The A4 study: β-amyloid and cognition in 4432 cognitively unimpaired adults
Funding Information
Work at the authors’ laboratory was supported by the Wallenberg Center for Molecular Medicine, The Knut and Alice Wallenberg Foundation, The Medical Faculty at Lund University, Region Skåne, the European Research Council, the Swedish Research Council, the Marianne and Marcus Wallenberg foundation, the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation, the Swedish Brain Foundation, the Parkinson foundation of Sweden, The Parkinson Research Foundation, the Swedish Medical Association, the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, the Skåne University Hospital Foundation, and the Swedish Federal Government under the ALF agreement. The A4 study is a secondary prevention trial in preclinical Alzheimer’s disease, aiming to slow cognitive decline associated with brain amyloid accumulation in clinically normal older individuals. The A4 study is funded by a public-private-philanthropic partnership, including funding from the National Institutes of Health-National Institute on Aging (U19AG010483; R01AG063689), Eli Lilly and Company, Alzheimer’s Association, Accelerating Medicines Partnership, GHR Foundation, an anonymous foundation and additional private donors, with in-kind support from Avid, Cogstate, Albert Einstein College of Medicine, US Against Alzheimer’s disease, and Foundation for Neurologic Diseases. The companion observational Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) study is funded by the Alzheimer’s Association and GHR Foundation. The A4 and LEARN Studies are led by Reisa Sperling at Brigham and Women’s Hospital, Harvard Medical School and Paul Aisen at the Alzheimer’s Therapeutic Research Institute (ATRI), University of Southern California. The A4 and LEARN Studies are coordinated by ATRI at the University of Southern California, and the data are made available through the Laboratory for Neuro Imaging at the University of Southern California. The participants screening for the A4 study provided permission to share their de-identified data in order to advance the quest to find a successful treatment for Alzheimer’s disease. We would like to acknowledge the dedication of all the participants, the site personnel, and all of the partnership team members who continue to make the A4 and LEARN Studies possible. The complete A4 study team list is available on: a4study.org/a4-study-team.
Abstract
Objective
To clarify the preclinical stage of Alzheimer’s disease by estimating when β-amyloid accumulation first becomes associated with changes in cognition.
Methods
Here we studied a large group (N = 4432) of cognitively unimpaired individuals who were screened for inclusion in the A4 trial (age 65–85) to assess the effect of subthreshold levels of β-amyloid on cognition and to identify which cognitive domains first become affected.
Results
β-amyloid accumulation was linked to significant cognitive dysfunction in cognitively unimpaired participants with subthreshold levels of β-amyloid in multiple measures of memory (Logical Memory Delayed Recall, P = 0.03; Free and Cued Selective Reminding Test, P < 0.001), the Preclinical Alzheimer’s Cognitive Composite (P = 0.01), and was marginally associated with decreased executive function (Digit Symbol Substitution, P = 0.07). Significantly, decreased cognitive scores were associated with suprathreshold levels of β-amyloid, across all measures (P < 0.05). The Free and Cued Selective Reminding Test, a list recall memory test, appeared most sensitive to β-amyloid -related decreases in average cognitive scores, outperforming all other cognitive domains, including the narrative recall memory test, Logical Memory.
Interpretation
Clinical trials for cognitively unimpaired β-amyloid-positive individuals will include a large number of individuals where mechanisms downstream from β-amyloid pathology are already activated. These findings have implications for primary and secondary prevention of Alzheimer’s disease.
Introduction
The preclinical stage of Alzheimer’s disease (AD) is thought to start with the accumulation of β-amyloid (Aβ) pathology and end with the onset of clinical symptoms. The failure of trials directed against symptomatic AD over the last few years has led to an increased interest among researchers and drug developers to study preclinical AD.1, 2 The hope is that early interventions, initiated before widespread neuronal damage, may be more likely to modify the disease course. To guide these interventions, a detailed understanding of the earliest phases of AD is needed.
In previous studies, preclinical AD has often been defined by abnormal levels of Aβ biomarkers (e.g., using positron emission tomography, PET), typically using thresholds associated with quite advanced Aβ pathology.3 We suggest that such overt changes are preceded by subthreshold changes that occur within the normal range of the biomarkers, but which may still have meaningful biological and clinical effects.4-7 A focus on biomarker positivity at conservative thresholds may therefore result in misleadingly late estimates for when preclinical AD starts. In this study, we tested how the principal risk factors for AD, including age and continuous levels of 18F-florbetapir (a tracer sensitive to fibrillar Aβ) PET, were related to very early changes in cognition, within the normal range.
