A comprehensive study of skeletal muscle imaging in FHL1-related reducing body myopathy
Abstract
Objective
FHL1-related reducing body myopathy is an ultra-rare, X-linked dominant myopathy. In this cross-sectional study, we characterize skeletal muscle ultrasound, muscle MRI, and cardiac MRI findings in FHL1-related reducing body myopathy patients.
Methods
Seventeen patients (11 male, mean age 35.4, range 12–76 years) from nine independent families with FHL1-related reducing body myopathy underwent clinical evaluation, muscle ultrasound (n = 11/17), and lower extremity muscle MRI (n = 14/17), including Dixon MRI (n = 6/17). Muscle ultrasound echogenicity was graded using a modified Heckmatt scale. T1 and STIR axial images of the lower extremity muscles were evaluated for pattern and distribution of abnormalities. Quantitative analysis of intramuscular fat fraction was performed using the Dixon MRI images. Cardiac studies included electrocardiogram (n = 15/17), echocardiogram (n = 17/17), and cardiac MRI (n = 6/17). Cardiac muscle function, T1 maps, T2-weighted black blood images, and late gadolinium enhancement patterns were analyzed.
Results
Muscle ultrasound showed a distinct pattern of increased echointensity in skeletal muscles with a nonuniform, multifocal, and “geographical” distribution, selectively involving the deeper fascicles of muscles such as biceps and tibialis anterior. Lower extremity muscle MRI showed relative sparing of gluteus maximus, rectus femoris, gracilis, and lateral gastrocnemius muscles and an asymmetric and multifocal, “geographical” pattern of T1 hyperintensity within affected muscles. Cardiac studies revealed mild and nonspecific abnormalities on electrocardiogram and echocardiogram with unremarkable cardiac MRI studies.
Interpretation
Skeletal muscle ultrasound and muscle MRI reflect the multifocal aggregate formation in muscle in FHL1-related reducing body myopathy and are practical and informative tools that can aid in diagnosis and monitoring of disease progression.
Introduction
Reducing body myopathy (RBM, OMIM #300717) is an ultra-rare, X-linked dominant myopathy that typically manifests with focal and asymmetric weakness, initially in a scapuloperoneal distribution, with a frequently rapid rate of progression.1 Respiratory failure, paraspinal muscle weakness, and rigid spine2 are also common phenotypic features. In addition, progressive and severe large joint contractures involving the neck, elbows, knees, and ankles often intensify the accruing functional loss due to muscle weakness. Muscle biopsies of RBM patients show characteristic intramuscular protein aggregates that have reducing activity on the menadione-nitroblue tetrazolium (NBT) stain to give the condition its name.1
RBM is caused by pathogenic missense variants in the LIM-2 domain of the FHL1 protein.1 However, a second class of pathogenic loss-of-function variants in FHL1 with X-linked recessive inheritance manifests with alternate phenotypes including Emery–Dreifuss muscular dystrophy (EDMD),3 X-linked myopathy with postural muscle atrophy (XMPMA),4 Uruguay syndrome,5 and isolated cardiomyopathy.6 Despite this general dichotomy, exceptional RBM cases with overlapping phenotypic features of both loss-of-function and gain-of-function variants have also been reported, in particular with missense variants in the FHL1 LIM-3 or LIM-4 domains.7-9
In rare myopathies like FHL1-related RBM, muscle imaging modalities hold promise as disease biomarkers to facilitate diagnosis and to help monitor disease progression and empower interventional clinical trials.10-12 In a few case studies of FHL1-related RBM patients, muscle MRI studies have suggested a distinct pattern of muscle involvement in the lower extremities: sparing of gluteus maximus muscle, and selective involvement of posteromedial thigh muscles, medial gastrocnemius, and soleus muscles.13-16 Muscle ultrasound and cardiac MRI studies are not extensively reported in the literature in patients with FHL1-related RBM.
Here, we provide clinical, genetic, and imaging data in a cohort of 17 patients with FHL1-related RBM and illustrate the feasibility of muscle imaging as a biomarker for this ultra-rare, progressive myopathy. In addition to confirming previous imaging studies, we highlight a distinctive pattern of muscle imaging changes associated with RBM. We explore the use of Dixon MRI to quantitatively assess muscle fat fraction in the lower extremity muscles of RBM patients. We also report cardiac MRI imaging in six patients in addition to routine cardiac studies to more definitively assess the possible presence of myocardial disease and fibrosis given the cardiomyopathy phenotype reported in patients with other FHL1 pathogenic variants, especially in individuals with loss-of-function variants.6 This constitutes the largest imaging case series to date, spanning the full range of phenotypic severity associated with RBM including mild, adult-onset scapuloperoneal myopathy.
