Test–retest reliability of the Friedreich’s ataxia rating scale
Funding Information
This work was funded by the Friedreich’s Ataxia Research Alliance (www.curefa.org).
Abstract
The modified Friedreich Ataxia Rating Scale (mFARS) is a disease specific, exam-based neurological rating scale commonly used as a outcome measure in clinical trials. While extensive clinimetric testing indicates it’s validity in measuring disease progression, formal test–retest reliability was lacking. To fill this gap, we acquired results from screening and baseline visits of several large clinical trials and calculated intraclass correlation coefficients, coefficients of variance, standard error, and the minimally detectable changes. This study demonstrated excellent test–retest reliability of the mFARS, and it’s upright stability subscore.
Introduction
Friedreich’s ataxia (FRDA) is a progressive, neurodegenerative disease that affects children and young adults with gait and limb ataxia, dysarthria, loss of reflexes, proprioceptive dysfunction, and muscle weakness, as well as non-neurological features of cardiomyopathy, diabetes, and scoliosis.1 FRDA is caused by a GAA repeat expansion in the FXN gene,2 leading to a marked reduction in frataxin, a mitochondrial protein that plays a vital role in energy production and iron homeostasis.
While FRDA currently has no treatment or cure, novel prospective therapeutic strategies are actively being studied in clinical trials, requiring the use of validated and clinically meaningful outcome measures. One commonly used disease specific measure Friedreich’s Ataxia Rating Scale (FARS), an exam-based neurological measure. The FARS was initially introduced in 2005.3 In addition to a standardized neurological exam, its initial validation included in parallel a patient reported activities of daily living scale (ADL), a functional disability staging (FDS), and time-based performance measures. The initial version of the neurological exam component of the FARS was comprised of five subscores: bulbar (11 points), upper limbs (36), lower limbs (16), peripheral nervous system (26), and upright stability (28), summed for a total maximum score of 117 points.3 Later, two-timed stance items without visual aid (standing with feet apart, eyes closed and standing with feet together, eyes closed) were added to the upright stability section, resulting in the FARS-neuro (FARSn) with a total of 125 points.4 This version has been used in multiple clinical trials as well as a prospective, longitudinal natural history, and clinical outcome measures study (FA-COMS, NCT03090789) that started in 2003.4 The FARSn has undergone further refinement as a clinical outcome measure, omitting items that do not directly assess functional abilities. Specifically, the peripheral nervous system subscore and two bulbar components were removed, leading to the modified FARS, or “mFARS” score (maximum total of 93 points5). Psychometric properties of the FARSn and the mFARS were recently summarized,5 but there remained a lack of published information specifically on test–retest reliability in these scales. In this study, we evaluated the test–retest reliability in the mFARS and the FARSn scales using data from recent clinical research in FRDA.
Material and Methods
Data source/ clinical studies
FARSn and mFARS scores were acquired from the FRDA integrated clinical database (FA-ICD) maintained by the Critical Path Institute (c-PATH). As of May 2020, these data included screening and baseline visits from the following clinical trials in FRDA: MICONOS (NCT00905268), IONIA6 (NCT00537680), LA-297 (NCT00530127), and EPI-7438 (NCT01728064). For the EPI-743 study and LA-29, the FA-ICD only included data from the placebo arm. Additional data were provided directly by sponsors: Reata Pharmaceuticals (MOXIe, NCT02255435) and Chiesi Canada (additional predose data from LA-29, NCT00530127). The scales analysed in this study were the mFARS, FARSn, and FARSn-117. For the test–retest analyses, we used all available data, as long as full exam results from two visits (screening and baseline) were available.
Statistical analysis/ Test–Retest-Reliability
Testing took place during screening and baseline visits of clinical trials, with the time between those visits generally not considered to lead to a relevant clinical decline of the condition. This was assessed by comparing means between the two visits using paired t-test and calculating the mean in-between change. In this specific situation, every patient was administered two exams by the same rater, allowing for assessment of the consistency between the ratings, but not between rater-variability. We, therefore, used the one-way analysis of variance ANOVA-based intraclass correlation coefficient (ICC2,1 by standard nomenclature 9, 10) to assess the test–retest reliability of the FARS scales. Also, we calculated coefficients of variance (CV), the standard error of the measurement (SEM), and the minimally detectable change at 90% confidence (MDC), reflecting the magnitude of change necessary in an individual to ensure that a change is not the result of random variation or measurement error. Group level MDC is usually of less interest due to sample size calculations in clinical trials.11 The MDC was then divided by the range of the individual measure to yield the relative amount of the random measurement error (MDC%). Bland-Altman plots were used to visualize the difference and mean score of each pair of measurements. All data derivation and analyses were conducted in R (www.r-project.org) utilizing the psych-package12 for calculation of the ICCs.