To further optimize early interventions against AD, we also need to understand the transition from purely asymptomatic preclinical AD into the clinical stage of the disease. Thresholds for cognition that define mild cognitive impairment (MCI) or dementia are too conservative to capture the first subtle changes in cognition. A traditionally identified preclinical AD population (Aβ-positive cognitively unimpaired) may therefore include a spectrum of participants ranging from no cognitive engagement at all to substantial cognitive dysfunction, albeit less than thresholds for MCI.8 The latter “preclinical” AD individuals may already be in a too advanced disease stage to optimally respond to anti-Aβ treatments (if downstream mechanisms have already been activated, e.g., tau propagation). We therefore tested how 18F-florbetapir levels were related to subtle cognitive dysfunction in multiple domains in 4432 participants who were screened for participation in the A4 trial.9 Specifically, we aimed to evaluate whether (1) there were significant reductions in cognitive scores prior to the threshold for Aβ-positivity and (2) whether there was a rank order of the decline of specific cognitive domains with respect to continuous levels of 18F-florbetapir. We hypothesized that Aβ would have subtle effects on cognition, indicating the start of cognitive decline in AD even in a population of participants who were classified as cognitively unimpaired, without MCI or dementia, with subthreshold levels of Aβ.
Materials and methods
Participants
Participants who were screened for inclusion in the A4 study9, 10 (https://clinicaltrials.gov/ct2/show/NCT02008357) were included in this study if they completed an 18F-florbetapir PET scan, had APOE genotype information, completed a battery of neuropsychological testing, scored between 25 and 30 on the Mini-Mental State Examination (MMSE), had a Clinical Dementia Rating of 0, and were between the ages of 65 and 85. Participants were excluded from the A4 study if they were (1) taking a prescription Alzheimer’s medication (acetylcholinesterase inhibitor and/or memantine); (2) had a current serious or unstable illness, including cardiovascular, hepatic, renal, gastroenterologic, respiratory, endocrinologic, neurologic, psychiatric, immunologic, or hematologic disease or other conditions that could interfere with the study; (3) had a history within the last 5 years of a serious infectious disease affecting the brain (including neurosyphilis, meningitis, or encephalitis) or head trauma resulting in protracted loss of consciousness; (4) had a history within the last 5 years of a primary or recurrent malignant disease, with the exception of resected cutaneous squamous cell carcinoma in situ, basal cell carcinoma, cervical carcinoma in situ, or in situ prostate cancer with normal prostate-specific antigen posttreatment; (5) had a known history of human immunodeficiency virus (HIV), clinically significant multiple or severe drug allergies, or severe posttreatment hypersensitivity reactions, including but not limited to erythema multiforme major, linear immunoglobulin A dermatosis, toxic epidermal necrolysis, or exfoliative dermatitis; (6) were at serious risk for suicide or had a history within the past 2 years of major depression or bipolar disorder; (7) had a history within the past 5 years of chronic alcohol or drug abuse/dependence; or (8) were residing in a skilled nursing facility or nursing home. Note that participants who did not demonstrate evidence of amyloid in the brain at screening were excluded from the randomized treatment in A4, but participants were included in the current study regardless of their PET scan result.
18F-florbetapir PET imaging
Amyloid PET imaging was done using 18F-florbetapir data, acquired 50–70 min postinjection. Images were realigned and averaged, and then spatially aligned to a standard space template. 18F-florbetapir, sampled in a global neocortical region for Aβ, was expressed as an standardized uptake value ratio (SUVR) with a cerebellar reference region.11 An Aβ-negative group was defined as participants with 18F-florbetapir PET SUVR < 1.10.12, 13 The quantitative threshold for Aβ+ in our analyses differs from the eligibility for randomization in the A4 study, which used an algorithm involving both SUVR thresholds and visual inspection.