Subjects and Methods
Participants and clinical research studies
Seventeen individuals (age 12–76 years) with pathogenic or likely pathogenic FHL1 variants underwent detailed clinical evaluation between July 2014 and November 2018 at the NIH Clinical Center (n = 12) or their local clinic (n = 5). Medical records, muscle imaging (if available), muscle biopsy slides, cardiac studies (electrocardiogram, echocardiogram), pulmonary function testing, and serum creatine kinase values were retrospectively reviewed. Genetic testing (direct FHL1 sequencing or as part of diagnostic panels) was performed as part of standard clinical care. Variant classification was based on American College of Medical Genetics criteria17 (Table S1). Additional imaging studies (muscle ultrasound, muscle MRI including Dixon, and cardiac MRI) were performed as part of a research protocol at the NIH Clinical Center.
Ethical standards
Research studies were performed after informed consent (and assent when appropriate) as part of the following IRB-approved research studies: National Institute of Neurological Disorders and Stroke (NINDS) protocol #12-N-0095 and National Heart, Lung, and Blood Institute (NHLBI) protocol #02-H-0090. Clinical photographs were taken after a separate informed consent/assent for clinical photos was obtained.
Muscle ultrasound
Ultrasound scans were performed in 11/17 patients, using an Acuson S2000 upgraded to S3000 (Siemens Healthineers) with a 6–18 MHz linear array probe. Transverse ultrasound images were acquired by optimal perpendicular imaging, using the bone echo as reference, if visible. Tissue compression was avoided by observation of skin outline on the image. Axial scans of mid-thigh (rectus femoris, vastus lateralis, sartorius, gracilis and hamstring muscles), upper arm (deltoid, biceps and triceps muscles) and lower leg (tibialis anterior, soleus, and medial and lateral gastrocnemius) were obtained when feasible. The echogenicity was graded based on a modified Heckmatt scale18 with grade 0 for normal appearance, grade 1 for mild increase in echointensity with distinct bone echo, grade 2 with a moderate increase in muscle echointensity and reduced bone echo, and grade 3 with severe increase in muscle echointensity and loss of bone echo. Two independent raters trained in neuromuscular ultrasound graded each muscle and any discrepancies were resolved after a discussion. In muscles that had a sectorial or “geographical” pattern of involvement (i.e. adjacent areas with different degrees of echointensity), an overall score was given based on the total affected area and rater's overall judgment.
Skeletal muscle MRI
Muscle MRI was performed using conventional T1-weighted spin echo and short tau inversion recovery (STIR) of the lower extremities (N = 14/17) and upper extremities (N = 6) on different clinical scanners: Siemens Verio 3.0T (N = 6), Siemens Aera 1.5T (N = 5), Philips Achieva 3.0T (N = 2), and GE Healthcare 1.5T (N = 1). Non-contrast images were obtained from upper arms, forearms, pelvis, thighs, and lower legs in the axial plane. Slices were 5–10 mm thick. The gap between slices ranged between 8 and 16 mm thick. The lower extremity images included the hips, thighs, and lower legs to the ankles in all but one patient (F8P15 only tolerated imaging of hips to knees) who underwent these studies.
Cardiac MRI
Cardiac MRI was performed on six patients on a 3T Siemens Skyra scanner and reviewed by expert cardiologists. Late gadolinium enhancement images were acquired in five patients (F1P1, F1P2, F1P4, F3P6, F5P10) with an intravenous gadolinium-based contrast agent (gadobutrol 0.15 mmol/kg) while the sixth (F2P5) only underwent a non-contrast cardiac MRI. The heart was imaged in standard cardiac imaging planes, consisting of four, three, and two-chamber long axis as well as ventricular short axis views. ECG-gated steady-state free precession cine imaging (N = 5) or real-time cine imaging (N = 1) was used to assess left ventricular mass, volume, and systolic function by tracing epicardial and endocardial borders in end-systole and end-diastole. Native tissue characterization sequences included T1 mapping (N = 6) and T2-weighted black blood imaging (N = 4). In the five patients who received gadolinium contrast, T1-weighted inversion recovery early (i.e. less than approximately 10 min after injection) and late (i.e. starting approximately 10 min after injection) gadolinium enhancement (EGE, LGE) imaging was performed. Tissue characterization images were qualitatively evaluated to assess for the presence and location of myocardial fibrosis (LGE, T1 mapping) or myocardial edema (EGE, T1 mapping, T2-weighted black blood imaging).
