and deep gray matter (the thalamus and striatum) between of the patient and controls (Fig. 2A2–A6).
In contrast, the FAs of the corticofugal tracts were reduced in ALS compared to controls: corticospinal tract (0.395 ± 0.015 vs. 0.435 ± 0.068, p = 0.003), corticostriatal tract (0.311 ± 0.0179 vs. 0.362 ± 0.069, p < 0.001), and corticothalamic tract (0.322 ± 0.018 vs. 0.371 ± 0.069, p < 0.001) (Fig. 2B1–B3). Similar findings were documented in ADs (corticospinal tract 1.345 ± 0.007 vs.1.628 ± 0.025, p < 0.001; corticostriatal tract 1.302 ± 0.010 vs. 1.557 ± 0.025, p < 0.001; and corticothalamic tract 1.311 ± 0.011 vs. 1.543 ± 0.024, p < 0.001) and MDs (corticospinal tract 0.967 ± 0.006 vs. 1.104 ± 0.018, p < 0.001; corticostriatal tract 1.003 ± 0.010 vs. 1.111 ± 0.017, p < 0.001; and corticothalamic tract 0.999 ± 0.011 vs. 1.092 ± 0.018, p < 0.001). In analysis of RDs, significant difference was only noted in corticospinal tract (0.778 ± 0.006 vs. 0.842 ± 0.0192, p = 0.002).
Relationship between gray matter volume and white matter fractional anisotropy
Since the integrity of the white matter is influenced by degeneration of the neuronal cell bodies in the gray matter, we examined the relationship of the degeneration between the two groups of structures (Table 2). There was a positive correlation between the gray matter volume and the FA of corticofugal tracts, in particular, the gray matter volume of the precentral gyrus and the FA of corticospinal tract. The gray matter volume of the precentral gyrus was significantly correlated with the FA of corticospinal tract, the cotricostriatal tract, and the corticothalamic tract. The FA of the corticostriatal tract had significant correlation with the gray matter volume of the cortices (precentral gyrus, superior frontal gyrus, and medial superior frontal gyrus) and subcortical structures (thalamus and striatum). Similarly, the FA of the corticothalamic tract was significantly correlated with all the sampled regions of the cortical and subcortical gray matter.
| Model: R2, p Area: β, t, p | Precentral gyrus | Superior frontal gyrus | Medial superior frontal gyrus | Middle frontal gyrus | Striatum | Thalamus |
|---|---|---|---|---|---|---|
| Corticospinal tract | 0.059, 0.008; 14.585, 2.68 0.008* | 0.080, 0.002; −5.816, −3.15, 0.002* | 0.114, <0.001, 6.869, 3.83, <0.001* | 0.001, 0.696; 2.983, 0.39, 0.696 | 0.004, 0.497; 4.421, 0.68, 0.497 | 0.028, 0.075; 18.781,1.80, 0.075 |
| Corticostriatal tract | 0.149, <0.001; 20.432, 4.47, <0.001* | 0.134, <0.001; −6.656, −4.20, <0.001* | 0.239, <0.001, 8.804, 5.99, <0.001* | 0.023, 0.105; 10.887, 1.63, 0.105 | 0.045, 0.022; 13.059, 2.32, 0.022* | 0.089, 0.001; 29.894, 3.34, 0.001* |
| Corticothalamic tract | 0.145, <0.001; 19.732, 4.40, <0.001* | 0.122, <0.001; −6.194, −3.97, <0.001* | 0.251, <0.001, 8.826, 6.19, <0.001* | 0.069, 0.004; 18.567, 2.92, 0.004* | 0.054, 0.012, 13.976, 2.22, 0.012* | 0.130, <0.001; 35.335, 4.13, <0.001* |
- R2, R-squared value; β, beta regression coefficients; t, t score.
- * p < 0.05.
Clinical correlates of structure changes in motor-associated gray matter
To investigate the clinical significance of gray matter volume change, we examined the relationship with functional and physiological markers according to (1) generalized parameters of ALSFRS-R, FVC, and MRC sum score; (2) upper motor neuron parameters of Penn score; and (3) lower motor neuron parameters of CMAP Z sum score (Supplementary Table S1). We analyzed with multivariate regression models in which the age, gender, and duration of disease served as covariates. FVC was significantly correlated with the volume of the precentral gyrus (R2 = 0.189, p = 0.032), superior frontal gyrus (R2 = 0.190, p = 0.031), and middle frontal gyrus (R2 = 0.178, p = 0.039). The MRC sum score was correlated with the gray matter volume of the cortical regions, including precentral (R2 = 0.256, p = 0.017), superior frontal gyrus (R2 = 0.267, p = 0.010), and middle frontal gyrus (R2 = 0.277, p = 0.006), along with the striatum (R2 = 0.235, p = 0.047).