Cognitive testing
Participants completed a neuropsychological test battery including the Preclinical Alzheimer's Cognitive Composite (PACC),14, 15 comprising the MMSE, a measure of global cognition, Logical Memory Delayed Recall, a narrative recall memory test, Free and Cued Selective Reminding Test (FCSRT96), a list recall memory test, and the Digit Symbol Substitution Test, a measure of executive function. To calculate the PACC, the individual components were centered on their means and scaled to their standard deviations and summed, calculated using all participants. This sum was then centered on the mean and standard deviation of the sum, calculated using only the Aβ-negative group. We evaluated the FCSRT96 formulation of the FCSRT as well as the Free Recall portion of the FCRST because of evidence of their sensitivity to early Aβ-related cognitive changes.14, 16-18
Statistical analysis
The main outcomes in this study were the PACC and its individual components. The relationship between cognitive scores and 18F-florbetapir PET, as well as interactions between demographics and 18F-florbetapir PET, was evaluated. Cognitive scores were modeled using ordinary least squares regression. To evaluate potential nonlinearity in the relationships with cognition, continuous 18F-florbetapir PET levels were parameterized using monotone cubic splines.19 Cubic splines are functions of polynomials allowing flexibility in the estimation of trajectories. Spline models included two knots, with the number of knots selected by the Akaike Information Criterion (AIC).20 Statistical significance of the associations between the outcome and predictors was tested using likelihood ratio tests and AIC. A lower value of AIC indicates a better fitting model. Models were adjusted for age, sex, and years of education. Because the range of the MMSE in this cognitively unimpaired population is restricted (25–30), a sensitivity analysis was done assuming a binomial distribution with 30 Bernoulli trials.
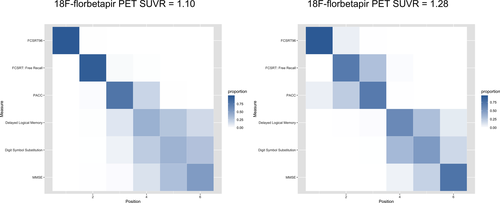
We also assessed the measures for their sensitivity to the earliest decreases in cognitive scores. We used a positional variance diagram to depict the ordering of the cognitive measures in terms of decreased score at 18F-florbetapir SUVR = 1.10 (the threshold for Aβ-positivity used for these analyses) and also at 18F-florbetapir SUVR = 1.28 (the median SUVR in Aβ+ participants). The cognitive measures were centered on their estimated means at the lowest 18F-florbetapir SUVR value and scaled to the standard deviation of the Aβ- group. The positional variance diagram shows the proportion of 1000 bootstrap samples in which a particular cognitive measure appears in a particular position in the central ordering, ranging from 0 (white or no shading, in Fig. 3) to 1 (blue shading). Cognitive measures are ordered by their most frequently estimated position with uncertainty captured by the transparency of the shading.
Additionally, permutation tests were performed to estimate the statistical significance of the magnitude of the decrease in cognitive scores at 18F-florbetapir SUVR = 1.10 and separately at SUVR = 1.28, compared with the scores at the lowest level of 18F-florbetapir, SUVR = 0.78. Spline model-estimated decreases in cognitive scores from SUVR = 0.78 to SUVR = 1.10 (and separately, to SUVR = 1.28) based on permuted values of 18F-florbetapir in each bootstrap sample were used to obtain a null distribution of cognitive changes. A null distribution results because the 18F-florbetapir values are attributed at random via permutation. P-values were then calculated as the proportion of cognitive changes from the null distribution that were equal or greater than the observed changes estimated using the true 18F-florbetapir values.
Associations between demographics and 18F-florbetapir PET SUVR were assessed using Spearman correlation for continuous variables and a Kruskal-Wallis test for categorical variables. Changes in AIC (ΔAIC) < −2 were considered a meaningful21 improvement with respect to the more parsimonious model and P-values < 0.05 were considered significant. All analyses were done in R v3.6.0 (www.r-project.org); splines were estimated using the splines2 package.
Standard protocol approvals, registrations, and patient consents
This study was approved by the Institutional Review Boards of all of the participating institutions. Informed written consent was obtained from all participants at each site.
Results
Cohort characteristics
A total of 4432 cognitively unimpaired adults were included in the study. They were 71.3 years old on average (interquartile range: 67.5–74.2), 59.4% female, had an average of 16.6 years of education (interquartile range: 15 to 18), and 34.1% were Aβ+. 18F-florbetapir SUVR was significantly correlated with increasing age (ρ = 0.08, P < 0.001) and APOE ε4 status (mean SUVR in ε4- = 1.05, mean SUVR in ε4+ = 1.18, P < 0.001), whereas only marginally associated with sex (mean SUVR in males = 1.09, mean SUVR in females = 1.10, P = 0.06). 18F-florbetapir SUVR was not associated with years of education (ρ = −0.009, P = 0.56).