Muscle MRI quantification
Fat fraction for each muscle image slice was plotted in violin plots indicating the mean and variability of fat fraction across the length of each muscle and in comparison to the contralateral side.
Results
Clinical phenotype, genotype, and muscle histological findings
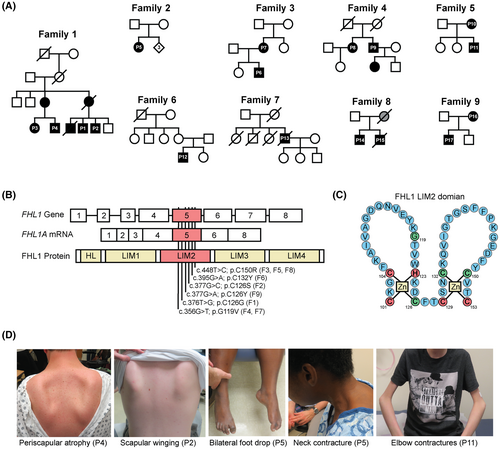
We identified 17 patients from nine independent families with genetically confirmed FHL1-related RBM (Fig. 1A). All affected individuals had missense variants in the FHL1 gene, exclusively situated in exon 5 (NM_001449.5), which encodes the LIM-2 domain of the protein (Fig. 1B, Table S1). Similar to prior reports,1, 19 substitution of the highly conserved cysteine or histidine residues of the LIM-2 domain were commonly pathogenic and associated with severe disease (Fig. 1C). In two independent families (F4 and F7), we identified a novel variant in the same exon [FHL1 NM_001449.5; c.356G>T; p.(G119V)] that was associated with a milder phenotype. Male patients with this variant (F4P9, F7P13) manifested with progressive scapuloperoneal weakness in late adulthood, and a female individual did not report subjective symptoms at 70 years of age (F4P8). She was found to have presymptomatic disease, however, with mild proximal and distal weakness upon detailed examination (Table 1, Text S1).
| Family/Patient | FHL1 Variant | Sex/Agea | Symptom onset (years) | Pattern of weakness | Ambulation status | Joint contractures | Creatine kinase (IU/L) | FVC % predicted |
|---|---|---|---|---|---|---|---|---|
| F1P1 | c.376T>G; p.C126G | M/32 | Mid-20's | Proximal and distal with asymmetry | Independent with cane | + | 1898 | 41%b |
| F1P2 | c.376T>G; p.C126G | M/30 | ~21 | Proximal and distal | Independent with cane | + | 870 | 63% |
| F1P3 | c.376T>G; p.C126G | F/34 | No subjective symptoms | Mild proximal weakness | Independent | − | 2000 | 99% |
| F1P4 | c.376T>G; p.C126G | M/32 | Early 20's | Proximal and distal with asymmetry | Rolling walker and motorized scooter | + | NA | NA |
| F2P5 | c.377G>C; p.C126S | F/37 | 18 | Proximal and distal | Uses Motorized chair | + | 600 | 23%b |
| F3P6 | c.448T>C; p.C150R | M/15 | 11 | Proximal and distal | Motorized chair | + | 774 | 95% |
| F3P7 | c.448T>C; p.C150R | F/36 | No subjective symptoms | Proximal and distal | Independent | − | NA | 94% |
| F4P8 | c.356G>T; p.G119V | F/70 | No subjective symptoms | Proximal and distal | Independent | − | 128 | 95% |
| F4P9 | c.356G>T; p.G119V | M/69 | 59 | Scapuloperoneal with asymmetry | Independent with cane and AFOs | − | 1218 | 102% |
| F5P10 | c.448T>C; p.C150R | F/44 | 32 | Proximal and distal with asymmetry | Independent | + | 170 | 105% |
| F5P11 | c.448T>C; p.C150R | M/14 | 9 | Proximal and distal | Independent | + | 745 | 64% |
| F6P12 | c.395G>A; p.C132Y | M/12 | 8 | Proximal and distal | Independent | + | 530 | 50% |
| F7P13 | c.356G>T; p.G119V | M/76 | 60's | Scapuloperoneal | Independent | − | 182 | NA |
| F8P14 | c.448T>C; p.C150R | M/28 | 10 | Proximal and distal | Motorized chair | + | ~1100 to 1600 | 7.1%b |
| F8P15 | c.448T>C; p.C150R | M/21 | 10 | Proximal and distal | Motorized chair | + | ~1000 to 2000 | Severeb,c |
| F9P16 | c.377G>A; p.C126Y | F/47 | 28 | Proximal and distal with asymmetry | Independent | + | ~380 to 550 | Normalc |
| F9P17 | c.377G>A; p.C126Y | M/18 | 6 | Proximal and distal | Independent | − | ~2000 | Severeb,c |
- FHL1 reference sequence NM_001449.5. CK: creatine kinase, normal range (39–308 U/L for males, 26–192 U/L for females).
