KCNA1 gain-of-function epileptic encephalopathy treated with 4-aminopyridine
Abstract
Precision medicine for Mendelian epilepsy is rapidly developing. We describe an early infant with severely pharmacoresistant multifocal epilepsy. Exome sequencing revealed the de novo variant p.(Leu296Phe) in the gene KCNA1, encoding the voltage-gated K+ channel subunit KV1.1. So far, loss-of-function variants in KCNA1 have been associated with episodic ataxia type 1 or epilepsy. Functional studies of the mutated subunit in oocytes revealed a gain-of-function caused by a hyperpolarizing shift of voltage dependence. Leu296Phe channels are sensitive to block by 4-aminopyridine. Clinical use of 4-aminopyridine was associated with reduced seizure burden, enabled simplification of co-medication and prevented rehospitalization.
Introduction
Improvements in accessibility, speed, and thoroughness of next-generation genetic sequencing provide new opportunities to improve clinical care of young patients with complex neurological disease, but formidable challenges remain.1, 2 We report an infant patient with a highly pharmacoresistant focal-onset epilepsy leading to recurrent months-long hospitalizations. Sequencing revealed a de novo KCNA1 missense change located in the voltage-sensing S4 transmembrane segment. KCNA1 variants in episodic ataxia type 1 (EA1) pedigrees, and in a very small number of children with generalized seizures and encephalopathy, are loss-of-function (LOF).3 We noticed that our patient's novel variant was exactly homologous to a previously described gain-of-function (GOF) variant in KCNA2,4-6 and was adjoining to sites of highly recurrent KCNQ2 and KCNQ3 GOF variants causing DEE.7-9 We hypothesized that his severe focal epilepsy was due to KV1.1 GOF. We tested this through voltage-clamp studies and adjunctive treatment with the K+ channel blocker 4-aminopyridine (4-AP).
Methods
Genetics
Proband and parental samples were analyzed in parallel by rapid-turnover whole exome sequencing (Critical Trio Exome, Baylor Genetics) with concurrent chromosomal microarray.2
Mutagenesis, expression, and voltage-clamp
Human KCNA1 cDNA (GenScript) was cloned into pGEMHE. Mutagenesis (Quickchange, Agilent Technologies) and Xenopus oocyte automated voltage-clamp recording (Roboocyte2, Multi-Channel Systems) were performed as reported previously.4-6
Data analysis and statistics
Conductances were obtained by normalizing peak current to Vtest−Vrev, with Vrev the calculated K+ reversal potential. Boltzmann equations were fit to activation and inactivation curves and the Hill equation to dose–response curves.6 Statistical analysis was performed using Prism (GraphPad).
Results
Case report
The patient was a former full-term male, previously healthy, with onset of recurrent focal seizures near 3 months of age. Seizures involved behavioral arrest, unresponsiveness, perioral cyanosis, posturing or tonic extension of both upper extremities, and prolonged ictal/postictal oxygen desaturation. On continuous EEG (cEEG), seizure onsets were initially marked by broad left temporal onset, with evolution to 2–3 Hz spike and wave discharges. Brain magnetic resonance imaging revealed no structural abnormalities. Blood, CSF, and urine tests including basic laboratory, metabolic screens, and viral panels were unremarkable. Seizures came under control during this initial hospitalization with dual fosphenytoin and levetiracetam treatment. Fosphenytoin was transitioned to oxcarbazepine, and patient had 2 weeks of seizure freedom prior to discharge home near 3.5 months of life.
Within 2 months of initial hospital discharge, however, seizures resumed, with frequent emergency care leading to rehospitalization near 6 months of age. On cEEG, seizure onsets were marked by rhythmic 12 Hz spikes independently in either the left or right temporal lobes (Fig. 1A,B). Interictal EEG showed multifocal epileptiform sharp wave discharges, most prominent in left and right temporal lobes. Treatment including maximal therapeutic doses of anti-seizure medications (levetiracetam, oxcarbazepine, valproic acid, lacosamide, phenytoin, clobazam, and vigabatrin) and a ketogenic diet (Fig. 1C, “hospital care”) failed to bring remission. Seizures, again accompanied by oxygen desaturation and cyanosis, recurred as frequently as 30 times per day. The longest seizure-free interval on cEEG between age 6 and 8 months was 2 days. Formal neuropsychological evaluation at 7 months of age showed slightly delayed development, approximated at a 5-month level, but noted use of sedating medications as potentially contributory.
