Characteristics of systemic inflammation and brain iron deposition in Parkinson's disease patients
Funding Information: This work was supported by the National Natural Science Foundation of China (Grant No.:81871847), the Project funded by China Postdoctoral Science Foundation (Grant No.:2021M703740), the Science and Technology Planning Key Project of Guangzhou (Grant No.: 201803010119), the Guangzhou health and medical collaborative innovation major projects (Grant No.: 201604020009), and the Natural Science Foundation of Guangdong Province China (Grant No.: 2018A030313277). The authors sincerely thank the subjects included in this study, Doctor Yan Liu of Department of Medical Imaging, Nanfang Hospital of Southern Medical University for MRI measurement, the Home for Researchers editorial team for the language polishment (www.home-for-researchers.com).
Abstract
Objective
This study aimed at determining the characteristics of systemic inflammation and brain iron deposition in Parkinson's disease (PD) patients.
Methods
Thirty two PD patients and 30 gender- as well as age-matched controls were enrolled. Serum interleukin (IL)-1β, IL-33, tumor necrosis factor (TNF)-α, IL-6, IL-10, ferritin, iron, and total iron binding capacity (TIBC) levels were assayed. Quantitative susceptibility mapping (QSM) was used to quantitatively analyze brain iron accumulation in the regions of interest (ROIs). Correlations between concentrations of inflammatory cytokines and biomarkers for peripheral iron metabolism, brain iron deposition were evaluated in the PD group.
Results
Serum concentrations of IL-1β and IL-33 were found to be significantly elevated in the PD group compared to the control group, and in early-stage PD group compared to advanced-stage PD group. Total QSM value for bilateral ROIs was significantly elevated in the PD group compared to the control group, and in advanced-stage PD group compared to early-stage PD group. There was a significant inverse correlation between serum IL-1β concentration and total QSM value for bilateral ROIs, between serum ferritin, iron, TIBC concentrations, and total QSM value for bilateral ROIs in PD patients. However, there was no significant correlation between serum IL-1β concentrations and serum ferritin, iron, TIBC concentrations in PD patients.
Interpretation
The inflammatory state and chronic brain iron deposition progression in PD patients might be asynchronous. Alterations in systemic inflammation were not correlated with peripheral iron metabolism and might not contribute to the aggravation of brain iron deposition in PD patients.
Introduction
Studies have reported that neuroinflammation is involved in PD pathogenesis.1 Inflammatory cells have been found in both substantia nigra of PD patients during autopsies2 and in PD animal models.3 Neuroinflammation is strongly associated with neuronal death in PD.1 Concentrations of peripheral inflammatory cytokines have been found to be elevated in PD patients,4 indicating that systemic inflammation is an important pathophysiological feature in PD.5 Moreover, inflammatory marker levels are correlated with PD disease progression.6
However, the relationship between some inflammatory biomarkers and PD is not specific,7 and implications of elevated serum inflammatory cytokines in different PD stages have not been established. Moreover, the mechanisms through which inflammation interacts with other pathogenesis factors for PD, such as iron metabolism and deposition have not been elucidated.
Elevated iron levels in the substantia nigra is an important pathophysiological feature for PD.8 Systemic iron metabolism in PD patients is also disrupted, just as it is in the brain.9 Excess redox-active iron induces hydroxyl radical generation,10 which is an important reactive oxygen species that generates oxidative damage and subsequent degeneration. Disrupted iron metabolism and oxidative damage can also be triggered by neuroinflammation.10 Since the mechanism associated with iron accumulation during neurodegeneration may be abnormal iron homeostasis, factors that may interfere with iron homeostasis should be evaluated. An in vitro study reported that TNF-α and IL-6 promote iron accumulation in neurons and microglia.11 Alterations in the expression of some iron-related factors that are mediated by pro-inflammatory cytokines may be involved in iron accumulation in the neurons of PD models.12 Furthermore, IL-1β levels in the cerebrospinal fluid are correlated with iron deposition in the substantia nigra of PD patients.13 However, it has not been well established whether elevated systemic inflammatory cytokines, disrupted peripheral iron metabolism, as well as enhanced brain iron deposition are correlated.
Imaging methods for evaluating brain iron deposition have been extensively used.14 In particular, due to its sensitive capacity for quantifying the magnetic susceptibility of brain tissue from the gradient echo signal phase, and providing excellent contrast to visualize iron-rich deep nuclei within neighboring tissues,15 applications of quantitative susceptibility mapping in PD patients have already been established.15 To the best of our knowledge, no clinical study has simultaneously investigated peripheral inflammatory cytokines, iron metabolism biomarkers, and brain iron deposition using QSM in a cohort of PD patients.