- AFO, ankle foot orthosis; F, female; FVC, forced vital capacity; M, male; NA, not available.
- a Age at the time of last examination (in years).
- b Respiratory support: F1P1 initiated non-invasive ventilation (NIV) in the form of BiPAP, F2P5 required a tracheostomy, F8P14 was placed on continuous NIV support but chose not to pursue a tracheostomy, F8P15 and F9P17 both were started on NIV support but ultimately required a tracheostomy tube and continuous ventilatory support.
- c FVC values not available.
Clinical manifestations of RBM in our cohort were compatible with previous reports.19 All patients had normal development in the motor domain as infants and young children; however, age at symptom onset was variable, ranging from six to 60 years of age (Table 1, Text S1). The late adult-onset cases in our cohort were similar in presentation and progression to a previously reported scapuloperoneal presentation associated with the FHL1 p.W122S pathogenic variant.20 Muscle weakness was focal and asymmetric at onset, often with an initial scapuloperoneal distribution. These symptoms were unrelenting in progression, ultimately resulting in a confluent pattern of weakness involving both distal and proximal muscles. Severe, often asymmetric and progressive large joint contractures in the elbows, neck, hips, knees, and ankles were a common clinical feature (Fig. 1D). Early onset and progressive respiratory insufficiency preceded loss of ambulation in most cases and typically occurred without severe scoliosis, reflecting the early involvement of diaphragm and other respiratory muscles (Table 1).
The phenotypic variability was in part FHL1-variant specific. For example, F1P3 and F2P5 are two women in their mid 30s with two different FHL1 variants involving the same residue (C126G for F1P3, C126S for F2P5). However, F2P5 used a motorized chair with a severely reduced forced vital capacity (23% predicted) while F1P3 was ambulatory and reported no subjective symptoms. The observed phenotypic variability also depended on the sex of the affected individuals: male individuals presented earlier and progressed faster than female individuals, even when carrying the same FHL1 variants, consistent with the X-linked dominant mode of inheritance of RBM. Illustrating this point, in contrast with their affected male relatives, three female individuals that carried FHL1 variants (F1P3, F3P7, and F4P8) did not report subjective symptoms and were found to have proximal and/or distal weakness only upon detailed clinical evaluation. EMG was obtained in a few patients and typically was consistent with an irritable myopathy with abnormal spontaneous activity in the form of fibrillation potentials and positive sharp waves, and early recruitment of small amplitude and short duration motor unit action potentials (Text S1). Serum creatine kinase levels were moderately elevated, typically 2.5–10 times the upper limit of normal range, including in one of the presymptomatic females (Table 1).
Muscle biopsies showed chronic myopathic changes with a distinct, focal pattern of protein aggregate formation. For example, fascicles of normal appearing muscle were found adjacent to severely diseased fascicles with severe myofiber atrophy and preponderance of cytoplasmic aggregates (Fig. 2A and B). Some of the cytoplasmic aggregates spontaneously reacted with the menadione-nitroblue tetrazolium (NBT) stain, consistent with sulfhydryl-rich reducing bodies (Fig. 2C), the pathologic hallmark of RBM. Ultrastructural studies identified electron-dense reducing bodies neighboring typical cytoplasmic bodies with a perinuclear and cytoplasmic distribution (Fig. 2D).