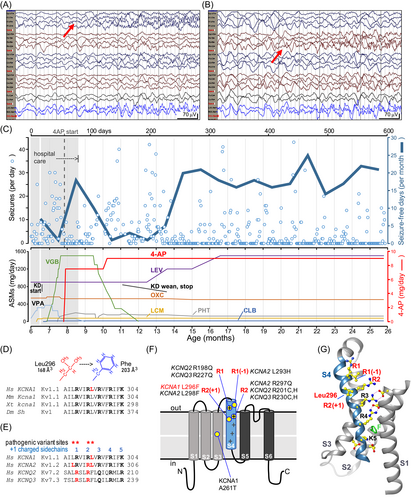
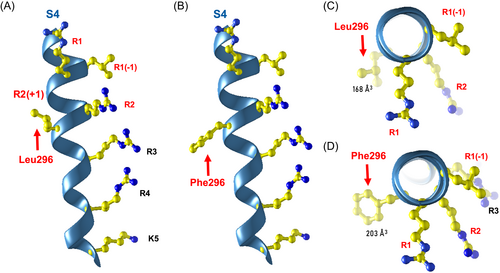
Trio WES demonstrated a novel heterozygous de novo KCNA1 variant, c.888G>T, p.(Leu296Phe) (L296F), in the extracellular-facing portion of the S4 helix (Fig. 1D–G). KCNA1 S4 sequences are completely conserved in fly, frog, mouse, and man (Fig. 1D). Alignment of selected human KV channel sequences revealed that L296 is adjacent to the R2 Arg residue, near nine known DEE pathogenic variants in three other KV channel subunits, notably including the homologous variant in KCNA2, KV1.2 L298F (Fig. 1E,F). All nine of these pathogenic variants confer GOF when assessed by electrophysiology.4-9 In models of “open state” KV1 channels, the S4 helix is translocated outward (Fig. 1F cartoon, 1G structure).10-12 During closing, modeling predicts that S4 moves inward ~15–20 Å, and R1-R2 replace R3, R4, and K5 at binding sites buried within the membrane.11, 12 Although the patient's phenotype was novel for KCNA1, it is well known that LOF and GOF in the same channel can cause distinct syndromes. We hypothesized that replacement of KCNA1 Leu296 with the slightly larger, planar Phe sidechain (Fig. 2) caused GOF and was thereby pathogenic. Given this analysis, recent evidence of beneficial effects of 4-AP as a targeted therapy in KCNA2 GOF variants,6 and the urgent clinical setting, we in parallel assessed variant function and initiated 4-AP after an interdisciplinary discussion and informed consent of the parents.
KCNA1 L296F causes a GOF by opening at more hyperpolarized voltages
Under Xenopus oocyte voltage-clamp, channels formed by L296F alone showed a very large hyperpolarizing shift (~−40 mV) in activation voltage dependence (p < 0.0001, Fig. 3A,C). Upon 1:1 co-expression of wild type (wt) and L296F to mimic the heterozygous state, we still observed a smaller, but significant, ~−10 mV shift (p = 0.0001). The mean current amplitude at +30 mV was not significantly increased for either L296F or the co-expression when compared to the wt (Fig. 3B).
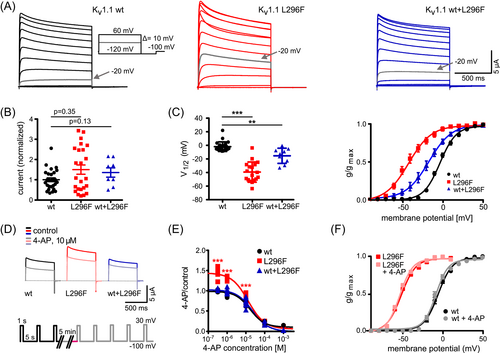
4-AP blocks L296F and wt channels with similar IC50
We tested five different concentrations of 4-AP, from 0.3 μM to 1 mM (Fig. 3D–F). Potassium currents carried by both wt and mutant channels were reduced with similar concentration dependence. IC50 were as follows: wt: 16.5 ± 2.7 μM 4-AP (n = 20), L296F alone: 13.2 ± 2.7 μM (n = 16), wt+L296F: 21.9 ± 4.9 μM (n = 18, Fig. 3E). At 0.3, 1 and 10 μM 4-AP, the variant expressed alone showed an unexpected increase in current amplitude, but this was not seen in cells co-expressing wt and the variant (p < 0.001, Fig. 3E).