Study Participants and Methods
Study participants
This study was approved by the Ethics Committee of Nanfang Hospital. Participants were enrolled according to a predetermined inclusion and exclusion criteria. They were required to provide a written informed consent prior to the start of the study. A total of 32 PD patients from Nanfang Hospital of and Guangdong 999 Brain Hospital were enrolled. The diagnosis of PD was performed by two experienced movement disorder specialists according to the Movement Disorder Society (MDS) clinical diagnostic criteria for PD.16 In addition, a total of 30 controls from Nanfang Hospital were recruited during the same period by experienced neurologists who were blinded to the study. The controls did not exhibit severe neurological deficits. Participants were from the Han ethnicity and anyone with high blood pressure, diabetes, abnormal liver functions, or on medications that could influence blood biochemistry, with the exception of anti-Parkinsonism drugs, were excluded.
Nervous system assessment and neuropsychological testing
The MDS-Unified PD Rating Scale17 and the Hoehn & Yahr Scale were used to assess PD patients when in “ON” state. Global cognitive functioning was assessed by CMMSE.18 PD patients were subdivided into either early-stage PD group (EP, H & Y stage 1–2.5) or advanced-stage PD group (AP, H & Y stage 3–5) according to the modified Hoehn & Yahr Scale.
Sample preparation and storage
After 8 h of fasting, blood samples were collected from participants and stored in a serum separation gel tube. Samples were centrifuged at 1500g for 10 min at 4°C after which top serum layers were collected and stored at −80°C.
Bio-plex cytokine and proteins for iron metabolism assay
Serum cytokine (IL-1β, IL-33, TNF-α, IL-6, and IL-10) and chemokine protein levels were assessed by the Bio-Plex Pro Human Chemokine 6plx EXP #17005393 kit (Bio-Rad Laboratories, Inc). All assays were performed in accordance with the corresponding protocol. Results were analyzed using the Bio-Plex 200 System and Bio-Plex Manager 6.1 software (Bio-Rad Laboratories). Ferritin, serum iron, and TIBC were assayed using a routine biochemistry test system.
Image acquisition
Participants were scanned using a clinical 3T MR imaging system (MK750, GE Healthcare, Milwaukee, WI) equipped with an eight-channel phased array head coil. 3D multi-echo gradient echo images were acquired in a T2 star-weighted sequence. Imaging parameters were: TE of first echo = 5.9 msec, echo spacing = 7.0 msec, number of echoes = 5, TR = 38 msec; matrix size = 183 × 156; slice thickness = 2 mm; FOV = 182 × 220 mm2; slice space = 0.6 mm; and flip angle = 10°. To reduce noise and head motions during MRI exams and to minimize adverse effects of the imaging scan, ear plugs, and fixed pillows were used. Before QSM and R2* reconstruction, two researchers who were blinded to the participants evaluated the quality of the magnitude images, and low-quality images were excluded.
Image reconstruction
As previously described,19 QSM was performed using the STI Suite software (Duke University) after phase images had been retrieved in a DICOM format from the MRI scanner. Phase images were unwrapped using a Laplacian-based phase unwrapping method that only relied on sine and cosine functions of the phase angle.20 Background phase was removed by sophisticated harmonic artifact reduction for phase data with varying kernel sizes (VSHARP).20 Then, streaking artifact reduction for QSM (STAR-QSM) was used to enhance the accuracy of magnetic susceptibility at the tissue edge. After the whole tissue field had been inverted from field to source, QSM images were finally obtained.
Regions of interest
The Regions of interest (ROIs) in the basal ganglia and midbrain included bilateral globus pallidus (GP), putamen (PUT), head of the caudate nucleus (CN), substantia nigra (SN), and red nucleus (RN). All ROIs were manually drawn on the susceptibility maps using the ITK-SNAP (www.itksnap.org). Manual drawing was performed by two experienced neuroradiologists who were blinded to the information of each participant. The ROIs for each nucleus were obtained from all visible sections (Fig. 1), except sections that represented the most inferior or most superior slice in which the nucleus was defined. To minimize partial volume effects, voxels at tissue boundaries were also excluded. Mean QSM value for all included sections of bilateral ROI was considered the QSM value for the corresponding ROI of each individual. The QSM value (total) of five-related regions was considered the total QSM value for the bilateral regions. The ROIs were confirmed by a radiologist with extensive neuroimaging experience.