We then analyzed the relationship of the gray matter volume and the parameters of the lower and upper motor neuron involvement, CMAP Z sum score and Penn score, respectively. The CMAP Z sum score was correlated with the gray matter volume in all examined motor-associated cortical regions: precentral (R2 = 0.312, p = 0.042), superior frontal gyrus (R2 = 0. 351, p = 0.005), medial superior frontal gyrus (R2 = 0.147, p = 0.001), and middle frontal gyrus (R2 = 0.318, p = 0.031). There was no significant correlation between the gray matter structures and the Penn score.
Clinical significance of white matter integrity
We further investigated the relationship between the white matter integrity in terms of FA, AD, RD, and MD and functional deficits in ALS patients with (1) global parameters of MRC sum score, ALSFRS-R, and FVC; and (2) focal parameters of CMAP Z sum score and Penn score (Table 3). Multivariate analysis revealed significant correlation between the MRC sum score and the FA of corticothalamic tract (R2 = 0. 264, p = 0.005), and corticostriatal tract (R2 = 0.237, p = 0.021). On the contrary, a significant correlation between the FVC and the corticothalamic (R2 = 0.166, p = 0.048) and corticostriatal (R2 = 0.175, p = 0.040) tracts was identified by multivariate analysis. Similar correlations of AD, RD, and MD with MRC sum score and FVC were also noted. There is no significant correlation between ALSFRS-R and these three parameters of the selected tracts.
| Fractional anisotropy (FA) | Radial diffusivity (RD) | Axial diffusivity (AD) | Mean diffusivity (MD) | |
|---|---|---|---|---|
| MRC sum score | ||||
| Corticospinal tract | −8.938, −0.29, 0.770 | −10.724, −1.07, 0.289 | −20.210, −1.92, 0.058 | −18.514, −1.61, 0.113 |
| Corticostriatal tract | 78.378, 2.36, 0.021* | −27.871, −3.62, 0.001* | −24.824, −3.35, 0.001* | −27.165, −3.56, 0.001* |
| Corticothalamic tract | 82.570, 2.89, 0.005* | −25.504, −3.70, <0.001* | −22.933, −3.34, 0.001* | −24.878, −3.60, 0.001* |
| FVC | ||||
| Corticospinal tract | 258.430, 0.90, 0.377 | −106.828, −1.14, 0.263 | −177.385, −1.87, 0.072 | −133.452, −1.39, 0.174 |
| Corticostriatal tract | 580.856, 2.15, 0.040* | −164.843, −2.59, 0.015* | −163.146, −2.49, 0.019* | −165.562, −2.57, 0.016* |
| Corticothalamic tract | 549.780, 2.06, 0.048* | −145.910, −2.53, 0.017* | −140.777, −2.45, 0.020* | −145.378, −2.51, 0.018* |
| ALSFRS-R | ||||
| Corticospinal tract | 224.411, 1.92, 0.069 | −59.792, −1.48, 0.155 | −41.107, −0.99, 0.336 | −55.145, −1.33, 0.199 |
| Corticostriatal tract | 230.817, 1.69, 0.108 | −44.300, −1.25, 0.226 | −32.955, −0.92, 0.369 | −40.792, −1.14, 0.267 |
| Corticothalamic tract | 215.910, 1.46, 0.160 | −36.241, −1.02, 0.322 | −24.150, −0.75, 0.464 | −32.024, −0.92, 0.367 |
| CMAP Z sum score | ||||
| Corticospinal tract | 62.675, 2.23, 0.029* | −33.570, −3.82, <0.001* | −28.116, −3.31, 0.001* | −33.559, −3.76, <0.001* |
| Corticostriatal tract | 81.841, 2.60, 0.011* | −28. 490, −3.86, <0.001* | −25.027, −3.58, 0.001* | −27.359, −3.79, <0.001* |
| Corticothalamic tract | 77.552, 2.84, 0.006* | −21.985, −3.29, 0.002* | −19.207, −2.88, 0.005* | −21.250, −3.17, 0.002* |
| Penn Score | ||||
| Corticospinal tract | −41.727, −2.86, 0.005* | 8.954, 1.80, 0.077 | 5.584, 1.17, 0.246 | 1.257, 0.21, 0.832 |
| Corticostriatal tract | −35.038, −2.08, 0.041* | 3.772, 0.90, 0.371 | 1.866, 0.47, 0.643 | 3.139, 0.76, 0.453 |
| Corticothalamic tract | −26.893, −1.81, 0.075 | 2.383, 0.63, 0.530 | 0.671, 0.18, 0.857 | 1.817, 0.48, 0.632 |
- ALSFRS-R, revised amyotrophic lateral sclerosis functional rating scale; CMAP, compound muscle action potential; FVC, forced vital capacity, predicted percentage; MRC sum score, Medical Research Council muscle strength sum score; Penn Score, University of Pennsylvania upper motor neuron score.