Muscle ultrasound
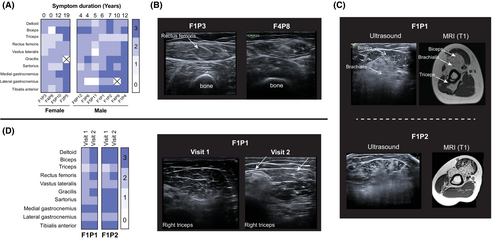
Muscle ultrasound was obtained in 11/17 patients and showed widely variable changes in echogenicity of skeletal muscle, partly reflecting the range of clinical severity and disease duration in our cohort (Fig. 3A). Illustrating the high sensitivity of muscle ultrasound, two clinically asymptomatic females with FHL1 variants who underwent ultrasound (F1P3 and F4P8) had mildly abnormal patterns of muscle echogenicity (Fig. 3A and B). Consistent with the focal histologic findings, the echogenicity pattern was also focal and “geographical” in most affected muscles, with neighboring areas within the same muscle showing strikingly different echointensities. In the biceps muscle, the increased echogenicity appeared more severe in deeper fascicles (that is, nearer to the humerus). This pattern was consistent with findings on upper extremity T1 axial MRI images (F1P1, F1P2) (Fig. 3C). This conspicuous differential involvement within defined areas of muscles was also noted in the tibialis anterior muscle and was a common finding across the cohort (Figure S1). In addition, in two patients with repeat ultrasound studies ~2.5 years apart, we noted increased echogenicity in a few muscles over time, in correlation with overall disease progression and functional deterioration (Fig. 3D).
Muscle MRI and fat fraction quantification
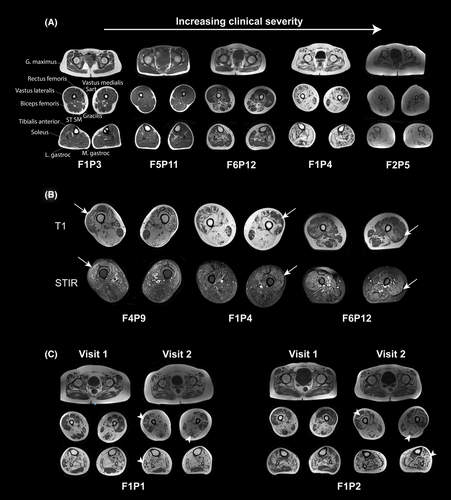
Similar to ultrasound studies, T1 axial MR imaging of the lower extremity muscles including the hips, thighs, and lower leg to the ankles, obtained in 13/17 patients (F8P15 unable to tolerate lower leg imaging and F3P7, F9P16, F9P17 without any lower extremity imaging), demonstrated variable degrees of contractile tissue remodeling, as reflected by areas of T1 hyperintensity, in correlation with overall clinical disease severity (Fig. 4A, Figure S2). When assessing selective involvement of muscles, we observed a relative sparing of gluteus maximus in the pelvis, the anterior thigh compartment (except for vastus intermedius) compared to hamstrings, gracilis muscle compared to hamstrings and adductors, and lateral gastrocnemius compared to medial gastrocnemius muscle (Fig. 4A). Side-to-side asymmetry, focal signal change, and heterogeneity within individual muscles were conspicuous features. In addition, short tau inversion recovery (STIR) sequence identified areas of increased signal without corresponding T1 hyperintensity (Fig. 4B). While the exact histopathologic correlate of these areas is not clearly understood, they are thought to represent areas of disease activity, tissue edema, or inflammation in myopathies.21 Two individuals (F1P1, F1P2) had serial MRIs obtained ~2.5 years apart that showed expanding areas of T1 hyperintensity in several muscles in parallel with worsening weakness and loss of motor function during this period (Fig. 4C).