Clinical treatment with 4-AP
After a day with four seizures, 4-AP was initiated, and seizures remitted for 48 h. Improved control persisted. After 4-AP dose increase to 0.9 mg/kg/day, one concurrent drug (valproic acid, Fig. 1C, lower) was tapered and the patient was discharged from the hospital. Over 18 subsequent months of 4-AP treatment, there have been bouts of seizure recurrence (some associated with detected subtherapeutic phenytoin levels, routine viral illness, or vaccinations). However, the patient has not required rehospitalization. He has demonstrated continued developmental progression and tolerated discontinuation of the ketogenic diet, divalproex, and vigabatrin (Fig. 1C). At age 15 months, 48-h cEEG monitoring demonstrated electroclinical seizure freedom, supporting the validity of the parent-reported seizure counts (Fig. 1C, upper). Serum concentration of 4-AP was obtained at intervals and was within the range found in adults treated for multiple sclerosis (Table 1). At age 24 months, he walks independently, grasps objects, brings objects to his mouth, and brings his hands together. Expressive language is delayed, with only a few words (approximately at a 12-month level).
| Age (months) | Dose and dosing interval | Daily dose (mg/kg/day) | Trough level (pre-med dose) | Estimated peak level (1-h post-med) |
|---|---|---|---|---|
| 7.9 | 1.25 mg, 3×/d | 0.4 | ||
| 8 | 2.5 mg, 3×/d | 0.9 | ||
| 8.5 | 1.875 mg, 4×/d | 0.9 | ||
| 9.5 | 1.875 mg, 4×/d | 13 ng/mL (138 nM) | – | |
| 9.5 | 1.875 mg, 4×/d | 11 ng/mL (116 nM) | 33 ng/mL (350 nM) | |
| 10 | 1.875 mg, 4×/d | 10 ng/mL (106 nM) | 39 ng/mL (414 nM) | |
| 10 | 1.875 mg, 4×/d | 13 ng/mL (138 nM) | a18 ng/mL (191 nM) | |
| 10.5 | 3 mg, 3×/d | 0.83 | ||
| 12.5 | 3 mg, 3×/d | 27 ng/mL (287 nM) | 65 ng/mL (691 nM) | |
- Extended-release dalfampridine tablets (10 mg, Ampyra) were cut in quarters or halves. Tablet pieces were crushed and dissolved in 4 or 5 mL of tap water. The dose volume was measured and provided orally with a syringe. Serum 4-AP levels were determined in a CLIA laboratory (NMS Labs, Horsham, PA). Where peak levels are shown, levels were obtained on same day with the paired trough, drawn before and approximately 1 h after dosing; this timing was based on adult immediate release 4-AP kinetics.20 Daily doses (mg/kg/day) are provided when patient was reweighed the same day.
- a One peak level was unexpectedly low given the paired, trough level recorded as drawn 1.5 h earlier,16 and timing of the 4-AP dose was uncertain.
Discussion
This study broadens the spectrum of KCNA1-related illness to include GOF-associated DEE, highlights the utility of incorporating paralogue-derived evidence into variant classification rules, provides an example of 4-AP use as an anti-seizure medication in a second K+ channel GOF disorder,6 and highlights some unanswered questions about 4-AP's mechanism of action and efficacy as a targeted therapy for Kv1.1 channel GOF.
KCNA1 EA1 has been linked to missense LOF variants. A few examples of KCNA1 DEE are known where functional analysis has shown more severe LOF.3 A recent study described a teenage patient with normal development and focal epilepsy beginning in childhood, fully controlled by carbamazepine monotherapy, in whom a de novo KCNA1 p.(Ala261Thr) variant (Fig. 1F) was found.13 Functional expression revealed a smaller hyperpolarization of voltage activation compared to that of our patient. Although the number of known patients is very low, it appears that severe KCNA1 LOF is associated with early onset DEE, milder LOF with EA1, and GOF with focal epilepsy whose severity may correlate to the extent of GOF.
