Impact of simultaneous subthalamic and nigral stimulation on dysphagia in Parkinson’s disease
Funding Information
This study was supported by SFB 936, C8 to M. Pötter-Nerger.
Abstract
Objectives
Dysphagia is a frequent and highly relevant symptom in Parkinson's disease (PD) due to high associated morbidity and mortality. To compare the effect of simultaneous stimulation of the subthalamic nucleus (STN) and substantia nigra (SNr) with conventional STN-stimulation on swallowing function in Parkinson's disease.
Methods
In this controlled, randomized, double-blind, cross-over clinical trial, 15 PD patients were assessed with DBS switched off (STIM OFF), STN-DBS, STN + SNr-DBS. Patients and 32 age-matched healthy controls were examined clinically and by flexible-endoscopic evaluation of swallowing (FEES) to evaluate the swallowing function. The primary endpoint was the assessment of residues, secondary endpoints were penetration/aspiration, leakage, retained pharyngeal secretions, drooling, and assessments of the patient’s self-perception of swallowing on a visual analog scale.
Results
Compared with healthy controls PD patients showed significantly more pharyngeal residues in STIM OFF and both DBS modes. Residues or aspiration events were found in 80% of the patients under STN-stimulation. Simultaneous STN + SNr-stimulation had no additional positive effect on objective dysphagia and self-reported swallowing function compared to STN-DBS.
Interpretation
Simultaneous STN + SNr-stimulation seems to have no additional beneficial effects on dysphagia when compared with conventional STN-stimulation, but did not deteriorate the swallowing function. If STN + SNr-stimulation is planned to be applied for the improvement of axial symptoms and gait disorders in PD patients, it can be considered safe in terms of dysphagia.
Introduction
Dysphagia is a well-known problem in Parkinson´s disease (PD) with a prevalence of 11 to 95% in PD patients.1, 2 The most common endoscopic pathologies are pharyngeal residues of firm consistencies followed by the aspiration of water.2, 3 Self-perception of swallowing is poor in many PD patients and even severe dysphagia is often not realized.4, 5 Dysphagia in PD has a negative impact on the quality of life and also on morbidity and mortality.6, 7 Aspiration pneumonia as a complication of dysphagia is one of the leading causes of death in PD patients.8, 9 The whole physiological act of swallowing including oral preparation and transport phase, pharyngeal and oesophageal phase, can be impaired.10 In particular, the oropharyngeal deglutition abnormalities are of clinical relevance since life-threatening aspiration events can arise in this phase. The pathological swallowing characteristics like aspiration (bolus enters trachea), penetration (bolus enters larynx), leakage (predeglutitive involuntary escape of the bolus into the deeper pharynx or larynx), and residues (remaining boluses after swallowing) may occur at different times of the swallowing act. In this regard, the swallowing reflex is essential. For example, leakage occurs prior to the swallowing reflex due to a malfunction in the oral phase, while aspiration from residues arises after the swallowing reflex.
The underlying pathophysiology of neuronal subcortical and cortical circuits of PD related to dysphagia has been recently assessed.11 The medullary swallowing central pattern generator with the dorsal motor nucleus of the glossopharyngeal nerve, vagus nerve, surrounding reticular activating system, and solitary tract in the brainstem seems to be of particular relevance.12 In PD, modified basal ganglia output of the globus pallidus internum (GPI) and substantia nigra pars reticulata (SNr) might alter the activity of the medullary swallowing central pattern generator via the interposed relay nucleus and the pedunculopontine nucleus12 or nuclei in the superior colliculus in the tectum.13 In animal models, gabaergic inhibitory nigro-tectal projections are proposed to be involved in the coordination of tongue propulsion and retraction during consummatory orofacial behavior.13
Therapeutically, there exists no suitable drug treatment for dysphagia in PD and the response to L-Dopa is very low.4, 14 Deep brain stimulation of the subthalamic nucleus (STN-DBS), which is otherwise quite effective in improving motor and nonmotor symptoms in PD,15, 16 was found to have inconsistent effects on swallowing.17, 18 Hypothetically, intensifying the modulation of basal ganglia-brainstem projections by increased disinhibition of brainstem centers such as the medullary swallowing central pattern generator by another mode of DBS could lead to improved deglutition in PD.
Recently, simultaneous stimulation of the subthalamic nucleus and substantia nigra (STN + SNr-stimulation) has been introduced in PD patients with the primary goal to improve the freezing of gait.19 The nigral co-stimulation is of particular interest due to dense interconnections with the pedunculopontine nucleus, the colliculus superior and consecutively, the downstream central swallow pattern generator.20-23 DBS within the SNr has been hypothesized to induce the enhancement of inhibitory synaptic plasticity and frequency-dependent cell firing suppression.24 Therefore, hypothetically combined STN + SNr-stimulation might be favorable for improving dysphagia in PD by increased disinhibition of basal-ganglia-brainstem projections.