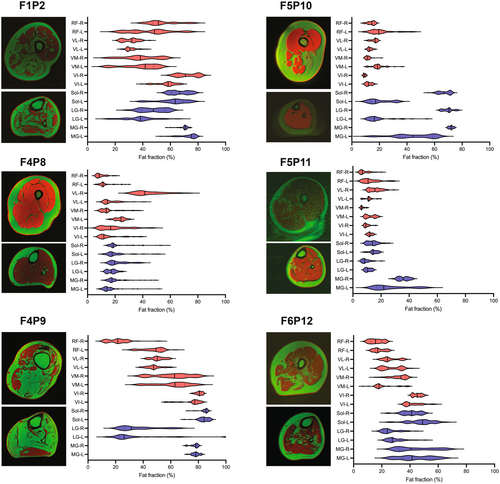
The heterogeneity and focal pattern of T1 signal hyperintensity within individual muscles and along their length as a characteristic feature of the disease made a qualitative grading of fat fraction on conventional T1 images difficult and unreliable. Thus, we resorted to Dixon imaging, albeit only in a subset of patients that had this modality available, to analyze fat fraction along the length of each muscle. Thigh muscle fat fraction quantification was performed in 6/17 patients using Dixon images of rectus femoris, vastus intermedius and vastus lateralis muscles in the thigh and lateral gastrocnemius, medial gastrocnemius, and soleus in the lower leg (Fig. 5). Side-to side asymmetry of muscle involvement and intramuscular variability of fat fraction in individual muscles was notable and consistent with the focal onset and progression of the disease.
Cardiac function assessments
Echocardiographic (N = 17) and electrocardiographic (N = 15) studies were largely without clinically important findings in this cohort (Table 2) except for one patient (F7P13) with left bundle branch block on ECG and left ventricular hypertrophy with mildly reduced global systolic function (left ventricular ejection fraction of 47%) on echocardiogram. This patient did not undergo cardiac MRI and later passed away at ~80 years of age. Six patients (F1P1, F1P2, F1P4, F2P5, F3P6, and F5P10) underwent cardiac MRI studies. Global left ventricular systolic function was normal in all six patients. Five patients (all except for F2P5) also underwent post gadolinium contrast cardiac MRI studies, and among these, one (F1P4) had possible myocardial edema based on a combination of early gadolinium enhancement, T1 mapping, and T2-weighted black blood images. However, there was no evidence of cardiac fibrosis on late gadolinium enhancement images (Figure S3), and these findings did not have any clinical correlates. None of the other patients had evidence of myocardial disease or myocardial fibrosis on late gadolinium enhancement images.
| Muscle MRI | Muscle Ultrasound | EKG | Echocardiogram (LVEF%) | Cardiac MRI | |
|---|---|---|---|---|---|
| F1P1 | Y | Y | Normal | RA pressure increased (60) | Normal |
| F1P2 | Y | Y | Normal | Normal (62) | Tricuspid regurgitation |
| F1P3 | Y | Y | Normal | Normal (60) | NA |
| F1P4 | Y | Y | Early precordial R/S transition | Normal (65) | Possible myocardial edema on T1, T2 and EGE |
| F2P5 | Y | Y | LVH | Normal (65) | Normal |
| F3P6 | Y | Y | Left axis deviation | Normal (65) | Normal |
| F3P7 | NA | NA | Normal | Normal (60) | NA |
| F4P8 | Y | Y | Normal | Focal thickening of aortic valve leaflets (65) | NA |
| F4P9 | Y | Y | Mild ST depression | Asymmetric basal septal hypertrophy and focal aortic valve leaflet thickening (65) | NA |
| F5P10 | Y | Y | Normal | Normal (63) | Mild thickening of the mitral leaflet tips without mitral stenosis |
| F5P11 | Y | Y | Normal | Normal (62) | NA |
| F6P12 | Y | Y | Normal | Normal (NA) | NA |
| F7P13 | Y | NA | Left bundle branch block | LVH (47%) | NA |
| F8P14 | Y | NA | Normal | Normal (55%) | NA |
| F8P15 | Ya | NA | Incomplete right bundle branch block | Normal (55%) | NA |
| F9P16 | NA | NA | NA | Normal (NA) | NA |
| F9P17 | NA | NA | NA | Normal (NA) | NA |
- EGE, early gadolinium enhancement; EKG, electrocardiogram; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; MRI, magnetic resonance imaging; NA, Not available; RA, right atrium; Y, yes.
- a No lower leg imaging available.
Discussion
FHL1-related RBM is a uniformly progressive disease. The age of onset and rate of progression of the disease, however, varies from very severe and often fatal infantile-onset cases to milder adult-onset disease. Sex differences account for some of this phenotypic variability. The expression of the mutant FHL1 off the X chromosome will cause disease in both males and females owing to its dominant negative effect that results in aggregate formation; however, in females, this process is initially ameliorated due to the presence of wildtype FHL1 on the other X chromosome, which owing to lyonization will be transcribed in about half of the muscle nuclei.