Cognition
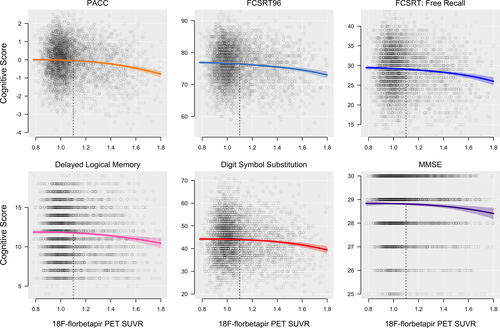
The PACC and each individual cognitive component were significantly associated with 18F-florbetapir SUVR (P < 0.01, ΔAIC < −8). Each cognitive domain trajectory is shown in Figures 1 and 2. In Table 1, mean cognitive scores at the threshold for Aβ+ (18F-florbetapir SUVR = 1.10) and at the median of the Aβ+ participants (18F-florbetapir SUVR = 1.28) are summarized, as well as the decreases in cognitive scores compared to the values at the floor of 18F-florbetapir PET (18F-florbetapir SUVR = 0.78). The results from the sensitivity analysis for MMSE assuming a binomial distribution were the same as the main analysis, with mean estimates and 95% confidence intervals almost perfectly overlapping.
| Mean (SUVR = 0.78) | SD | Mean (SUVR = 1.10) | Δ 1.10 * | P | Effect size (Δ1.10/SD) | Mean (SUVR = 1.28) | Δ 1.28 | P | Effect size (Δ1.28/SD) | |
|---|---|---|---|---|---|---|---|---|---|---|
| PACC | 0.008 | 1.00 | −0.04 | −0.05 | 0.01 | −0.05 | −0.13 | −0.14 | 0.001 | −0.14 |
| FCSRT96 | 76.93 | 5.81 | 76.44 | −0.49 | <0.001 | −0.09 | 75.94 | −0.99 | <0.001 | −0.17 |
| FCSRT (Free Recall) | 29.47 | 5.50 | 29.06 | −0.41 | <0.001 | −0.08 | 28.63 | −0.84 | <0.001 | −0.15 |
| Delayed logical memory | 11.86 | 3.17 | 11.76 | −0.10 | 0.03 | −0.03 | 11.58 | −0.29 | 0.003 | −0.09 |
| Digit symbol substitution | 44.20 | 8.96 | 43.98 | −0.23 | 0.07 | −0.03 | 43.49 | −0.71 | 0.01 | −0.08 |
| MMSE | 28.83 | 1.20 | 28.81 | −0.02 | 0.11 | −0.02 | 28.77 | −0.07 | 0.05 | −0.06 |
- * Refers to the change in cognitive score from SUVR 0.78 to SUVR 1.10.
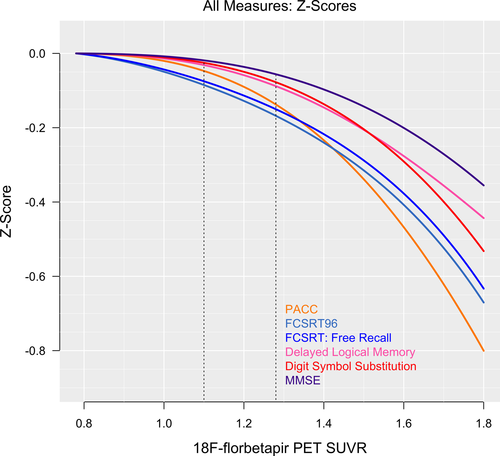
Delayed Logical Memory, FCSRT96, FCSRT Free Recall, and the PACC all showed significant subthreshold decreases from SUVR 0.78 to SUVR 1.10 (P < 0.03, Table 1), and Digit Symbol Substitution showed a marginally significant decrease (P = 0.07). All cognitive measures decreased significantly from SUVR 0.78 to SUVR 1.28 (P < 0.05).
Positional variance diagrams, depicting the order of all cognitive measures in terms of decrease in scores with increasing SUVR, are shown in Figure 3. At the threshold for Aβ+, FCSRT96 consistently (99% of the time) showed the greatest decrease in cognitive score, followed by the FCSRT Free Recall and the PACC (also seen in Figs. 1 and 2). At the median 18F-florbetapir uptake in Aβ+ (SUVR 1.28), the order remained the same. MMSE was consistently in the fifth position.