The aim of the current study was to assess the impact of combined STN + SNr on swallowing function in PD and to compare the effect with conventional STN-DBS.
Methods
Study design
This single-center, controlled, randomized, double-blind, cross-over clinical trial was conducted at the University Medical Center Hamburg-Eppendorf and compares the effect of STN-DBS and combined STN + SNr-DBS as described previously.25 The study was approved by the local ethics committee and was conducted in agreement with the Code of Ethics of the World Medical Association (Declaration of Helsinki, 1967). Written informed consent was obtained from all participants. Patients were seen by an unblinded therapeutic movement disorder specialist, who performed the programing of the stimulation condition and by a blinded rater at three visits with a time interval of 3 weeks in between (baseline recording with DBS switched off (STIM-OFF), phase I and phase II, see Figure 1). Patients were blinded for their stimulation mode (conventional STN stimulation or combined STN + SNR stimulation). All visits were performed in medication-on condition. Examinations and assessments were performed by blinded investigators at the end of phase I and II. After testing thresholds for side effects in the SNr, defined stimulation settings for the STN and STN + SNr stimulation were fixed for the course of the experiment. The STN settings were not different from those before the study. Afterward, stimulation of the STN or combined STN + SNr was set by a nonblinded investigator in a randomized manner for the following 3 weeks; that is, 7 of 15 PD patients received first a conventional STN stimulation (the control stimulation for placebo effects) and after 3 weeks the combined STN + SNr stimulation, while the other eight patients received first the combined STN + SNr stimulation followed by conventional STN stimulation (see Figure 1). After completion of phase I, the second visit was performed with the reprograming of stimulation parameters in a cross-over manner for the following 3 weeks (starting phase II). The third visit was performed after 6 weeks when phase II was completed. In the end, patients were unblinded to their stimulation mode and the preferred stimulation mode was programed as permanent therapeutic stimulation. Medication and stimulation parameters were held constant during the phase I and II of the study. Only in one case, the stimulation amplitude in the SNr had to be reduced after 2 days due to dyskinesias.
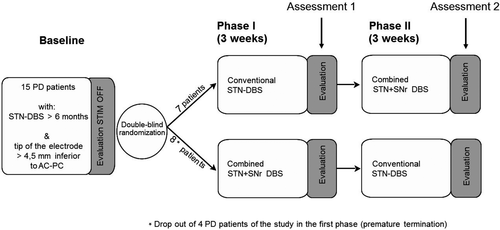
Subjects
Participants’ demographic and clinical characteristics are summarized in Table 1 and Table 2. About 15 patients suffering from idiopathic PD participated in the study. The 32 healthy controls were at least 50 years of age (half of them> 75 years) and without the history of swallowing problems (negative six item dysphagia-screening questionnaire) or any known disease of the central nervous system. All patients had a bilateral STN-DBS with the deepest contacts of the electrodes within the dorsal aspects of the SNr along image-based electrode reconstruction (>4.5 mm below AC-PC). The study in- and exclusion criteria, as well as the selection criteria for DBS surgery, have been reported in detail previously.25 The location of the electrode position was controlled by stereotactic coordinates based on MR imaging and intraoperative microrecording. The sensorimotor STN was identified by cell responses to passive and active movements and a high prevalence of oscillating unit activities in the range between 10 and 30 Hz. Differentiation of STN from SNr was based on the decrease of background noise and the replacement of irregular STN unit activity by tonic regular high-frequency spiking of SNr neurons marked the ventral exit of the STN and dorsal aspect of the SNr, respectively. The stereotactic coordinates of the ventral most DBS contact relative to the mid-commissural point (MCP; mean ± standard deviation in mm) were x = 10.4 ± 0.9, y = 2.8 ± 1.3, z = 6.3 ± 1.0 for the left hemisphere and x = 10.1 ± 1.7, y = 2.7 ± 1.5, z = 5.7 ± 1.4 for the right side (x = lateral to midline, y = posterior to MCP, z = inferior to AC-PC level).