- * p < 0.05.
There were significant correlations between CMAP Z sum score and the FA of all three tracts (corticospinal tract, R2 = 0.301, p = 0.029; corticothalamic tract, R2 = 0.179, p = 0.006; and corticostriatal tract, R2 = 0. 317, p = 0.011). Similar correlation existed for AD (corticospinal tract, R2 = 0.351, p = 0.001; corticothalamic tract, R2 = 0.330, p = 0.005; and corticostriatal tract, R2 = 0.365, p < 0.001), RD (R2 = 0.378, p < 0.001, corticothalamic tract, R2 = 0.3503, p = 0.002, and corticostriatal tract, R2 = 0.3810, p < 0.001), and MD (corticospinal tract, R2 = 0.361, p = 0.001; corticothalamic tract, R2 = 0. 344, p = 0.002; and corticostriatal tract, R2 = 0. 377, p < 0.001). In contrast, Penn score was associated with the FA of the corticospinal (R2 = 0.229, p = 0.005) and corticostriatal (R2 = 0.190, p = 0.041) tracts in the multivariate model, but not associated with the AD, RD, and MD of the white matter tracts.
Discussion
The study investigated the brain microstructures and integrity by applying diffusion tensor imaging and demonstrated (1) reduced gray matter volume in the precentral gyrus and (2) reduced FA, AD, RD, and MD of the corticofugal tracts, and their clinical significance, that is, correlation with lower motor neuron degeneration measured with CMAP Z sum score.
Pathological processes of upper and lower motor neurons in ALS had been thought to have a relationship with clinical and imaging findings. Although previous neuroimaging studies have indicated the association of clinical symptoms and signs,6, 31 most studies only focused on the correlation with generalized presentation or upper motor neuron involvement regardless the simultaneous involvement of lower motor neurons in ALS patients.5, 7, 32 This current report provides evidence for the correlation of brain imaging signatures of reduced gray matter volume and white matter microstrcutural changes with biomarkers of lower motor neuron degeneration. An intriguing finding is that the gray matter volume of the selected motor-associated areas except precentral gyrus did not differ significantly between the ALS and control group in the present study, although the gray matter volume of these areas was correlated with the global functional measurements. This may be due to the heterogeneity within the ALS patients, and a more detailed classification and longitudinal follow-up of the subjects is necessary in future studies.
Beside the traditional marker of FA, which may represent Wallerian degeneration,33 we also found significant decremental changes of AD and MD in the white matter tracts of ALS patients as compared to controls, while there are no significant differences in terms of RD. Although increases in AD and MD had been considered to represent axonal loss and neurodegeneration,33 the findings had been variable in ALS patients.34 The findings may be also different based on the structures measured, which may be compatible with the spreading pathological pattern of the disease. Whether the AD and MD changes in ALS may represent a disease-specific pathological process should also be investigated in the future.
This study also documented that clinical upper motor neuron signs measured by Penn score were correlated to the degeneration of the corticospinal tract and corticostriatal tract. The presence of diffuse clinical upper motor neuron degeneration is a hallmark of ALS,35 and clinical manifestations included hyperreflexia, spasticity, and presence of long tract signs such as Hoffman sign and Babinski sign. Previous reports implied the correlation of corticospinal tract degeneration with indirect measures of upper motor neuron dysfunction such as finger tapping speed,36 the number of body regions involved,5 or a parameter that is only based on presence-or-absence of signs.7 Our study specifically demonstrated direct evidence for the associations of upper motor neuron impairment with the degeneration of corticospinal tract according to the validated Penn score consisting of direct clinical parameters of upper motor neuron dysfunction.
In addition to degeneration of the corticospinal tracts, similar relationships were evident in the extrapyramidal tracts, indicating a diffuse degeneration pattern in ALS in addition to the changes in the primary motor neurons, which was rarely reported in previous studies.37 The observation of reduced FA in the corticostriatal tract is supported by and corroborates with postmortem examinations on the brain of ALS.38 Pathological studies on the molecular mechanisms of ALS indicate extensive involvement of the brain in ALS, that is, not only the motor cortex and corticospinal tract, but also the extra-motor areas and tracts, including the striatum and corticofugal pathways,39 this study showed that similar degeneration patterns could also be observed using non-invasive pre-mortem image studies. The correlation of the corticostriatal tract with Penn score implies the concomitant involvement of the extra-motor or extrapyramidal tract in ALS and is supported by the autopsy observations of ALS pathology, for example, stage-dependent deposition of pTDP-43 in the striatum.10, 38 The effect of pathology-specific treatments on the upper motor neuron-related symptoms in ALS, such as spasticity and disinhibition, warrants further investigation in the future.
