Cerebellar connectivity in Parkinson's disease with levodopa-induced dyskinesia
Funding information
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (grant number: NRF-2016R1A2A2A05920131) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; Ministry of Science, ICT & Future Planning) (grant number: 2016R1A2B3016609).
Abstract
Objective
The precise pathogenesis or neural correlates underlying levodopa-induced dyskinesia (LID) remains poorly understood. There is growing evidence of the involvement of the cerebellum in Parkinson's disease (PD). The present study evaluated the role of motor cerebellar connectivity in determining vulnerability to LID.
Methods
We enrolled 25 de novo patients with PD who developed LID within 5 years of levodopa treatment, 26 propensity score-matched PD patients who had not developed LID, and 24 age- and sex-matched healthy controls. We performed a comparative analysis of resting-state functional connectivity (FC) between the motor cerebellum and whole brain between the groups.
Results
The patients with PD had increased FC bewteen the motor cerebellum and posterior cortical and cerebellar regions, while no gray matter regions had decreased FC with the motor cerebellum compared to the control participant. The patients with PD who were vulnerable to the development of LID had a significantly higher FC between the motor cerebellum lobule VIIIb and the left inferior frontal gyrus than those who were resistant to LID development. The connectivity of the motor cerebellum and left inferior frontal gyrus was negatively correlated with the latency from PD onset to the occurrence of LID.
Interpretation
Increased FC between the motor cerebellum and left inferior frontal gyrus in de novo patients with PD could be an important determinant of vulnerability to LID.
Introduction
Although levodopa is the most effective treatment for Parkinson's disease (PD), its chronic administration can be complicated by disabling involuntary movement, levodopa-induced dyskinesia (LID). The likelihood of developing LID is 40% within 4–6 years of levodopa therapy,1 and its cumulative incidence increases up to 90 % in patients with PD taking levodopa for more than 10 years.2 LID is one of the most debilitating effects of levodopa therapy, and is associated with a poor quality of life and health-related costs.3
The exact pathogenesis or neural correlates underlying LID remain poorly understood. Presynaptic dopaminergic deafferentation to the striatum due to nigrostriatal degeneration is a prerequisite for the development of LID.4 The pulsatile stimulation of dopamine receptors and alteration of nondopaminergic neurotransmitters simultaneously contribute to the development of LID via abnormal plasticity in the striatum, cortex, and their connections.5, 6 Structural and functional magnetic resonance images have found increased prefrontal volume and its altered connectivity with the putamen to be associated with the condition.7, 8 Aberrant dopaminergic modulation of the connectivity between the putamen and motor areas has been supposed as a possible substrate and predictor for the development of LID.9
Apart from the traditional concept of the cortico-basal ganglia-thalamo-cortical loop as a motor circuit in PD, the role of the cerebellum has recently been emphasized; several studies have identified reciprocal links between the basal ganglia and cerebellum in animals10 and in vivo evidence of alteration in the cerebello-thalamo-cortical pathway in PD.11 In terms of the cerebellar involvement in LID, deep brain stimulation of the subthalamic nucleus or globus pallidus has been reported to normalize metabolism in the cerebellum,12, 13 and transcranial magnetic stimulation (TMS) over the cerebellum has shown promising results in alleviating LID.14 Despite many studies investigating the mechanisms of LID, there have been no previous studies to evaluate the relationship between cerebellar connectivity and LID in patients with PD.
In the present study, we hypothesized that drug-naïve PD patients who were vulnerable to the future development of LID would demonstrate a different pattern of functional connectivity (FC) of the motor cerebellar seed compared to those who were resistant to LID. Thus, to elucidate the role of the motor cerebellum in the pathophysiology of LID, we explored the FC between the motor cerebellum and whole brain areas in de novo patients with PD who were followed for more than 5 years during levodopa treatment.