|
Case Gender Age |
Age at onset | Disease duration [years] | Time with DBS [months] | LEDD [mg] |
DBS System |
STN-DBS parameters | Combined STN + SNr-DBS parameters | X, Y, Z coordinates |
|---|---|---|---|---|---|---|---|---|
|
Left electrode (1. row) Right electrode (2. row) |
Left electrode (1. row) Right electrode (2. row) |
Left electrode (1. row) Right electrode (2. row) |
||||||
| 1 M 61 | 38 | 23 | 54 | 1150 | MD |
1- 2- G+, 3.5V, 60 µs, 125 Hz 9- 10- G+, 2.7 V, 60 µs, 125 Hz |
1- 2- G+, 3.5V, 60 µs, 125 Hz; 0- G+, 2.0 V, 60 µs, 125 Hz 9- 10- G+, 2.7 V, 60 µs, 125 Hz, 8- G+, 2.0 V, 60 µs, 125 Hz |
10.9, 2.2, 4.7 10.5, 3.8, 4.7 |
| 2 M 63 | 40 | 23 | 105 | 860 | MD |
1- G+, 1.9 V, 60 µs, 125 Hz; 2- G+, 2.9 V, 60 µs, 125 Hz 9- G+, 1.9V, 60 µs, 125 Hz; 10- G+, 3.3 V, 60 µs, 125 Hz |
2- G+, 2.9 V, 60 µs, 125 Hz; 1- 0- G+, 1.9 V (1.5 V), 60 µs, 125 Hz 10- G+, 3.3 V, 60 µs, 125 Hz; 8- 9- G+, 1.9 V (1.5 V) 60 µs, 125 Hz |
11.2 1.9, 5.6 8.3, 5.5, 4 |
| 3 M 56 | 47 | 9 | 36 | 880 | MD |
1 + 2- G + 2.2 V, 60 µs, 125 Hz 10- G+, 4.3 V, 60 µs, 125 Hz |
2-G+, 2.2V, 60 µs, 125 Hz; 0- G+, 1.0 V, 60 µs, 125 Hz 10- G+, 4.3V, 60 µs, 125 Hz, 8- G+, 1.0 V, 60 µs, 125 Hz |
9.5, 2.8, 6.4 11.2, 1.4, 7.2 |
| 4 M 67 | 51 | 16 | 60 | 600 | MD |
1- G+, 1.5 V, 60 µ, 125 Hz 9- 10- G+, 3.9 V, 60 µs, 125 Hz |
1- G+, 1.5 V, 60 µ, 125 Hz; 0- G+, 2.0 V, 60 µs, 125 Hz 9-10- G+, 3.9 V, 60 µs, 125 Hz; 8- G+, 2.0 V, 60 µs, 125 Hz |
9.6, 4.7, 6.6 11.7, 3.1, 3.2 |
| 5 M 65 | 56 | 9 | 9 | 300 | MD |
1- G+, 2.8 V, 60 µs, 125 Hz 9- G+, 3.0 V, 60 µs, 125 Hz |
1-G+, 2.8 V, 60 µs, 125 Hu; 0- G+, 1.5 V, 60 µs, 125 Hz 9-G+, 3.0 V, 60 µs, 125 Hz; 8- G+, 1.5 V, 60 µs |
10.9, 1.4, 7.7 11.1, 2.7, 6.7 |
| 6 M 74 | 65 | 9 | 9 | 360 | MD |
1- G+, 2.7 V, 130 Hz 9- G+, 2.6 V, 60 µs, 130 Hz |
1- G+, 2.7 V, 60 µs, 125 Hz; 0- G+, 1.5 V, 60 µs, 125 Hz 9- G+, 2.9 V, 60 µs, 125 Hz; 8- G+, 1.5 V, 60 µs, 125 Hz |
10.7, 2.6, 4.9 10.2, 2.5, 4.5 |
| 7 M 51 | 42 | 9 | 15 | 900 | BS |
2- 30%, 3- 70%, 3,4 mA, 60 µs, 125 Hz 10- 20%, 11- 80%, 4,0 mA, 60 µs, 125 Hz |
1- 23%, 2- 23%, 3- 54%, 4.4 mA, 60 µs, 125 Hz 9- 20%, 10- 16%, 11- 64%, 5.0 mA, 60 µs, 125 Hz |
8.81, 3.38, 7.37 7.04, 4.28, 6.41 |
| 8 M 57 | 50 | 7 | 18 | 580 | BS |
3- 70%, 4- 30%, 4,5 mA, 60 µsec, 130 Hz 12- 100% 3,8 mA, 60 µsec, 130 Hz |
3- 61%, 4- 26%, 1- 13%, 5,2 mA, 60 µsec, 130 Hz 12- 85%, 9- 15%, 4,5 mA, 60 µsec, 130 Hz |
11.85, 3.37, 6.09 11.63, 2.69, 5.9 |
| 9 M 71 | 60 | 11 | 13 | 950 | MD |
1- G+, 3,5 V, 60 µsec, 125 Hz 9- G+, 2,7 V, 60 µsec, 125 Hz |
1- G+, 3,5 V, 60 µsec, 125 Hz, 0- G+, 1,0 V, 60 µsec, 125 Hz 9- G+, 2,7 V, 60 µsec, 125 Hz; 8- G+, 1,0 V, 60 µsec, 125 Hz |
11.34, 2.18, 6.24 12.23, 0.2, 5.22 |
| 10M 66 | 54 | 13 | 6 | 1000 | MD |
1- G+, 3.8 V, 60 µsec, 130 Hz 9- G+, 3.6 V, 60 µz 130 Hz |
1- 3.8 V 60 µsec 125 Hz 0- 1.0 V 60 µsec, 125 Hz 9- G + 3.6 V, 60 µsec, 125 Hz 8- G + 1,0 V 60 µsec, 125 Hz |
11.24, 6.46, 6.6 10.53, 4.23, 5.09 |
| 11 W 66 | 56 | 10 | 5 | 1245 | BS |