This study documented the correlation of MRC sum score with gray matter volume and FA of the corticospinal tracts, in addition to the correlation of FVC with gray matter volume. Such an observation carries important clinical implications. First, MRC sum score is a routine evaluation in clinical practice and FVC is the most common parameter to represent function of respiratory muscles. Both are easy to use compared to other clinical scores. Furthermore, the strong correlation of these clinically feasible measures provides a surrogate marker of imaging alteration of the ALS brain including the gray matter volume and white matter integrity. In summary, the implementation of global measurements, such as muscle strength of MRC sum score and respiratory function of FVC, and a focal measure CMAP Z sum score, as shown in our study, serve as surrogate markers assessing the gray matter volume and white matter integrity of ALS. This clinical-image combined approach may also be useful to advance our understanding of the recently proposed disease category of mild motor impairment, or prodromal ALS, which had been recently characterized to have different degrees of impairment in the upper motor neurons and lower motor neurons at the earliest stages of ALS based on their underlying etiology and genetic profiles.40 On the other hand, the poor correlation of the structural parameters with the ALSFRS-R implied that changes in ALSFRS-R may be non-specific and had poor relationship with the pathological process in ALS. Since the ALSFRS-R is a wildly used parameter for outcome in modern clinical trials, a more disease-specific parameter, such as image and electrophysiological parameters, should be included in the design of future clinical trials to better identify the efficacy of potential treatments in ALS.
ALS patients usually present with both upper and lower motor neuron signs clinically. Although upper motor neuron impairment was considered to accelerate the loss of lower motor neurons,41 the relationship between the dysfunction and progression of upper motor neuron and lower motor neuron was not established with contradictory findings reported.35, 42 The relationship between the two clinical parameters of lower motor neuron degeneration and MR imaging (gray matter volume and white matter tract integrity) had also not been systematically examined. Our findings documented that in patients with classical ALS, the degeneration of the lower motor neurons, as represented by the changes in CMAP amplitude, paralleled the degeneration of the upper motor neurons and their corresponding fiber tracts assessed by changes in the white matter tract integrity, and with the reduction of cortical gray matter volume. These observations suggest parallel degeneration of upper and lower motor neurons. Alternatively, upper motor neuron degeneration might influence the degeneration of lower motor neurons, or vice versa. This implies that an effective treatment of ALS may preserve the integrity of the gray matter, white matter, and the lower motor neurons. Further studies are required to investigate (1) temporal pattern of neurodegeneration between the upper and lower motor neurons and (2) the interactions between upper and lower motor neurons.
There are limitations that await studies in the future. First, this report was a cross-sectional study, and there was a lack of temporal changes in our patients. A longitudinal follow-up of the images and clinical manifestations in a prospective design will constitute comprehensive pictures of ALS for prognosis prediction. Second, the analysis of brain MRI was performed by one evaluator, and there is a lack of the second evaluator to check the interrater variability. The evaluations across patients over time and by different clinicians might also lead to potential variability. Third, there might be selection bias in the enrollment of ALS patients because only patients fulfilling the El Escorial criteria were recruited and patients with extensive disease involvement or respiratory failure were excluded due to intolerance to MRI examination. Additionally, the inclusion of disease controls for comparison in future studies may be essential in determining the specificity of the structural changes in the ALS patients. The current report focuses on imaging signatures and the correlation with clinical and physiological parameters. Given the technological advancement, future studies to incorporate fluid biomarkers will provide a battery of assessments to improve the care of ALS.1 Nevertheless, this study demonstrates that simple assessments of muscle strength and routine electrophysiology examinations of CMAP Z sum score provide robust surrogate biomarkers for neuroimaging degeneration of the motor cortex and corresponding tracts in ALS.
Acknowledgements
The authors would like to thank all the participants and their caregivers for participating in the study.
Author Contributions
Conception and design of the study: S.-J. H., C.-C. C., W.-C. W., S.-T. H. Acquisition and analysis of data: S.-J. H., C.-C. C., T.-F. C., Y.-F. C., H.-W. H., L.-K. T., W.-C. W., S.-T. H. Drafting a significant portion of the manuscript or figures: S.-J. H., C.-C. C., W.-C. W., S.-T. H. Corresponding authors: S.-T. H., W.-C. W, and C.-C. C.
Funding Information
This work was supported by grants from the Ministry of Science and Technology, Taiwan (110-2320-B-002-072 and 111-2320-B-002-079 to S.-T, H; 109-2314-B-002-031-MY3 to W.-C.W.) and National Science and Technology Council (NSTC111-2634-F-002-017 to S.-T. H). The funders had no role in data collection or analysis and did not participate in writing or approving the manuscript.
Conflict of Interest
The authors reported no potential conflict of interest.