In our patient, clinical suspicion and management were influenced by broad teamwork including pediatric epileptologists, clinical and clinical laboratory geneticists, research laboratory-based channel physiologists, and channelopathy clinical researchers. A key step in assessing the novel KCNA1 variant was inclusion of information about its location in a conserved functional domain (Fig. 1D) bearing nine GOF variants at identical or nearby positions of related KV channel genes (Fig. 1E–G). This suggested a mutational intolerance hotspot and common molecular mechanism, GOF. This knowledge, the prior beneficial use of 4-AP in KCNA2-GOF DEE,6 and the demonstrated resistance to conventional medications, led to the care team's decision to begin 4-AP. Despite the exact homology of KCNA1 L298F and KCNA2 L296F, it is noteworthy that the KCNA2 variant's phenotype (DEE with severe generalized epilepsy and ataxia)5-7 is much different from our patient's, and the KCNA2 variant causes a 10-fold increase in current amplitudes in oocytes, unlike KCNA1 L296F (Fig. 3B).4 Although paralogue evidence is not well established in clinical genetics laboratory classification protocols, informatics techniques to exploit channel paralogue datasets are being developed.14, 15 Training of such programs can be helped as more empirical studies of variant pairs, as provided here, become available.
The 4-AP serum concentrations obtained (Table 1) raise questions regarding the drug's targets and therapeutic mechanisms. In our patient, trough and peak levels were similar to those previously reported in adults under treatment for multiple sclerosis (13–27 ng/mL, trough; 30–60 ng/mL, peak).16 The clinical dose range is far below the concentrations required for KV channel half-blocking as determined by conventional biophysical recordings. Strong voltage dependence and state dependence may make 4-AP binding more potent in firing neurons, since binding is diminished by long-lasting strong membrane depolarizations used in vitro, and unbinding from predominantly closed channels at resting membrane potentials is extremely slow.17 Nonetheless, it seems most likely that the therapeutic action of 4-AP is the outcome of a small fractional reduction in channel currents. In vivo actions of 4-AP may be partly mediated by effects on related channels, including KV3.1, that exhibit somewhat higher binding affinity.18 Additional study of the effects of 4-AP, including modeling in more realistic neuronal and network contexts, may guide development of more precise medicine with greater potency, safety, and efficacy.
We acknowledge the limitations of our study arising from the genetic and phenotypic novelty of this single patient. Seizure remission with 4-AP was incomplete; the causes of periods of excellent or poor seizure control (Fig. 1C) on 4-AP were mostly uncertain. It is not possible to know if 4-AP significantly reduced the seizure burden, whether the natural history for our patient's novel variant is for seizures to continue or improve with age, or if the other agents used were primarily responsible for our patient's improvement. We hope this first description of the variant, molecular consequences, and phenotype will promote the reporting of additional patients and enable controlled clinical trials in this ultrarare disease.
In summary, our results highlight the benefits of rapid genetic testing in the diagnosis of early onset epilepsies,2, 4, 6 and the current need for pipelines enabling timely variant functional characterization.1, 3, 19 Treatment with 4-AP appeared well tolerated during an 18-month course of therapy beginning in infancy, providing motivation for identification of additional KCNA1-2 GOF patients and translational studies of the 4-AP mechanism of action.
Author Contributions
All authors contributed to the conception and design of this study. PM performed the electrophysiology. DST led and coordinated clinical assessment. PM, DST, UH, HL, and ECC drafted and initially revised the manuscript and figures. All authors contributed to case discussions, acquisition and analysis of data, and all reviewed and edited the manuscript.
Acknowledgments
We are grateful for the family for participating in this study. This work was supported by the research unit FOR-2715 of the German Research Foundation (DFG, grants HE8155/1-2 and LE1030/16-2), the German Federal Ministry of Education and Research (BMBF, Treat-ION 01GM1907A and 01GM2210A), the Else Kröner-Fresenius-Stiftung College for Clinician Scientists program, Precise.net, NINDS NS108874, the Jack Pribaz Foundation, and the Miles Family Fund.
Conflict of Interest
JAC has served as a consultant to Xenon Pharmaceuticals; a company developing drugs for potassium channel forms of developmental and epileptic encephalopathy. These relationships have been reviewed and approved by Baylor College of Medicine according to its policies on research conflict of interest. HL has nothing to report which is related directly to this study. ECC has received grants and served as a consultant to Xenon Pharmaceuticals and Knopp Biosciences; both companies are developing drugs for potassium channel forms of developmental and epileptic encephalopathy. These relationships have been reviewed and approved by Baylor College of Medicine according to its policies on research conflict of interest. All other authors have nothing to report.