5-6-7- (Ring)G + 2.2 mA, 60 µsec, 130 Hz 13-14-15- (Ring) G+, 2.4 mA 60 µsec, 130 Hz |
5- (23%) 6- (23%) 7- (23%) 1- (31%) G + 2.9 mA, 60 µsec, 130 Hz 13-(24%) 14- (23%) 15-(23%) 9-(30%) G+, 3.1mA, 60 µsec, 130 Hz |
10.86, 2.51, 5.69 10.42, 0.44, 5.23 |
| Mn 63.4 | 50.8 | 12.6 | 30.0 | 802.3 | ||||
| SD 6.7 | 8.5 | 5.7 | 31.4 | 305.8 |
- "Disease duration [years]" is calculated from the date of the first diagnosis to the date of baseline measurement of the experiment. “DBS parameters” include: Active contacts, amplitude (volts or mA), pulse width (microseconds) and stimulation frequency (Hz), for the left and right electrode, respectively. Electrode coordinates are given as mm lateral to the midline (X), posterior to the mid-commissural point (Y) and inferior to the intercommissural plane (Z). Note that the deepest contacts were contact 0 and 8 (Medtronic) or contact 1 and 9 (Boston Scientific).
- LEDD, levodopa equivalent daily dose; ME, Medtronic. BS, Boston Scientific.
|
Patients (n = 11) N (%) |
Controls (n = 32) N (%) |
P values pat con |
|
|---|---|---|---|
| Age (y) | 63.4 ± 6.7 | 68.1 ± 10.7 | 0.179a |
| Men | 10 (91%) | 16 (50%) | 0.03b |
| History of pneumonia | 0 | 3 (9%) | <0.001b |
| MOCA at baseline | 25.7 ± 2.5 | 25.3 ± 3.0 | 0.612a |
| Cognitive deficits (MOCA < 26 points) | 4 (36 %) | 17 (53%) | 0.49b |
- MOCA, Montreal Cognitive Assessment. Values are mean ± SD unless otherwise indicated. Inter-group differences were tested with aStudent’s t-test or bFisher’s exact test.
Four patients withdrew from the study due to side effects of combined STN + SNr-DBS. All of them terminated the study under a combined STN + SNr-DBS regime, suggesting that for these patients this stimulation mode was not adequate or even disadvantageous. In detail, side effects were worsening of motor functions as well as a lack of beneficial effects of levodopa, akathisia, general uncomfortable feeling, aggressiveness, and increased confusion and hallucinations. From the baseline electrode position, these patients did not differ from the other PD patients who completed the study. The results of the 11 patients, who completed the full study protocol, were considered.
Assessments and outcome measures
All subjects underwent a standardized otorhinolaryngological examination and a flexible-endoscopic evaluation of swallowing (FEES) by experienced otorhinolaryngologists, blinded with respect to the DBS parameters. FEES was performed using a 2.6-mm-diameter high-definition rhino-laryngo-videoscope (ENT-V3, Olympus Medical Systems Corp., Tokyo, Japan) as described before.2 During FEES, standardized test boluses were given in a fixed order: first one teaspoon of pudding, second one teaspoon of water, third 90 ml of water to be drunk with a straw (quickly, but not hastily), fourth 1 biscuit (Ø 91 mm, weight 20 g), and fifth half slice of bread with butter (≈94 × 90 × 9 mm, weight 28 g).