Methods
Participants
We retrospectively reviewed the database from the Movement Disorders outpatient clinic at the Yonsei University Health System from September 2008 to August 2013. We enrolled 27 patients with PD who developed LID within 5 years of levodopa treatment. Among patients with PD who had not developed LID within 5 years of levodopa treatment, we recruited 27 patients using a propensity score matching method to match each patient 1:1 in terms of age, sex, disease duration, and parkinsonian motor score. All patients with PD underwent baseline brain magnetic resonance imaging (MRI) at the de novo state and were followed for more than 5 years. The diagnosis of PD was based on the clinical diagnostic criteria of the United Kingdom PD Society Brain Bank, and only patients diagnosed with PD who responded to dopaminergic medication during the follow-up period (≥6 months) were included in the present study. All patients with PD had undergone N-(3-[18F]fluoropropyl)-2β-carbon ethoxy-3β-(4-iodophenyl) nortropane (18F-FP-CIT) positron emission tomography (PET) imaging at the time of their PD diagnosis, which revealed decreased uptake in the posterior putamen. Patients with PD visited outpatient clinic every 3 to 6 months, and two movement disorders experts (Y.H.S., P.H.L.) carefully examined for the presence of LID based on history from patients and caregivers or direct neurological examination at every visit. We regarded the date on which the PD patients or their caregivers reported the occurrence of LID or the date it was first observed in the clinic as the date of LID occurrence. Since the cumulative incidence of LID increases with the duration of levodopa intake and the mean latency from the initiation of levodopa treatment to LID in patients with PD was approximately 5 years,15 we divided the PD patients into two groups: a LID-resistant group (PD-LID-) who did not develop LID within 5 years of levodopa treatment and a LID-vulnerable group (PD-LID+) who developed LID within 5 years of levodopa administration. Due to image quality/process assurance, one patient in the PD-LID- group and two patients in the PD-LID + group were further excluded; therefore a total of 51 patients with PD (PD-LID- group, n = 26; PD-LID + group, n = 25) were included in the study. Twenty-four healthy control individuals matched for age and sex with no focal neurologic diseases and normal cognitive function (scores ≥ 26 on Korean version of the Mini-Mental State Examination [K-MMSE]) were also recruited. The exclusion criteria included an evidence of atypical parkinsonism; focal brain lesions, severe white matter hyperintensities,16 multiple lacunes in the basal ganglia, or hydrocephalus on MRI; other neurological, psychiatric, or metabolic illnesses; or patients with PD who displayed parkinsonian symptoms before age 40 because young-onset PD patients are extremely high risk for LID.17
Parkinsonian motor symptoms were assessed during the drug-naïve state at the time of 18F-FP-CIT PET acquisition using the Unified PD Rating Scale (UPDRS) motor (part III) subscale. The levodopa dose was calculated according to the method described in a previous study.18 Patients who were taking amantadine at LID onset were excluded because it exerts an antidyskinetic effect. The K-MMSE was used to assess general cognition.
The present study was approved by the Institutional Review Board of Yonsei University College of Medicine and informed consent was obtained from all participants.
Neuroimaging acquisition
All subjects underwent MRI scanning with a 3.0 Tesla MRI scanner (Achieva, Philips Medical System, Best, Netherlands). A detailed description is provided in Data S1.
Preprocessing of functional MRI data
The resting-state functional MRI data were processed using Analysis of Functional NeuroImages (AFNI) software (http://afni.nimh.nih.gov/afni).19 A detailed description is provided in Data S2.
FC analyses
The motor cerebellum was defined by anterior lobe (lobule I–V) and lobule VI, VIIb, VIIIa, and VIIIb according to previous studies,20-22 within both cerebellar hemispheres and vermis, using masks created with Probabilistic atlas of the human cerebellum.23 A detailed description is provided in Data S3.
Group comparisons of FC
In comparisons of FC of motor cerebellum, we used age, sex, years of education, and total K-MMSE score as covariates to adjust potential confounders that can affect FC and to exclude the influence of cognitive function on FC in all group comparisons. First, we compared the FC of motor cerebellum between controls and patients with PD to investigate PD-specific changes in FC from motor cerebellum. For five motor cerebellar seeds, we performed a two-sample t-test on the group gray mask with above covariates. We further performed a correction for five comparisons for five seeds. The corrected significance level was set at Pα-value < 0.05/5 (uncorrected per-voxel height threshold of two-tailed P-value < 0.005 with a minimum cluster size of 267 voxels). Second, we compared the FC of motor cerebellum among the control, PD-LID-, and PD-LID + groups to investigate LID-related change in FC from motor cerebellum. For five motor cerebellar seeds, we performed analysis of variance on the group gray mask with the same covariates with threshold at uncorrected P < 0.05 (Fig. S1). Then, we performed subgroup comparison between the two groups for five FC maps. We further performed a correction for three post-hoc subgroup comparisons and five comparisons for five motor cerebellar seeds. The corrected significance level was set at Pα-value < 0.05/15 (uncorrected per-voxel height threshold of two-tailed P-value < 0.005 with a minimum cluster size of 120 voxels).
Statistical analyses
For baseline demographics and clinical characteristics of all participants, we used a two-sample t-test or an analysis of variance for continuous variables and Pearson's χ2 test for categorical variables. A partial correlation analysis using age, sex, disease duration, and parkinsonian motor score as covariates was conducted to evaluate the relationship between the latency from levodopa start to the development of LID and the FC from the motor cerebellar seed. The data were analyzed using SPSS software version 23 (IBM Corporation, Armonk, NY, USA). P-values less than 0.05 were considered significant.