In addition, the specific molecular consequences of the variants on the FHL1 protein function account for part of the phenotypic variability as well. Variants that disrupt the zinc coordinating cysteine and histidine residues of the two zinc fingers of the FHL1 LIM-2 domain are typically pathogenic with early-onset and severe phenotypes.1 The variant with the mildest phenotype in our cohort, [FHL1:NM_001449.5; c.356G>T; p.(G119V)], was identified in two independent families and manifested with a late adulthood-onset, progressive scapuloperoneal myopathy. Glycine 119 is near the highly conserved histidine residue (H123) that has been reported in a severe infantile-onset phenotype of RBM1 (Fig. 1C). Thus, the degree of disruption of the critical cysteine and histidine residues in the LIM-2 domain may also be predictive of disease severity. Consistent with this notion, individuals with W122 substitutions, right adjacent to the critical histidine 123, also present with a scapuloperoneal, adult-onset myopathy with histological reducing bodies,20 similar to the newly identified G119V patients in our cohort.
Focal onset and asymmetric weakness was a conspicuous feature in our cohort. Focal aggregate formation was evident on histological analysis (Fig. 2). Reflecting this initial, focal nature of reducing body aggregate formation, muscle MRI and muscle ultrasound also illustrated a heterogeneous pattern of increased signal or echogenicity within individual muscles, often with normal appearing areas adjacent to T1 hyperintense areas within the same muscle (Fig. 3). This unique multifocal and sectorial pattern of muscle remodeling resembled the appearance of land separated by bodies of water as might be depicted on a geographic map and thus we refer to it as a “geographical” pattern. The multifocal disease and intramuscular variability of fatty replacement along the length of each muscle was also notable on quantitative fat fraction analysis (Fig. 5).
Interestingly, such a “geographical” pattern has been reported in other protein aggregate myopathies such as VCP-associated myopathy22, 23 and may suggest aggregate formation starts from seed regions before spreading more widely within individual muscles. We also identified a distinctive appearance of increased echointensity with selective involvement of deeper fascicles of muscles such as biceps brachii and tibialis anterior (Figure S1) in the majority of the patients. The specificity of this unique pattern of intramuscular echointensity is unclear, but it can potentially serve as a non-invasive diagnostic sign to help direct genetic testing or aid in variant interpretation in the right clinical setting.
The overall degree of muscle remodeling in muscle MRI studies appeared to qualitatively correlate with global functional status such as ambulatory status and respiratory function, assessed by forced vital capacity (Table 1). For example, patients F1P3 (34 years), and F5P11 (14 years) demonstrated minimal levels of muscle involvement in the MRI studies and were independently ambulatory. At the most severe end of the spectrum, almost complete fatty replacement of all muscles in the lower extremities was present in F2P5 (37 years) who relied on a motorized wheelchair for ambulation, with little discernible voluntary movement in the lower extremities (Fig. 4A). In addition, serial MRIs and ultrasound studies in F1P1 and F1P2 (~2.5 years apart) showed expanding areas of T1 hyperintensity over time, in parallel with increasing difficulty in independent ambulation (Figs. 3D, 4C, Text S1).
Muscle ultrasound and MRI, which are now becoming increasingly available, may also hold promise as non-invasive diagnostic biomarkers. For example, the characteristic pattern of intramuscular fatty replacement associated with FHL1-RBM may provide supportive evidence for confirmation of pathogenicity of variants of uncertain significance and complement other clinical and ancillary diagnostic evaluations. Additionally, patients with greater progression of fatty replacement of muscles may anticipate need for assistance in mobility and support to maintain quality of life and thus help with proactive clinical management. Since muscle MRI and ultrasound qualitatively change in parallel with overall disease progression in RBM they also hold promise as biomarkers of disease progression. However, a prospective study with functional clinical outcome assessments over time is needed to establish the utility of these muscle imaging modalities, their reliability, and sensitivity to change in RBM.
Echocardiography and cardiac MRI did not identify any clinically important abnormalities in our patients. One patient (76 years old) had nonspecific left ventricular hypertrophy by echocardiography, which could also be ascribed to unrelated underlying medical conditions. Very subtle changes suggestive of myocardial edema in the absence of myocardial fibrosis in one patient who underwent cardiac MRI were also without clinical correlates. In contrast, the EDMD patients with loss-of-function FHL1 variants commonly develop notable cardiac abnormalities including focal myocardial hypertrophy24 and late gadolinium enhancement.9 Given the small number of patients in our cohort who underwent cardiac MRI studies, it is difficult to make definitive conclusions about the value of this modality in the anticipatory guidance and management of FHL1 RBM patients at this time. However, our observations do not exclude the possibility of development of more cardiac pathology in late stages of the disease.