For swallow analysis, residues, penetration and aspiration and leakage were evaluated for each consistency and for each participant.10 Residues were classified from 0 to 5. The occurrence of a build-up phenomenon, a special variant of residues that goes along with an increase of firm consistencies in the valleculae after each swallow, was also recorded. Penetration and aspiration were assessed on the Rosenbek Penetration-Aspiration Scale (PAS) and leakage on the “scale for bolus location”.26 To better illustrate the clinical impact, pharyngeal residues were classified into three categories according to their clinical relevance: "slight" (0-2), "moderate" (3-4), and "severe” (5). The PAS-scores were grouped into three categories: "non-pathological" (PAS 1-2), "laryngeal penetration" (PAS 3-5), and "aspiration" (PAS 6-8). For each patient, a change in PAS and residue category at baseline compared with both stimulation modes was determined. The capability to swallow pills was rated on a 4-point scale (Table S1). Four different placebo-pills were administered to each participant in a random order: a) small round pill (∅ 7 mm), b) big round pill (∅10 mm), c) dragee (∅ 9.8 mm), and d) capsule (length 18 mm). Retained pharyngeal secretions were rated by the "Murray Score" based on the “short version of the four-point Secretion Severity Rating Scale.” Drooling was assessed using the “Drooling Severity and Frequency Scale” (DFSS) ranging from 1 to 5 for severity and from 1 to 4 for frequency. Both values are added to one resulting score (Score 2 = no drooling and score 9 = maximal drooling). A drooling score ≥ 6 was defined as severe drooling.
The swallowing duration of 90 ml of water, of one biscuit and half slice of bread was measured from first to the last gulp. For evaluating the self-perception of swallowing function under the three conditions, the patients were asked to rank themselves on a visual analog scale (VAS) with a vertical line from "no swallowing problems" to "strongest imaginable swallowing disorder."
Statistics
The primary endpoint was “residues of bread” as they are a characteristic pathology in PD patients.3 A sample-size calculation based on the mean values of residues along the 0-5 residue classification was performed with SD of 0.76, an expected effect size of 1 point, and a P-value of 0.05 resulting in a requirement of 11 patients per group to achieve a power of 80 %. Therefore, the number of PD patients assessed in our study seems to be sufficient.
For interval-scaled data, mean values and standard deviations (Mean ± SD) were calculated. In the boxplots median, interquartile range (IQR), whiskers (highest/ lowest values of the data set within 1.5 times of the IQR), and outliers (highest/lowest values of the data set outside the whiskers and within 3 IQR above/below the whiskers) are given. Values above 3 IQR are regarded as extremes. Significant differences in the mean values between patients and controls were tested with the t test for independent samples. For nominal and ordinal-scaled data, frequencies were calculated and compared by Fisher's exact test. The Friedman test was used to detect the influences of the three stimulation conditions on PAS and residues ranking for the patient group. Distribution differences in PAS and residue ranking between patient and control groups were tested by the Mann–Whitney U test. All statistical tests were two-tailed. To compare patients and controls, the significance level (alpha) was set to 0.05. In cases of multiple comparisons between patients, food consistencies and stimulation conditions, a Bonferroni correction was applied to the alpha level.
Statistical analyses were carried out with the statistical software package SPSS, version 19 (IBM, USA).
Results
Effect of DBS mode on endoscopic swallowing parameters
Residues of bread and biscuit were more frequently and severely present in PD patients compared with controls at baseline and both under stimulation conditions (P < 0.005). Residues of water, as well as penetration and aspiration of all consistencies, were comparable between PD patients and controls (Table 3).