Results
Demographic and clinical characteristics
The baseline demographics and clinical characteristics of the participants are summarized in Table 1. Age, sex, years of education, and total K-MMSE score were comparable among the controls and PD patients. Between the PD-LID- and PD-LID + groups, there were no differences in the disease duration, follow-up duration, levodopa duration, levodopa dose at the last follow-up, or UPDRS motor score. In the PD-LID + group, the latency from levodopa start to LID development was 0.5–4.9 years and its mean and standard deviation was 2.5 ± 1.3 years.
| Variables | Control | PD-LID- | PD-LID+ | P-value |
|---|---|---|---|---|
| Number | 24 | 26 | 25 | |
| Age, years | 66.1 ± 8.9 | 67.8 ± 8.4 | 65.7 ± 9.0 | 0.625 |
| Sex, female, n (%) | 13 (54.2) | 13 (50.0) | 12 (48.0) | 0.908 |
| Education, years | 12.2 ± 3.4 | 10.6 ± 4.8 | 9.6 ± 4.5 | 0.110 |
| Total K-MMSE score | 27.3 ± 1.1 | 26.2 ± 2.4 | 26.7 ± 2.9 | 0.101 |
| Disease duration, months | 14.2 ± 10.4 | 18.0 ± 13.1 | 0.236 | |
| Follow-up duration, years | 6.0 ± 0.8 | 6.1 ± 0.8 | 0.617 | |
| Duration of levodopa therapy, year | 6.0 ± 0.7 | 5.9 ± 0.7 | 0.593 | |
| Levodopa dose at the last follow-up, mg | 509.1 ± 204.4 | 516.0 ± 148.9 | 0.891 | |
| UPDRS motor score | 20.4 ± 9.5 | 24.1 ± 11.4 | 0.395 | |
| Latency from levodopa start to LID development, years | 2.5 ± 1.3 |
- Values are expressed as mean ± standard deviation or number (percentage).
- PD, Parkinson's disease; LID, levodopa-induced dyskinesia; MRI, magnetic resonance imaging; CCSIT, Cross-Cultural Smell Identification Test; BDI, Beck Depression Inventory; K-MMSE, the Korean version of the Mini-Mental State Examination; UPDRS, Unified Parkinson's Disease Rating Scale
Comparison of FC from the motor cerebellum between the control and PD groups
The patients with PD had increased FC between cerebellar lobule VI and the right middle temporal gyrus (Fig. 1A). In the seed of VIIIb, the patients with PD exhibited increased FC with the left cerebellum, posterior cingulate cortex, and left inferior parietal region (Fig. 1B). No regions had altered FC from cerebellar lobules I–V, VIIb, and VIIIa in the PD group compared with the control group.
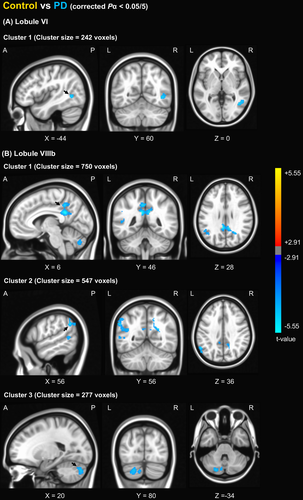
Comparison of FC from the motor cerebellum between the control, PD-LID-, and the PD-LID + groups
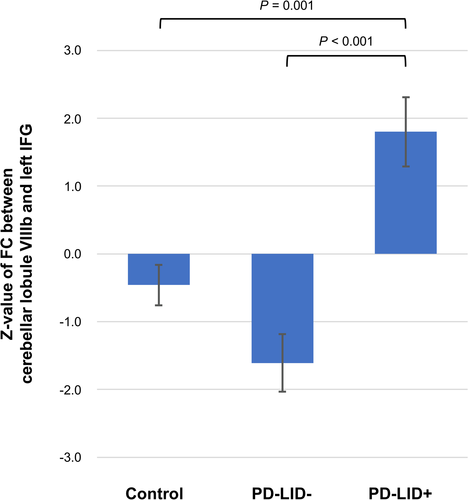
Compared to the controls, the PD-LID- group had increased FC between lobule VI and right superior parietal lobule and between lobule VIIIb and posterior cingulate cortex, left inferior parietal lobule, left calcarine gyrus, left cerebellum, and right angular gyrus compared to the control group (Fig. S2), while the PD-LID + group had increased FC between lobule VI and left paracentral lobule and right superior occipital gyrus, between lobule VIIb and right calcarine gyrus, between lobule VIIIa and right fusiform gyrus, and between lobule VIIIb and right superior parietal lobule, left middle temporal gyrus, and left calcarine gyrus (Fig. S3). In a direct comparison of FC from the motor cerebellar seed between the PD-LID- and PD-LID + groups, the PD-LID + group had significantly increased FC between cerebellar lobule VIIIb and the left prefrontal cortex, maximally with the left inferior frontal gyrus (Fig. 2). The averaged z-value of FC between cerebellar lobule VIIIb and the left inferior frontal gyrus in the PD-LID + group was higher than the control or PD-LID- group (Fig. 3).