Results
mFARS and FARSn test–retest data were available from 172 patients from the IONIA and MOXIE studies (Table 1). Only in these studies did the neurological exams include the two stance items performed with eyes closed,5 necessary to calculate mFARS and FARSn. For the remaining studies (EPI-743, LA-29, and MICONOS), only FARSn-117 data were available. In addition, the EPI-743 study only included one predose visit. Therefore, (excluding EPI-743) data from a total of 405 patients could be evaluated for the test–retest analysis of FARSn-117. Visit timing indicates values foreseen in study protocols as exact dates were only on hand for the LA-29 study (median time between visits was 42 d, range 26 d to 97d, Table 1).
| Study | Scales Evaluated | Time from Screening to Baseline | N 1 | Comments |
|---|---|---|---|---|
| MOXIE |
mFARS FARSn FARSn-117 |
up to 1 month | 103 | |
| IONIA |
mFARS FARSn FARSn-117 |
within 8 weeks | 69 | |
| EPI-743 | FARSn-117 | – | 20 | Study included only 1 predose visit. |
| MICONOS | FARSn-117 | within 8 weeks | 161 | |
| LA-29 | FARSn-117 | less than 3 months | 72 |
- 1 Participants with full scale data from two visits available.
ICC values above 0.90 usually indicate excellent test–retest reliability;13 the present results overall demonstrate this for the mFARS scale with an ICC of 0.95 (95%CI 0.94–0.96). Mean values for both visits were 42.3 (SD 10.8) and 42.3 (SD 10.7), and the mean change was −0.1 (SD 3.3), showing that no relevant change in the scores has occurred between the visits. ICC and other corresponding values for FARSn and the FARSn-117 confirm these results (Table 2). All P-values from paired t-tests were nonsignificant (i.e. larger than 0.05). The minimally detectable change for mFARS was 5.51 points, which corresponds to a percentage MDC of 6%, likewise an excellent value.11, 13
| Parameter | max. score | n |
Mean (SD) D1 |
Mean (SD) D2 |
d | P* | ICC | 95%CI | CV | SEM | MDC |
MDC (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mFARS | 93 | 172 | 42.3 (10.8) | 42.3 (10.7) | −0.1 (3.3) | 0.956 | 0.95 | (0.94–0.96) | 5.57 | 2.36 | 5.51 | 6 |
| FARSn | 125 | 172 | 52.2 (12.2) | 52.2 (12) | 0 (4.3) | 0.995 | 0.94 | (0.92–0.95) | 5.75 | 3.02 | 7.05 | 6 |
| FARSn-117 | 117 | 405 | 48.1 (18.1) | 48.1 (17.8) | 0 (4.8) | 0.969 | 0.96 | (0.96–0.97) | 6.99 | 3.39 | 7.91 | 7 |
| Subscores | ||||||||||||
| Bulbar | 5 | 172 | 0.7 (0.6) | 0.8 (0.6) | 0.1 (0.5) | 0.251 | 0.73 | (0.65–0.79) | 42.89 | 0.33 | 0.77 | 15 |
| Upper Limbs | 36 | 172 | 12.6 (4.7) | 12.5 (4.7) | −0.1 (2.2) | 0.806 | 0.89 | (0.85–0.92) | 12.57 | 1.58 | 3.68 | 10 |
| Lower Limbs | 16 | 172 | 7.1 (2.6) | 7.2 (2.6) | 0.1 (1.5) | 0.790 | 0.83 | (0.78–0.87) | 15.07 | 1.07 | 2.49 | 16 |