| Patient-ID | Residues | PAS | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| water | bread | pudding | biscuit | water | bread | pudding | biscuit | |||||||||||||||||
| OFF | STN | SNr | OFF | STN | SNr | OFF | STN | SNr | OFF | STN | SNr | OFF | STN | SNr | OFF | STN | SNr | OFF | STN | SNr | OFF | STN | SNr | |
| 1 | 0 | 0 | 2 | 4 | 4 | 4 | 0 | 0 | 0 | 3 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 0 | 0 | 0 | 4 | 4 | 3 | 0 | 2 | 0 | 4 | 4 | 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 3 | 0 | 0 | 5 | 3 | 3 | 0 | 0 | 0 | 3 | 3 | 3 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5 | 0 | 0 | 0 | 5 | 5 | 5 | 0 | 0 | 0 | 5 | 5 | 5 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 3 | 1 | 1 |
| 6 | 3 | 4 | 4 | 4 | 4 | 5 | 0 | 2 | 3 | 4 | 4 | 4 | 1 | 6 | 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 |
| 7 | 2 | 4 | 4 | 5 | 4 | 5 | 3 | 0 | 4 | 5 | 4 | 4 | 3 | 5 | 5 | 2 | 2 | 3 | 1 | 1 | 3 | 1 | 3 | 1 |
| 8 | 0 | 0 | 0 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9 | 0 | 4 | 4 | 4 | 4 | 4 | 0 | 0 | 0 | 3 | 4 | 4 | 1 | 8 | 7 | 1 | 2 | 3 | 2 | 1 | 5 | 8 | 2 | 2 |
| 10 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| 11 | 4 | 0 | 0 | 3 | 4 | 4 | 0 | 0 | 0 | 4 | 4 | 4 | 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Friedman test (OFF vs. STN vs. SNr, p) | n.s. (0.61) | n.s. (0.42) | n.s. (0.44) | n.s. (0.85) | n.s. (0.40) | n.s. (0.67) | n.s. (0.16) | n.s. (1.0) | ||||||||||||||||
| Mann-Whitney test vs. control OFF (p [1]) |
n.s. 0.18 |
n.s. 0.34 | n.s. 0.17 |
sign. <0.001 |
sign. <0.001 | sign. <0.001 |
sign. <0.001 |
sign. <0.001 | sign. <0.005 |
n.s. 0.29 |
n.s. 0.05 | n.s. 0.28 |
n.s. 0.57 |
n.s. 0.32 | n.s. 0.54 |
n.s. 0.54 |
n.s. 0.29 | n.s. 0.30 | ||||||
| patients (median) | 0.00 | 0.00 | 0.00 | 4.00 | 4.00 | 4.00 | 0.00 | 0.00 | 0.00 | 3.00 | 4.00 | 4.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Controls (median) | 0.00 | 1.50 | NA | 1.50 | 1.00 | 1.00 | NA | 1.00 | ||||||||||||||||
- PAS, Penetration-Aspiration Scale; OFF, baseline (stimulation-off); STN, Deep Brain Stimulation of the subthalamic nucleus alone; SNr, simultaneous stimulation of the subthalamic nucleus and substantia nigra.
- 1 Bonferroni correction: Alpha = 0.0055.
Under STN-stimulation moderate or severe pharyngeal residues of bread were found in 9/11 (80%) patients and of biscuit in eight patients. Three of them also showed penetration/aspiration of water. Affecting 6 of 11 patients also the build-up phenomenon (a special variant of residues which goes along with an increase of firm consistencies in the valleculae after each swallow) was common in the cohort. Simultaneous STN + SNr-stimulation had no additional positive effect on pharyngeal residues and penetration/aspiration. The three patients with penetration/aspiration had PAS-scores of 6, 5, and 8 under STN resp. 8, 5 and 7 under STN + SNr-DBS (no clinically relevant influence). DBS itself (compared with STIM-OFF) did not lead to a clear change in residue severity. Eight of the nine patients with bread residues under STN-stimulation showed no change in combined STN + SNr-stimulation. Considering simultaneous STN + SNr-stimulation, no significant effect on the other endoscopic swallowing parameters like build-up, pharyngeal secretion, leakage, and swallowing capability of pills was found.
Interestingly, PAS-scores of most patients were not influenced by DBS mode. However, the outcome was variable with PAS worsening for water under both stimulation modes in three patients. One of them demonstrated improvement of swallowing biscuit and another patient showed a substantially better PAS (from 8 to 1) under both stimulation modes.
The individual descriptive data, the mean values of residues and PAS under the stimulation modes and the results from Friedmann tests are given in Table 3. There was no significant change in the mean values of swallowing ability at baseline compared with STN-DBS or STN + SNR-DBS. Looking at the individual category change, most patients showed no change in penetration-aspiration ("non-pathological," "laryngeal penetration," and "aspiration") or pharyngeal residues ("slight,” "moderate," and "severe”) due to DBS mode (neither STN-DBS nor STN + SNr-DBS) for all tested consistencies. In both DBS modes, some patients exhibited improvement and some other impairment of swallowing without a significant difference. The influence of DBS mode on category change is illustrated for penetration-aspiration and residues in Figure 2A and B.
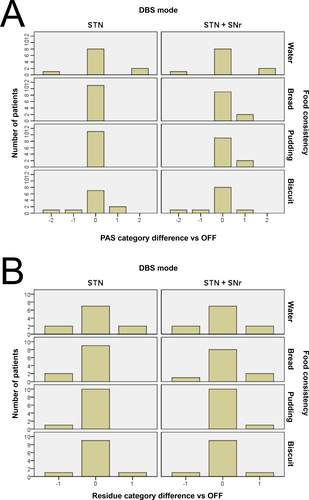
The build-up phenomenon was not influenced by DBS mode. In six patients (four with and two without build-up), no change due to DBS was observed. In the other patients, the presence of build-up was highly variable considering the DBS mode.