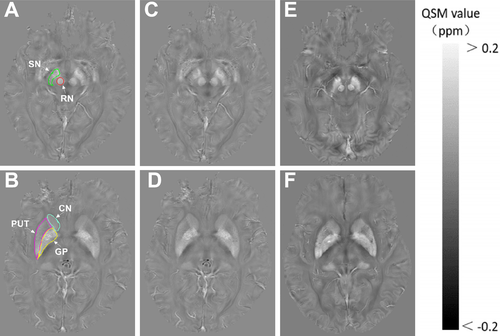
Statistical analyses
Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as frequencies and percentages. Since the data for serum IL-1β, IL-33, TNF-α, IL-6, and IL-10, ferritin, TIBC were asymmetrically distributed, group mean values were compared using nonparametric tests (Mann–Whitney U test). Group mean values were compared using general linear model if data were normally distributed according to the homogeneity of variance. Partial correlation coefficient (adjusted age and sex) was determined to evaluate the relationship between serum levels of inflammatory cytokines and iron metabolism biomarkers, QSM values of the ROIs. p < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v. 20.0 (IBM, Chicago, IL, USA), and graphical depictions were made using GraphPad Prism v. 8.02 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Demographic and clinical characteristics of the study participants are presented in Table 1. The number of patients in the two groups were well matched. There was no significant difference in age and sex between PD patients and the controls.
| Control group | PD | p Value | |
|---|---|---|---|
| N | 30 | 32 | / |
| M/F, n | 13/17 | 15/17 | 0.7791 |
| Age, y | 55.77 ± 12.89 | 61.22 ± 8.54 | 0.0572 |
| Duration, y | / | 5.40 ± 4.02 | / |
| Hoehn-Yahr stages | / | 2.59 ± 0.93 | / |
| UPDRS-III | / | 44.72 ± 20.43 | / |
| LEDD, mg/day | / | 372.91 ± 110.39 | / |
- Data are mean ± SD.
- F, female; LEDD, Levodopa equivalent daily dose; M, male; N, numbers; UPDRS-III, MDS- Unified Parkinson's Disease Rating Scale III).
- 1 Pearson chi-square test.
- 2 Independent-samples T test.
Concentrations of serum inflammatory markers in PD patients and controls
Mean IL-1β and IL-33 levels were elevated in PD patients when compared to controls (p < 0.01). There were no significant differences in mean IL-6, IL-10, or TNF-α levels between PD patients and the controls. Subgroup analysis indicated that mean IL-1β levels were higher in EP patients than in AP group patients (p < 0.01). Moreover, mean IL-33 levels were significantly elevated in EP compared to AP group patients (p < 0.01; Table 2, Fig. 2A). To evaluate the underlying relationships between levels of serum inflammatory cytokines and medications in PD patients, we performed correlational analyses. There were a significant inverse correlation between serum IL-1β levels and Levodopa equivalent daily dose(LEDD) (r = −0.442, p = 0.014), whereas there were no significant correlations between serum IL-1β levels and dose of MAO-B inhibitor in PD patients, and neither were there any correlations between serum IL-33 levels and LEDD, dose of MAO-B inhibitor (Fig. 2A, D–G).
| Control group | PD | p Value | |
|---|---|---|---|
| Inflammatory cytokines (pg/mL) | |||
| IL-1β | 1.120 ± 0.074 | 1.176 ± 0.079 | 0.009 |
| IL-33 | 30.443 ± 5.132 | 34.890 ± 7.943 | 0.006 |
| IL-6 | 8.476 ± 1.305 | 9.883 ± 4.719 | 0.165 |
| IL-10 | 8.500 ± 1.830 | 8.861 ± 1.613 | 0.153 |
| TNF-α | 13.350 ± 3.500 | 13.935 ± 2.973 | 0.369 |
| Indicators of iron metabolism | |||
| Ferritin (μg/L) | 162.743 ± 51.698 | 140.085 ± 38.437 | 0.027 |
| Serum iron (μmol/L) | 16.840 ± 2.798 | 16.125 ± 2.803 | 0.236 |
| TIBC (μmol/L) | 50.243 ± 5.317 | 49.541 ± 5.494 | 0.398 |
| QSM value of ROIs | |||
| SN | 6.649 ± 1.013 | 8.344 ± 1.493 | 0.000 |
| RN | 5.538 ± 1.545 | 7.761 ± 2.068 | 0.000 |
| CN | 2.432 ± 0.632 | 2.605 ± 0.755 | 0.371 |
| GP | 7.118 ± 1.161 | 7.979 ± 1.870 | 0.015 |
| PUT | 2.821 ± 0.913 | 3.756 ± 1.182 | 0.001 |
| Total QSM value | 49.114 ± 5.270 | 60.889 ± 9.435 | 0.000 |
- CN, the head of caudate nucleus; GP, globus pallidus; PUT, putamen; QSM, quantitative susceptibility mapping; RN, red nucleus; SN, substantia nigra; TIBC, Total iron binding capacity; Total QSM value, Total QSM value of bilateral ROIs.