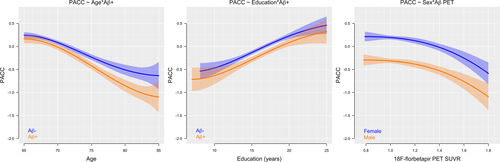
Aβ demographic interactions to predict the PACC
There was a significant interaction between 18F-florbetapir and age to predict decreasing PACC scores, where steeper decline with age was observed in the Aβ+ participants (P = 0.02, ΔAIC = −3.8). The association between 18F-florbetapir and decreasing PACC scores did not differ by years of education (P = 0.99, ΔAIC = 6.0) or sex (P = 0.95, ΔAIC = 5.6), although for a given level of 18F-florbetapir, females had higher scores compared with males. All interactions are shown in Figure 4.
Discussion
The main finding of this study was that subtle decline in cognition had already occurred as a function of subthreshold levels of Aβ in cognitively unimpaired individuals. Among included tests, average scores on the FCSRT96 decreased first, with respect to Aβ accumulation. This suggests that the effects of Aβ on cognition start early, in specific memory tests, with over half of individuals in a traditionally recruited “preclinical AD” population being at risk for subtle cognitive effects already at baseline. If the goal is to interfere very early in the disease process before downstream pathways are activated, primary or secondary AD prevention trials may therefore both (1) aim at younger people (below age 65–70 years) and (2) exclude individuals with high levels of Aβ accumulation.
The effect of Aβ on cognition in this cognitively unimpaired population is in line with previous large-scale studies. For example, in a recent meta-analysis of three large studies (not including A4), we found that Aβ-positive cognitively unimpaired individuals had significantly lower scores on several cognitive tests compared to Aβ-negative individuals already at baseline.22 This is no surprise, both because there are no clear boundaries between the clinical stages of AD,23 and because one may expect performance on cognitive tests to change gradually within the normal range due to AD before reaching the threshold for an MCI diagnosis, indicating cognitive impairment. Although cognitive changes in the subthreshold range of Aβ are quite small, and not clinically meaningful in terms of effect size, they mark the initial descent of cognitive decline. This large sample enables the observation of the gradual and continuous decrease in cognitive scores with increasing Aβ, leading to the more substantial decrease in cognitive scores at the median level of the Aβ-positive group and beyond. It is also possible that these initial signs of cognitive dysfunction are due primarily to synaptic dysfunction caused by Aβ toxicity, prior to overt neuronal degeneration.24 However, if the decline in cognition is driven not by Aβ accumulation, but rather by changes in tau and neurodegeneration,25 the earliest changes in cognition may indicate the point in the disease when mechanisms downstream to Aβ have become activated. This may also be a point when anti-Aβ treatments are less likely to successfully modify the disease course. This may pose a significant problem for ongoing and planned anti-Aβ trials focused on preclinical AD, since they may include significant numbers of participants with too advanced disease to respond to this class of treatments.
The FCSRT96, included in the PACC for its sensitivity to early memory changes,14, 16-18 appeared more sensitive to Aβ-associated cognitive decline compared with the other components of the PACC. Notably, the FCSRT96, a list recall memory test, outperformed the narrative recall memory test, Delayed Logical Memory (Figs. 1-3). The effect size of the FCSRT96 of the reduction in average cognitive score between the lowest 18F-florbetapir SUVR levels and the 1.10 threshold for Aβ-positivity was −0.09 (Table 1), more than double the effect size of any of the other three components of the PACC, including Delayed Logical Memory. Because the PACC is an average of the four cognitive tests, the early decline in PACC scores, with respect to 18F-florbetapir SUVR, appeared largely driven by the early changes in the FCSRT96. It was not until the trajectories of the remaining three cognitive tests showed an accelerated descent with respect to 18F-florbetapir SUVR that the PACC converged and eventually overtook the FCSRT96 near Aβ SUVR = 1.4 (Fig. 2).