Most subjects in our cohort did not show relevant leakage and disturbed pharyngeal saliva management. Neither at baseline nor with STN-DBS, any patient exhibited a leakage. Under STN + SNr-DBS, only one patient showed a slight leakage of water and pudding and one patient a mild leakage of biscuit. Slight abnormalities of pharyngeal saliva management were found in only three patients and without relation to DBS mode.
The swallowing capability of pills was disturbed in four patients. All of them had a severe malfunction. One patient had a substantial impairment for all four pills, the remaining three patients had problems with one or two pills regarding all four pills equally. There was no significant impact of the factor DBS mode or pill type (Figure S1).
Effect of DBS mode on self-reported swallowing function and clinical parameters
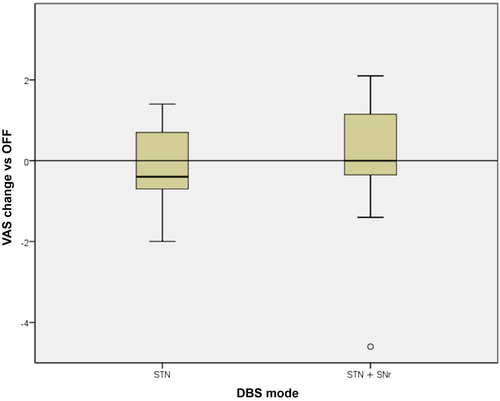
Almost all patients indicated no or only slight dysphagia on the VAS (mean values: baseline 1.77; STN-DBS 0.94; STN + SNr-DBS 1.6). No significant influence of the DBS mode was found on the subjective impression (Figure 3), but the self-perception was very inconsistent in our cohort. Some patients reported improvement, other impairment of swallowing due to both DBS modes.
At baseline, only two patients showed drooling at all. One patient had moderate (DSFS 6) and one mild (DSFS 4) drooling, which was each absent in both DBS modes. Another patient developed moderate drooling (DSFS 6) only under STN + SNr-DBS.
The time differences for swallowing water, bread, and biscuit were not significantly different for both DBS-modes compared with baseline (Figure S2; Table S2).
Discussion
This study analyzed the impact of combined stimulation of STN + SNr compared with conventional STN-DBS on swallowing function in PD patients. The results indicate that (i) self-reported swallowing function, drooling, and endoscopic swallowing parameters such as residues, penetration/aspiration, and leakage are not influenced by DBS itself or stimulation mode in the majority of patients, (ii) combined STN + SNr-stimulation is not suited to improve existing dysphagia but does not impair swallowing function.
A major finding of nearly all patients were at least moderate residues, in particular caused by firm consistencies. This is consistent with previous studies also describing residues as the leading finding in PD.2, 10 Interestingly, PAS scores did not differ significantly in PD patients and controls, and the overall PAS score was relatively low in the study cohort. Even the proportion of patients with penetration/aspiration was lower compared with a normally distributed PD population.1, 2 This potential floor effect could explain the lack of an overall effect of DBS on penetration and aspiration in this study, but not on the missing effect on pharyngeal residues. An explanation for the low PAS scores in this cohort compared to others might be, that patients of this study were moderately affected by PD.
In the interpretation of the results, one needs to consider the limits of the study. The small number of patients can hinder the detection of differences and four patients who initially received the combined stimulation, stopped the study prematurely due to side effects. The fact, that we included PD patients regardless of dysphagia is a weakness of this study. Nevertheless, 9 of 11 patients had moderate to severe residues, meaning clinically relevant dysphagia. Another constraint may be the fact that the impact of DBS on subjective dysphagia and clinical symptoms like drooling or aspiration is hard to determine as they were rare in our cohort.
Residue- and PAS-scores were not significantly influenced by the stimulation mode in most of the patients. Neither conventional STN nor combined STN + SNr-stimulation significantly improved or worsened the swallowing function compared with the STIM OFF condition. No change due to STN + SNr-stimulation was found.