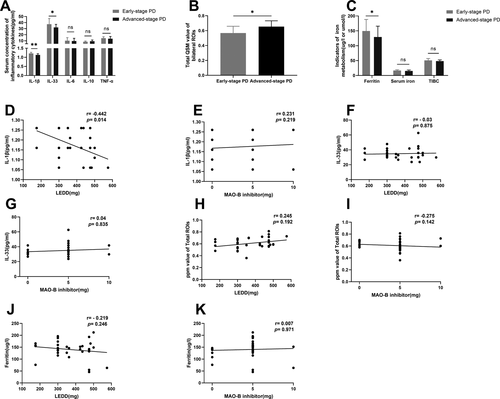
Comparisons of QSM values
Mean QSM values for bilateral substantia nigra, red nucleus, globus pallidus, and putamen for the PD group were significantly elevated compared to those of the control group (p < 0.01 or p < 0.05). We also found a significant difference between the EP and the AP groups in terms of the total QSM value of the bilateral ROIs (p < 0.05, adjusted by age, sex, and MAO-B inhibitor dose). There was no significant difference in mean QSM values of the bilateral CN between the PD and control groups (p > 0.05), although the value for the former group was higher than that of the latter group (Table 2, Fig. 2B). There were no significant correlations between total QSM value of the bilateral ROIs and LEDD, dose of MAO-B inhibitor in PD patients (Fig. 2H,I).
Concentrations of serum iron metabolism markers in PD patients and controls
Mean ferritin levels were decreased in PD patients when compared to controls (p < 0.05). There were no significant differences in mean iron, TIBC levels between PD patients, and the controls. Subgroup analysis indicated that mean ferritin levels were higher in EP patients than in AP group patients (p < 0.05, Table 2, Fig. 2C). There were no significant correlations between ferritin levels and LEDD, dose of MAO-B inhibitor in PD patients (Fig. 2J,K).
Correlations among levels of serum inflammatory cytokines, total QSM value of bilateral ROIs, and peripheral iron metabolism biomarkers in PD patients
To evaluate the underlying relationships among levels of serum inflammatory cytokines, QSM value of bilateral ROIs, and peripheral iron metabolism biomarkers that were observed in PD patients, we performed correlational analyses. There were a significant inverse correlation between serum IL-1β levels and total QSM value of the bilateral ROIs (r = −0.365, p = 0.047), whereas there was no significant correlations between serum IL-1β levels and serum ferritin, iron, TIBC levels. We also found significant inverse correlations between total QSM value of the bilateral ROIs and serum ferritin levels (r = −0.497, p = 0.005), serum iron levels (r = −0.584, p = 0.001), and serum TIBC levels (r = −0.606, p = 0.000) in PD patients (Fig. 3). There were no significant correlations between serum IL-33 levels and total QSM value of the bilateral ROIs in PD patients, and neither were there any correlations between serum IL-33 levels and serum ferritin, iron, TIBC levels (data not shown).
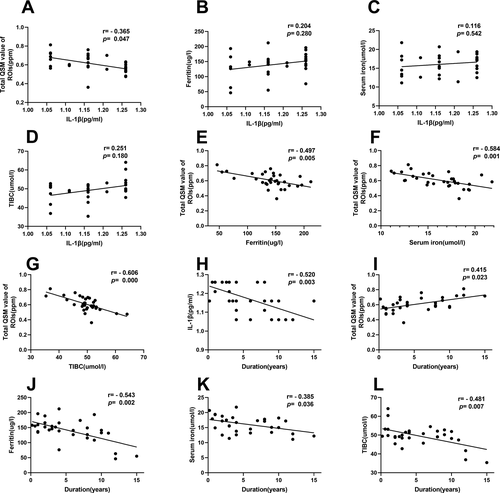
Correlations among disease duration and levels of serum inflammatory cytokines, QSM value of brain ROIs, peripheral iron metabolism in PD patients
Correlational analyses were performed to determine whether the aforementioned indicators were associated with disease duration. Suppressed serum IL-1β levels were associated with extended disease duration (r = −0.520, p = 0.003), whereas a significantly elevated total QSM value of the bilateral ROIs was associated with extended disease duration (r = 0.415, p = 0.023). In addition, there were significantly inverse correlations between serum ferritin, iron, TIBC levels, and disease duration (r = −0.543, p = 0.002; r = −0.385, p = 0.036; r = −0.481, p = 0.007, respectively). (Fig. 3).