Our study has a few limitations. The small sample size is predominantly due to the rarity and severity of the disease which prevented participation of some patients in imaging studies. In addition, the cross-sectional and observational nature of our study precludes definitive correlation of disease severity and progression with imaging studies. Lastly, lack of standardized research imaging evaluations such as Dixon MRI or muscle ultrasound across different centers resulted in missing data points. Future prospectively designed studies will allow for standardization and coordination of imaging protocols.
In summary, we characterize a cohort of patients with FHL1-related RBM, using muscle MRI and ultrasound. We report a novel likely pathogenic variant, p.(G119V), that provides additional evidence for a focal and regional onset of the disease based on histological and imaging studies, in keeping with FHL1-related RBM as a paradigmatic aggresomal disease of muscle. We propose unique features on muscle ultrasound and muscle MRI as diagnostic biomarkers and demonstrate the feasibility of using quantitative skeletal muscle MRI (Dixon) and ultrasound in this patient population. While these imaging modalities need to be formally validated in prospectively designed studies in parallel with formal clinical outcome assessments, they hold promise as surrogate outcome measures in rare myopathies such as RBM.
Acknowledgments
The authors thank the patients and their families for participating in the study. Further, we would like to thank Christopher Mendoza, Gilberto “Mike” Averion, Katherine Carney, and Marsha Block for coordinating and overseeing the patient visits. This research was supported in part by the Intramural Research Program of the NIH, NINDS (C.G. Bönnemann, protocol #12-N-0095) and NHLBI (A.E. Arai, protocol #02-H-0050).
Conflict of Interest
In his position at the NIH, Carsten G. Bönnemann serves on several scientific advisory boards (MDUK Neuromuscular Center, NeuroMyoGene Lyon, Genethon, Sarepta Therapeutics, CureCMD, CMD-IR, RYR1 Foundation, MTM and CNM Registry) and receives no personal compensation for his contributions. Vinay Chaudhry reports the following disclosures: Consultant to Department of HHS and Department of Justice – Vaccine Injury and Compensation Program. Royalty for Total Neuropathy Score (TNS) patent through Johns Hopkins University—from Levicept, Genenetech, AstraZenec, Fundacion, RWS Life sciences, Medimmune, Disarm Therapeutics, and Passage Bio. John Brandsema reports the following disclosures: Consultant for Audentes, AveXis/Novartis, Biogen, Cytokinetics, Genentech, Marathon, Momenta/Janssen, NS Pharma, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, WaVe, Speaker for AveXis/Novartis and Biogen, Medical advisory council member for Cure SMA, Site investigator for clinical trials with Alexion, Astellas, AveXis/Novartis, Biogen, Catabasis, CSL Behring, Cytokinetics, Fibrogen, Genentech, Ionis, Lilly, Pfizer, PTC Therapeutics, Sarepta, Summit, WaVe. The other authors report no conflicts of interest.
Author Contributions
Payam Mohassel contributed to the study concept, design, data acquisition, analysis, interpretation of data, and drafting of the manuscript. Pomi Yun also contributed to the analysis, interpretation of data, and drafting of the manuscript. Payam Mohassel and Pomi Yun contributed equally to this paper. The following authors were responsible for the interpretation of clinical data and revision of manuscript: Safoora Syeda, Abhinandan Batra, Andrew J. Bradley, Sandra Donkervoort, Soledad Monges, Julie S. Cohen, Doris G. Leung, Francina Munell, Carlos Ortez, Angela Sánchez-Montáñez, Peter Karachunski, John Brandsema, Livija Medne, Vinay Chaudhry, Giorgio Tasca, and Glenn A. Walter. Authors A. Reghan Foley, Bjarne Udd, and Andrew E. Arai contributed through supervision of the acquisition and interpretation of clinical data in addition to critical revision of the manuscript. Corresponding author and guarantor, Carsten G. Bönnemann, was responsible for overall supervising the acquisition and interpretation of clinical data in addition to critical revision of the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.