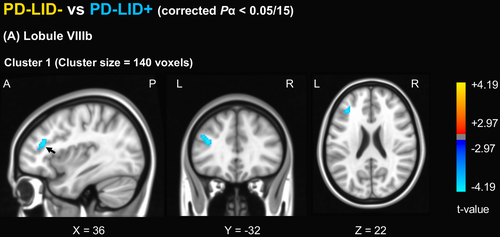
Correlation between motor cerebellar connectivity and latency to develop LID
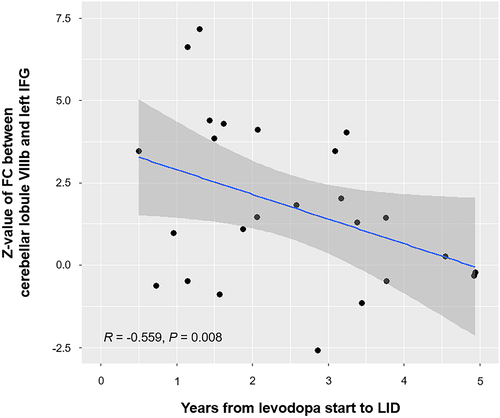
In the PD-LID + group, the z-value of FC between cerebellar lobule VIIIb and the left inferior frontal gyrus was negatively associated with the latency from levodopa start to the development of LID (r = −0.559, P = 0.008) after adjusting for age, sex, disease duration, and parkinsonian motor score (Fig. 4). There were no correlations between the z-value of FC and age, UPDRS motor score, or total K-MMSE score.
Discussion
The present study analyzed the association between the development of LID and FC of the motor cerebellum in de novo patients with PD. The major findings were as follows. First, patients with PD had increased FC between the motor cerebellum and posterior cortical regions compared to the control subjects. Second, PD patients who were vulnerable to the development of LID had a significantly higher FC between the motor cerebellum and left inferior frontal gyrus than those who were resistant to its development. Third, the connectivity of the motor cerebellum and left inferior frontal gyrus was negatively correlated with the latency from PD onset to the occurrence of LID.
Increasing evidence suggests that the cerebellum and its connectivity are altered in PD, which reported increased connectivity or hyperactivity in cerebellum. Liu et al. found that the cerebellum showed enhanced connectivity with the bilateral dentate nucleus in de novo patients with PD.24 Festini et al. reported dopamine-dependent cerebellar connectivity, which showed increased cerebellar connectivity in off-medication and decreased connectivity in on-medication compared with the control subjects.25 Increased cerebellar activation in PD was evident during motor execution or motor learning26, 27 as well as resting state.28 In the present study, we investigated the FC between the motor cerebellum and cortical gray matter in PD patients without exposure to dopaminergic medication. We found that the motor cerebellum, especially in lobule VI and VIIIb, had increased FC with posterior cortical areas in drug-naïve PD patients. Intriguingly, no motor areas in cortical and subcortical regions showed altered FC with motor cerebellum. Given that posterior cerebral hypometabolism or altered FC is related to cognitive dysfunction in PD,29, 30 one possible explanation is that increased networks in these areas may be attributable to the cognitive role of the seeds, rather motor dysfunction in PD, although we controlled for years of education and total K-MMSE score for covariates. Of the motor cerebellar regions, anterior lobe (lobule I–V) and lobules VIIIb are consistently involved in motor function, while whether cerebellar lobule VI, VIIb, and VIIIa are responsible for motor or cognitive functions varies from study to study.20, 31, 32 Thus, it can be inferred that precise anatomical segregation of motor and cognitive cerebellum is difficult and the FC of cerebellum is more predominant in cognitive aspect in early PD than motor aspect. Further studies are necessary to address whether altered motor cerebellum connectivity is a compensatory mechanism for motor dysfunction or an overall reflection of a pathologic change in PD.33
Interestingly, the present study demonstrated that the motor cerebellar connectivity to the left inferior frontal gyrus was enhanced at the time of the diagnosis in PD-LID + patients compared to PD-LID- patients. The role of the prefrontal cortex in the development of LID is not yet elucidated. Cerasa et al. reveal that the prefrontal cortex is critical in the pathophysiology of LID.7, 8 PD patients with LID had increased gray matter volume in the bilateral inferior frontal gyrus, which was more prominent on the right side, and underactivation of the right inferior frontal gyrus compared with those without LID. Contrarily, Herz et al. found that dopaminergic modulation of resting-state connectivity between the putamen and primary sensorimotor cortex or supplementary motor area, not the inferior frontal gyrus, predicted the presence or severity of LID.9 In the present study, the connectivity between the cerebellum and left inferior frontal gyrus was comparable between the patients with PD and control participants, but it was significantly higher in the PD-LID + group than the control and PD-LID- groups. Moreover, the connectivity of the motor cerebellum and left inferior frontal gyrus was negatively correlated with the latency from PD onset