| Upright Stability | 36 | 172 | 21.9 (6.3) | 21.8 (6.4) | −0.1 (2) | 0.892 | 0.95 | (0.93–0.96) | 6.48 | 1.40 | 3.26 | 9 |
| Items (mFARS only) | ||||||||||||
| A4 cough | 2 | 172 | 0.1 (0.3) | 0.1 (0.3) | 0 (0.2) | 0.705 | 0.75 | (0.68–0.81) | 129.30 | 0.14 | 0.33 | 16 |
| A4 speech | 3 | 172 | 0.6 (0.5) | 0.7 (0.5) | 0.1 (0.4) | 0.226 | 0.65 | (0.56–0.73) | 45.71 | 0.29 | 0.68 | 23 |
| B1 finger to finger | 6 | 172 | 1.5 (1) | 1.6 (1.1) | 0.1 (0.8) | 0.439 | 0.72 | (0.63–0.78) | 36.97 | 0.56 | 1.30 | 22 |
| B2 nose to finger | 8 | 172 | 2.3 (1) | 2.2 (1.1) | 0 (0.8) | 0.782 | 0.70 | (0.61–0.77) | 26.17 | 0.57 | 1.32 | 17 |
| B3 dysmetria | 8 | 172 | 2.8 (1.3) | 2.7 (1.3) | −0.1 (1) | 0.447 | 0.68 | (0.59–0.75) | 26.81 | 0.76 | 1.78 | 22 |
| B4 rapid movements | 6 | 172 | 2.9 (1.3) | 3 (1.3) | 0 (0.9) | 0.837 | 0.76 | (0.68–0.81) | 21.99 | 0.66 | 1.55 | 26 |
| B5 finger taps | 8 | 172 | 3.1 (1.5) | 3 (1.5) | −0.1 (1.1) | 0.519 | 0.72 | (0.64–0.79) | 25.92 | 0.80 | 1.88 | 23 |
| C1 heel shin slide | 8 | 172 | 4.2 (1.4) | 4.1 (1.4) | −0.1 (1.1) | 0.677 | 0.72 | (0.64–0.79) | 18.19 | 0.76 | 1.78 | 22 |
| C2 heel shin tap | 8 | 172 | 2.9 (1.6) | 3.1 (1.6) | 0.1 (1) | 0.419 | 0.80 | (0.73–0.84) | 24.09 | 0.73 | 1.71 | 21 |
| E1 sitting position | 4 | 172 | 0.7 (0.5) | 0.8 (0.5) | 0.1 (0.4) | 0.362 | 0.64 | (0.55–0.72) | 41.88 | 0.32 | 0.75 | 19 |
| E2A stance feet apart | 4 | 172 | 0.6 (1.4) | 0.6 (1.3) | 0 (0.6) | 0.989 | 0.90 | (0.87–0.92) | 71.62 | 0.43 | 1.00 | 25 |
| E2B (eyes closed) | 4 | 172 | 2.8 (1.7) | 2.8 (1.7) | 0 (0.7) | 0.924 | 0.91 | (0.88–0.93) | 18.61 | 0.52 | 1.22 | 30 |
| E3A stance feet together | 4 | 172 | 1.9 (1.8) | 1.9 (1.8) | 0.1 (0.9) | 0.797 | 0.87 | (0.83–0.9) | 34.55 | 0.65 | 1.52 | 38 |
| E3B (eyes closed) | 4 | 172 | 3.9 (0.4) | 3.9 (0.4) | 0 (0.2) | 0.734 | 0.89 | (0.85–0.92) | 3.61 | 0.13 | 0.31 | 8 |
| E4 tandem stance | 4 | 172 | 3.6 (0.9) | 3.4 (1.3) | −0.2 (1) | 0.104 | 0.60 | (0.49–0.69) | 20.15 | 0.60 | 1.39 | 35 |
| E5 stance dominant foot | 4 | 172 | 3.9 (0.4) | 3.9 (0.4) | 0 (0.3) | 0.772 | 0.82 | (0.77–0.87) | 4.65 | 0.18 | 0.41 | 10 |
| E6 tandem walk | 3 | 172 | 2.4 (0.7) | 2.4 (0.7) | 0 (0.5) | 0.708 | 0.75 | (0.67–0.81) | 15.21 | 0.37 | 0.87 | 29 |
| E7 gait | 5 | 172 | 2 (1.2) | 2 (1.2) | 0 (0.5) | 0.895 | 0.92 | (0.9–0.94) | 16.61 | 0.34 | 0.80 | 16 |
- * p-value for paired t-test comparing the means between both visits
The mFARS subscores and, in particular, upright stability showed similar preferable results with ICCs of 0.95 (upright stability), 0.89 (upper limbs), 0.83 (lower limbs), and 0.73 (bulbar). Among the individual items in the scale, this ensures that the essential stance items (E2A, E2B, and E3A), as well as the gait item, have excellent to good ICCs.