The variable influence of STN-stimulation on swallowing ability in PD was reported previously. All experimental studies were performed on patients, who had an STN-DBS, except for one study that evaluated the influence of DBS of the caudal zona incerta on swallowing. Data about simultaneous STN + SNR-stimulation and dysphagia are missing. In some studies, STN-DBS revealed beneficial effects particularly during the pharyngeal phase of swallowing, such as decreased pharyngeal transit time, improved pharyngeal composite scores, decreased leakage, and reduced aspiration frequency for a short time.17, 18, 27-29 Although small studies and case reports described a beneficial impact of STN-DBS on single swallowing parameters, there were also negative DBS-induced effects reported. Overall, none of the few experimental studies showed a clinically significant improvement or decline of the swallowing function with DBS. Some studies investigated different phases of swallowing (oral or pharyngeal phase) and also use different parameters such as PAS, oral transit time or maximal hyoid bone excursion due to the different examination techniques (FEES or videofluoroscopy). DBS-specific parameters such as the number of leads (one-sided vs. bilateral), lead locations, suitable stimulation washouts, and programing stability were not controlled or reported in the studies as well as the patients’ PD-specific parameters. The methodological differences with other studies, as well as the lack of suitable examination techniques such as the FEES, limit the comparison between this study and precedent ones, and a general statement about the influence of DBS on swallowing.
Different trouble-shooting options of DBS can be considered to improve dysphagia. Previously, the use of low-frequency stimulation with 60Hz STN-DBS has been proven to be beneficial to ameliorate dysphagia, but with subsiding long-term effects.29 Other therapeutic approaches could be the application of bipolar stimulation or possibly short-pulse stimulation,30 when dysphagia is supposed to be a stimulation-induced side effect of unintended co-stimulation of the capsula interna. In our study, it was assumed that STN + SNr-stimulation is a possible trouble-shooting option for the treatment of primary dysphagia by intensified modulation of basal ganglia-brainstem projections to the medullary central swallowing pattern generator.
The hypothesis of an improvement of swallowing function due to combined STN + SNr-stimulation could not be confirmed. Most PD patients with pathological residue- and PAS-scores under conventional STN-stimulation showed unchanged scores under simultaneous STN + SNr-stimulation. Interestingly, three patients with penetration/aspiration of water under conventional STN-stimulation (patients 6,7 and 9) had almost normal PAS-scores under STIM OFF. They showed a serious decline of PAS with penetration/aspiration of water under both conventional STN and simultaneous STN + SNr-stimulation. Moreover, one single patient with a silent aspiration of water under STIM OFF had improved PAS scores under both DBS modes.
The number of cases of this study is small and there was no clear trend regarding dysphagia due to combined STN + SNr-stimulation; but at an individual level, there were stimulation-induced differences with improvement, but also worsening of dysphagia. Therefore, PD patients with DBS should always be evaluated by FEES or videofluoroscopy and treated individually. Further studies on DBS with larger case numbers are, therefore, required.
In conclusion, objective swallowing function, as well as subjective perception of the majority of patients, were not influenced by DBS itself or DBS mode. Neither conventional STN-DBS nor simultaneous STN + SNr-DBS significantly enhanced or worsened swallowing function. It can be stated that combined stimulation is not suited to improve existing dysphagia. But the results of the present study suggest that the application of STN + SNr-DBS to improve axial symptoms, such as freezing of gait, in PD patients, does not necessarily impair the swallowing function.
Acknowledgment
This study was supported by SFB 936, C8 to M. Pötter-Nerger.
Ethical standards
The study was approved by the local ethics committee of the Medical Council Hamburg and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments; written informed consent was obtained from all persons.
Author contributions
Christina Pflug: Acquisition of data, drafting/revising the manuscript, including medical writing for the content, analysis or interpretation of data, statistical analysis, study design, and supervision or coordination. Monika Pötter-Nerger: Study concept and design, acquisition of data, drafting/revising the manuscript, including medical writing for the content, analysis or interpretation of data, statistical analysis, study supervision or coordination. Carsten Buhmann: Conception and drafting/revising the manuscript, including medical writing for the content. Daniel Weiss: Study design, drafting/revising the manuscript, including medical writing for the content. Wolfgang Hamel: Conception of the study and acquisition and analysis of data. Christian K.E. Moll: Conception of the study and acquisition and analysis of data. Almut Niessen: Conception of the study and acquisition and analysis of data. Julie C. Nienstedt: Conception and drafting/revising the manuscript, including medical writing for content, acquisition of data. Till Flügel: Design of the study and acquisition and analysis of data. Ute Hidding: Study conception and acquisition of data, study supervision and coordination. Jana-Christiane Koseki: Design of the study and acquisition and analysis of data. Eik Vettorazzi: Study planning/conception and statistical analysis, analysis and interpretation of data. Frank Müller: Study planning/conception statistical analysis, analysis, and interpretation of data. Alessandro Gulberti: Study design and acquisition of data, drafting/revising the manuscript, study supervision or coordination. Christian Gerloff: Conception of the study, drafting/revising the manuscript, including medical writing for the content.
Conflict of Interest
There are no financial disclosures relevant to the manuscript (no other study funding) and no conflicts of interest.