Discussion
Although both systemic inflammation and iron dyshomeostasis play important roles in PD progression, peripheral levels of some immune markers have been frequently used to investigate differences between PD patients and healthy controls. Few clinical studies have addressed whether systemic inflammation, disrupted peripheral iron metabolism, and brain iron deposition in PD patients are correlated. For the first time, we simultaneously investigated peripheral inflammatory cytokines, iron metabolism, and brain iron deposition using QSM in PD patients. Concentrations of inflammatory cytokines (IL-1β and IL-33) in serum and mean QSM values of deep brain nucleus were significantly elevated in PD patients compared to those of the control group. Subgroup analysis revealed that peripheral inflammatory cytokines were elevated in the EP group while total QSM values of bilateral ROIs were elevated in the AP group. Moreover, serum IL-1β concentrations were negatively correlated with iron deposition, but were not correlated with biomarkers of peripheral iron metabolism in PD patients. These findings implied that the marked inflammatory state and chronic brain iron deposition progression in PD patients might be asynchronous. Altered systemic inflammation was not correlated with peripheral iron metabolism and might not contribute to the aggravation of brain iron deposition in PD patients.
In this study, mean serum levels of IL-1β in PD patients were significantly elevated compared to those of the controls, in line with the results of a large longitudinal cohort study4 involving PD patients. To analyze alterations in expression levels of inflammatory cytokines in PD patients at different stages, we allocated PD patients into the early-stage group and advanced-stage group according to the H&Y stage. Mean serum levels of IL-1β in EP patients were significantly elevated compared to those of AP patients. A similar finding was reported in a study of early PD patients defined by disease duration.21 In addition, we found that serum IL-33 levels were significantly elevated in PD patients. Studies have elucidated on the diverse and pleiotropic role IL-33 plays in certain aspects of CNS function and disease through interactions between glia, neurons, and immune cells.22 Some studies have reported that IL-33 might be beneficial for AD,23 while IL-33 may play an important role in multiple sclerosis development by inhibiting CNS myelination.24 However, it has not been established whether IL-33 has detrimental or beneficial effects on PD progression. It has been reported that IL-33 stimulates the secretion of IL-10, TNF-α, and IL-1β.25 A recent study revealed that IL-33 expression was elevated in the midbrain and striatum of PD patients compared to control individuals.26 However, serum levels of IL-33 in PD patients had not been previously determined. We found that elevated IL-33 levels were consistent with activated inflammatory states of PD patients.
Regarding iron dyshomeostasis and brain iron deposition, we found that peripheral iron metabolism was disrupted in PD patients, although there were no significant differences in serum levels of iron, TIBC between PD patients and controls. Iron metabolism biomarkers have been reported to be dysregulated in PD,9 and dysregulated iron metabolism in central and peripheral systems are associated with iron accumulation in PD.13 We found that total QSM values of bilateral ROIs for PD patients were elevated compared to those of controls, and they inversely correlated with serum levels of iron metabolism biomarkers. These findings imply that the more brain iron depositions, the lower the serum iron metabolism biomarker levels. Progressive iron accumulation has been investigated in advanced-stage PD patients.27 Subgroup analysis in this study showed similar outcomes. Our previous study revealed that dysregulated peripheral iron metabolism was enhanced in advanced-stage PD patients.28 In this study, we found that levels of peripheral iron metabolism biomarkers and total QSM values of bilateral ROIs in PD patients were inversely correlated with disease duration. These findings show that brain iron deposition through local distribution of iron throughout the brain is chronic, occurs progressively and is accompanied by disrupted peripheral iron metabolism.