There was an age-dependent effect of Aβ on PACC scores, with increased separation between Aβ- and Aβ+ groups with older age. This is likely due to older age representing a longer time spent with a significant Aβ burden, or due to the presence of comorbidities in older age which may lower the threshold for when Aβ pathology leads to cognitive decline. All participants in this study have remained cognitively unimpaired to at least 65 years of age, suggesting that none of them are on an early AD path where Aβ-positivity at a younger age might be expected to be associated with more aggressive disease and more rapid progression.26 In contrast, Aβ-related reduction of PACC scores did not differ by sex. This is consistent with some, but not all, reports of sex-related associations with Aβ and cognition.22, 27 While females generally outperform males in most cognitive domains,28 in the current setting, the decrease in PACC scores with increasing levels of Aβ occurred in parallel with males and females. In a longitudinal setting where participants progress beyond the preclinical stage, tau accumulation, neurodegeneration, and rates of cognitive decline may become sex-dependent, resulting in the sex differences observed in other cohorts.27, 29
This study has several limitations. Inclusion criteria restricted participation to those over 65 years of age, and a restricted performance range on cognitive testing, limiting analyses to the effect of emerging Aβ pathology within late-onset preclinical AD. Although the study sample size was large, without tau PET or measures of cerebrovascular disease, we were limited to only part of the picture of cognitive changes in preclinical AD. Importantly, without longitudinal information, these results do not apply to individual trajectories of amyloid deposition or changes in cognition. Longitudinal follow-up of middle-aged individuals, with low and intermediate levels of amyloid, will be required to further clarify the earliest cognitive changes associated with Aβ accumulation. Finally, participation in the A4 study may represent a unique cohort that may reduce the generalizability of the results outside of clinical trials.
With a large sample size, subtle estimates of cognitive dysfunction can be estimated with high precision, as is required to detect the small cognitive changes associated with subthreshold increases of Aβ pathology. These findings suggest that subthreshold changes that occur within the normal range of Aβ have meaningful biological and clinical implications for defining preclinical AD. An alternative threshold for Aβ-positivity, prior to the onset of subtle cognitive dysfunction, may be required to identify individuals in the earliest stages of the disease, for whom early treatment may be the most beneficial.
Acknowledgments
Work at the authors’ laboratory was supported by the Wallenberg Center for Molecular Medicine, The Knut and Alice Wallenberg Foundation, The Medical Faculty at Lund University, Region Skåne, the European Research Council, the Swedish Research Council, the Marianne and Marcus Wallenberg foundation, the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation, the Swedish Brain Foundation, the Parkinson foundation of Sweden, The Parkinson Research Foundation, the Swedish Medical Association, the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, the Skåne University Hospital Foundation, and the Swedish Federal Government under the ALF agreement. The A4 study is a secondary prevention trial in preclinical Alzheimer’s disease, aiming to slow cognitive decline associated with brain amyloid accumulation in clinically normal older individuals. The A4 study is funded by a public-private-philanthropic partnership, including funding from the National Institutes of Health-National Institute on Aging (U19AG010483; R01AG063689), Eli Lilly and Company, Alzheimer’s Association, Accelerating Medicines Partnership, GHR Foundation, an anonymous foundation and additional private donors, with in-kind support from Avid, Cogstate, Albert Einstein College of Medicine, US Against Alzheimer’s disease, and Foundation for Neurologic Diseases. The companion observational Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) study is funded by the Alzheimer’s Association and GHR Foundation. The A4 and LEARN Studies are led by Reisa Sperling at Brigham and Women’s Hospital, Harvard Medical School and Paul Aisen at the Alzheimer’s Therapeutic Research Institute (ATRI), University of Southern California. The A4 and LEARN Studies are coordinated by ATRI at the University of Southern California, and the data are made available through the Laboratory for Neuro Imaging at the University of Southern California. The participants screening for the A4 study provided permission to share their de-identified data in order to advance the quest to find a successful treatment for Alzheimer’s disease. We would like to acknowledge the dedication of all the participants, the site personnel, and all of the partnership team members who continue to make the A4 and LEARN Studies possible. The complete A4 study team list is available on: a4study.org/a4-study-team.
Author Contributions
PSI contributed to the conception and design of the study, analysis of the data, and drafting of the manuscript and the figures. NM contributed to the conception and design of the study and drafting of the manuscript. RS and MCD contributed to the acquisition of the data and drafting of the manuscript. OH contributed to the drafting of the manuscript.
Conflict of Interest
Mr Insel, Mattsson, and Hansson report no conflict of interest. Donohue reports grants from NIH, grants from Eli Lilly and Co, grants from Alzheimer's Association, grants from Accelerating Medicines Partnership, from GHR Foundation, nonfinancial support from Avid, nonfinancial support from Cogstate, during the conduct of the study; personal fees from Biogen, grants and other from Janssen, personal fees from Neurotrack, personal fees from Roche, outside the submitted work. Sperling reports personal fees from AC Immune, personal fees from Biogen, personal fees from Janssen, personal fees from Neurocentria, grants from Eli Lilly, grants from Janssen, grants from NIA, grants from Alzheimer’s Association, personal fees and other from Novartis, personal fees and other from AC Immune, outside the submitted work.