to the occurrence of LID. The inferior frontal gyrus, either left or right, is critical for inhibitory control over motor responses.34, 35 One study using TMS found a decreased inhibitory control of the inferior frontal cortex over primary motor area.36 Therefore, it can be postulated that alterations or plasticity in the inferior frontal gyrus might disinhibit abnormal cortico-basal ganglia circuit or aggravate motor cortex plasticity. Based on the evidence that the prefrontal cortex is activated in PD patients with LID, Rektorova et al. administered TMS over the left dorsolateral prefrontal cortex and found improvement of LID and decrement in the amplitude of motor-evoked potential, suggesting a prefrontal contribution to motor cortex inhibition.37 Several studies have demonstrated the existence of the intrinsic connectivity between the lateral prefrontal region and cerebellum, which suggests the role of the cerebellum in cognitive function.38 Therefore, we provided new evidence that abnormally increased lateral prefrontal-cerebellar connectivity can have a role in ineffective motor control, inferring that the inferior frontal gyrus and its connectivity play an important role in the pathogenesis of LID.
The role of the cerebellum in LID has been strongly supported by the research of TMS over the cerebellum, which showed promising results in alleviating LID.14 Inhibitory cerebellar stimulation is thought to facilitate cerebello-thalamo-cortical afferent input to the primary motor cortex and improve responsiveness of the primary motor cortex and sensorimotor plasticity, thereby reducing LID.39 In conjunction with our findings, inhibitory cerebellar stimulation may reduce the enhanced prefrontal-cerebellar connectivity and modulate motor complications in PD. Since baseline connectivity is important in determining when LID will occur, we also anticipate delaying the occurrence of LID if we select PD patients with increased connectivity and apply TMS over the cerebellum in the de novo state.
The present study had several limitations. First, this study was based on a relatively small sample, which limits the generalizability of our results. However, we applied strict exclusion criteria to enroll drug-naïve PD patients who were susceptible to LID and performed a propensity score match to enroll those who were resistant to LID. Therefore, the results of the altered cerebellar FC may purely reflect the influence of LID. Second, the patients with PD were arbitrarily divided into the LID-vulnerable and LID-resistant groups based on the occurrence of LID within 5 years of levodopa treatment. The cutoff years were based on one study that the mean time from levodopa initiation to the onset of LID was approximately 5 years.15 Third, the onset of LID was determined by retrospective reports by the patients or observations made during regular follow-up evaluations in the outpatient clinic for movement disorders. Thus, the onset of these complications may have occurred earlier than reported. Fourth, we did not conduct functional MRI at the onset of LID, which would better reflect the changes in FC in LID patients. However, we cannot exclude the influence of chronic levodopa administration or disease progression on altered FC. Measuring FC in the absence of dopaminergic medication and in the early stages would be an effective method to study the exclusive functional changes associated with the future development of LID. Fifth, it would be preferable to measure the severity of LID as well as the onset timing of LID together to investigate the association between cerebellum connectivity and LID.
To conclude, we demonstrated that abnormally increased connectivity between the motor cerebellum and left inferior frontal gyrus in de novo PD patients could affect the likelihood of developing early LID during long-term levodopa treatment. These results provide a new perspective in the investigation of the role of the motor cerebellum in PD and its contribution to the pathophysiology of LID.
Acknowledgments
None.
Conflict of Interests
The authors report no competing interests.
Author Contributions
HSY and YHC had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Statistical analysis was conducted by HSY, YHC, and SJC. Study design was done by HSY, JML, and PHL. Drafting the manuscript was done by HSY, YHC, YHL, and PHL. Literature search, data interpretation, and revising the manuscript were done by HSY, YHC, BSY, YHS, JML, and PHL. All authors read and approved the final version of the manuscript.