It has been reported that IL-1β levels in the CSF are positively correlated with brain iron deposition, as measured by susceptibility weighted imaging in patients with PD,13 and changes in iron metabolism may partly be attributed to chronic inflammation in PD.29 In this study, elevated serum levels of IL-1β were negatively correlated with total QSM values of bilateral ROIs in PD patients. This outcome can be explained by: (i) Elevated patterns of peripheral pro-inflammatory cytokines differ from brain iron deposition in PD patients. That is, an inflammatory state may occur earlier than extensive brain iron deposition. Early PD patients may manifest a marked inflammatory state before chronic inflammation, whereas formation of significant brain iron deposition through local iron distribution throughout the brain is a chronic progressive process. A brain pathological study of PD revealed that elevated inflammatory cytokine levels are region- and course-dependent, as a majority of inflammatory cytokines were downregulated in the substantia nigra from Braak stage four onward.30 (ii) Brain iron deposition may not cause serious secondary inflammation and would, therefore, not lead to continuous elevation of IL-1β levels in advanced PD patients. Wang et al. reported that iron deposition in microglia alone is not sufficient to trigger the secretion of pro-inflammatory cytokines, including IL-1β,11 though a study indicated IL-1β secretion may be enhanced by accumulated iron in SN of PD patients.13 (iii) A dual function of inflammation during the onset of PD development cannot be ignored. We did not find a significant correlation between systemic inflammation and peripheral iron metabolism biomarkers, though some studies suggested that exacerbated inflammatory state might contribute to iron deposition in PD.31 Since IL-33 might be involved in CNS myelination and a recent study32 showed that iron accumulation was modulated by myelin breakdown, we next investigated whether the observed iron accumulation was associated with the elevated IL-33 levels in the PD group. We found that elevated serum levels of IL-33 were not correlated with total QSM values of bilateral ROIs in PD patients. As IL-33 could stimulate the secretion of both anti-inflammatory cytokines (i.e., IL-10) and pro-inflammatory cytokines (i.e., TNF-α, IL-1β), its dual function during the onset or progression of PD could not be ignored. What is more, Ir should be noted that the effect of medications (e.g., MAO-B inhibitors) on iron dyshomeostasis and deposition should be further studied, as oxidative stress is an important trigger factor for iron deposition. Whereas in the study, except the LEED correlate inversely with serum IL-1β levels, there were no significant correlation between medications and total QSM values, peripheral iron metabolism markers.
This study has some limitations. First, in the cross-sectional study, we did not evaluate dynamic changes in levels of inflammatory cytokines and QSM values in each PD patient and thus could not make a clear conclusion on the association between systemic inflammation and iron deposition. Longitudinal studies should be performed in next-step. Second, the small sample size may limit the statistical power of our findings. Third, QSM also has its own drawbacks, including the influence of fibers orientations on derived values, which may interfere with the measured values. Last but not least, iron metabolism biomarkers in the CSF of PD patients were not evaluated in this study. Therefore, more studies should aim at elucidating the association between systemic inflammation and peripheral iron metabolism, brain iron deposition in PD.
Conclusions
We found that the marked inflammatory state and chronic brain iron deposition progression in PD patients might be asynchronous. These findings imply that alterations in systemic inflammation are not correlated with peripheral iron metabolism and may not contribute to the aggravation of brain iron deposition in PD patients.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Grant No.:81871847), the Project funded by China Postdoctoral Science Foundation (Grant No.:2021M703740), the Science and Technology Planning Key Project of Guangzhou(Grant No.: 201803010119), the Guangzhou health and medical collaborative innovation major projects(Grant No.: 201604020009), and the Natural Science Foundation of Guangdong Province China (Grant No.: 2018A030313277). The authors sincerely thank the subjects included in this study, Doctor Yan Liu of Department of Medical Imaging, Nanfang Hospital of Southern Medical University for MRI measurement, the Home for Researchers editorial team for the language polishment (www.home-for-researchers.com).
Author Contributions
(1) Research Project: (A) Conception, (B) Organization, (C) Execution; (2) Statistical Analysis: (A) Design, (B) Execution, (C) Review; (3) Manuscript Writing: (A) Writing the First Draft, (B) Review and Critique.
Jinghui Xu, 1A, 1C, 2A, 2B, 3A; Xiaofei He, 1B, 1C, 2B, 3A; Yunqi Xu, 1A, 1B, 2C, 3B; Xi Chen, 1B, 2C; Mingyue Li, 1B, 2C; Liying Zhang, 1B, 3B; Xiaodi Fu, 1C; Mengqiu Pan, 1C; Qun Wang, 1A, 1B, 2C, 3B; and Xiquan Hu, 1A, 1B, 2C, 3B. The final manuscript was read and approved by all authors.
Conflict of Interest
The authors declare no competing interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.