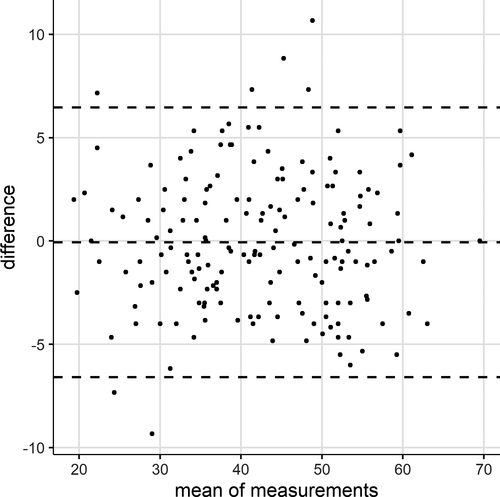
A Bland-Altman plot for the assessments of mFARS (Fig. 1) shows no clear trends for less reliability at any mean scores. Outliers occurred both at low and at average overall scores.
Discussion
The present study shows that the mFARS and FARS exams have excellent test–retest properties as need for use in therapeutic trials and other clinical studies. The FARS was first introduced in 20053 as a disease-specific clinical rating instrument to capture functional abilities related to neurological aspects of FRDA. Further optimization and focus on functional, patient relevant items, together with psychometric analyses of the FARSn5 has further refined this instrument to the “mFARS” score, which has been used both as primary and secondary endpoint in contemporary clinical trials of FRDA. Compared to the FARSn, the mFARS appears less prone particularly to floor effects, and shows a better dimensional structure, while retaining an adequate level of internal consistency.5 While mild ceiling effects are retained, particularly the gait subscore of the mFARS was shown to captures well the progressive function loss associated with loss of ambulation in FRDA.14 The results in the present work complement these features.
Validity of the FARS scales has been assessed in many observational studies, demonstrating its high correlation with age of onset, genetic burden of disease (GAA repeat length), and patient reported outcome measures such as the ADL scale.4, 15, 16 It also correlates with other rating scales such as the Scale for the Assessment and Rating of Ataxia (SARA) score, a modified version of Barthel Index, and the Functional Independence measure.17, 18 The mFARS/FARS associates not only with walking speed, cadence, and stability indexes,19 but also with physio-pathological aspects of the disease, including iron clustering at the cerebellum,20 atrophy in cerebellar peduncles,21, 22 degeneration of the spinal cord,23 and FXN expression.24 The FARSn and mFARS scales have shown adequate internal consistency, and inter-rater reliability.3, 5
Test–retest reliability however requires short-term follow-up data, which requires nontrivial efforts to obtain. In this study, we filled this gap using data from recent clinical studies and successfully proved the test–retest reliability of the overall scale. This study demonstrated excellent test–retest reliability of the mFARS, the FARSn, and the FARSn-117 scores as well as the mFARS upright stability score. In isolation, upper - and lowerlimb subscores still showed good ICCs, while the bulbar subsection had a moderate ICC. A potential limitation of the current study is the lack of intraday retesting, although such testing could conceivably be associated with practice effects. In addition, the fatigability of FRDA patients could confound same day testing. Also, in specific studies (e.g. LA-29) the between-test interval was fairly long, but the impact is considered low, given the (relatively) slow progressive nature of the disease. Due to the inherent variability of all FRDA patients, future clinical trials will likely focus on targeted subpopulations, and further work hopefully will provide evidence that the excellent overall qualities of the mFARS scale apply also to these dedicated patient subgroups.
Acknowledgments
This work was funded by the Friedreich’s Ataxia Research Alliance (www.curefa.org). We acknowledge Reata Pharmaceuticals and Chiesi Canada for providing additional predose data from clinical studies.
Author Contributions
TAZ, CR, and DRL contributed to conception and design of the study. TAZ, CR, DRL, SPL, JMF, and MP contributed to the acquisition and analysis of data and review of the manuscript. CR and TAZ drafted text, tables, and the figure.
Conflict of Interest
None declared.




