Recovery from cortical blindness with mepivacaïne
Funding Information
This study was funded by the “Investissements d’avenir” program (ANR-10-IAIHU-06) to the Brain and Spine Institute.
Abstract
We report the case of a patient suffering from cortical blindness following bilateral occipital stroke, who recovered normal vision in his right visual field following injection of the local anesthetic mepivacaïne. The effect was transient but reproducible, allowing the patient to lead a normal life. Effect duration increased after adjunction of paroxetine. We provide anatomical and functional brain imaging correlates of this improvement, showing particularly how functional connectivity is restored between intact perilesional cortex and distant brain regions. This serendipitous finding may potentially benefit patients suffering from visual but also nonvisual handicap following brain lesions.
Introduction
Stroke is a major public health problem and a frequent cause of severe sensorimotor and cognitive handicap. Poststroke rehabilitation mainly resorts to training and occupational methods, supplemented by appropriate technical devices, while drugs have shown very limited efficacy.1, 2 This general dearth of effective treatments also concerns visual field loss, which affects about 30% of stroke survivors.3 We report the case of a patient suffering from almost complete cortical blindness following bilateral occipital stroke, who recovered normal vision in his right visual field following injection of the local anesthetic mepivacaïne.
Medical History
A 52-year-old man underwent surgery for a herniated cervical disc. Following surgery, he suffered from dissection of a vertebral artery, resulting in bilateral occipital infarcts, and cortical blindness. He was subsequently cared for in an institution for the rehabilitation of the blind. Two to four years later, he underwent two minor interventions on his left upper limb, under brachial plexus block with mepivacaïne, an amino-amide anesthetic. In both instances, the right hemianopia disappeared a few minutes after the anesthesia, and relapsed 3 days later. After informal testing against placebo, the patient took systematic injections of mepivacaïne (54 mg), self-injected subcutaneously upon each relapse of cortical blindness. This allowed him to live a normal life, including tinkering, gardening, and reading. The patient recorded the duration of the effect of injections over a period of 2 years (Fig. S1). To treat moderate anxiety, the patient received paroxetine starting 9 months after the onset of the logging, which doubled the mean duration from 7.4 to 15.2 days (t = 3.8, P < 0.001), with an increase in variability from SD = 2.0 to SD = 10.3 days (F = 26.4, P < 0.001).
Clinical Status
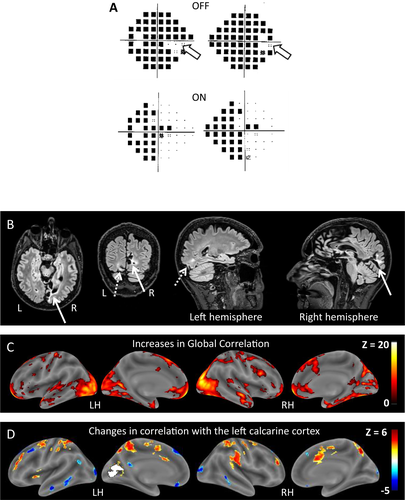
During off periods, on confrontation and using Humphrey automated perimetry, there was complete blindness except for a small region in the right periphery. During on periods, the whole right hemifield returned to normal, while hemianopia persisted in the left hemifield (Fig. 1A). An extensive battery of tests of high-level vision and general cognition performed during on periods were normal (Table S1). General neurological and psychiatric examination was normal.
Brain Imaging
All details on anatomical and functional imaging methods are provided as Data S1.
Anatomical MR images showed an infarct affecting most of the right calcarine cortex, causing the permanent left hemianopia. There was a much smaller left occipital infarct with underlying white matter hyperintensity (Fig. 1B). A study of hemifield activation with functional MRI and visual evoked potentials was unfortunately not performed.
Right-sided vision recovery suggested restoration of function in the left occipital cortex, which we assessed with resting state functional MRI, a technique used to evaluate correlations across brain regions during rest, and to infer patterns of distant interactions. First, we studied the changes in correlation induced by treatment with no anatomical a priori. Global Correlation, that is, the average correlation with the rest of the brain, increased bilaterally in the intact occipital and occipitotemporal cortex (Fig. 1C). Second, we assessed specifically the changes in the connectivity of the left calcarine cortex (Fig. 1D). There was an increase in correlation with bilateral frontoparietal regions, a network controlling attention to external events. Conversely, there was a decrease in correlation with the so-called default-mode network, involved in internally oriented cognition. As a control, we checked that the right-hemispheric calcarine region showed negligible changes in correlation (Fig. S1).
Finally, we assessed metabolic changes under mepivacaine with 18FDG PET scan. Off-mepivacaine, there was low metabolism in the areas lesioned on anatomical MRI, extending to apparently intact left occipital regions (Figs. S1 and S2 and Table S2). On-mepivacaine, there was a left-predominant decrease in the volume of low metabolism (39% and 13% decrease in the left and right occipital lobes, respectively), while the hypometabolic clusters in anatomically intact cortex returned to normal. In the absence of control subjects receiving mepivacaine, a statistical comparison of metabolic changes between the patient and controls was not possible.
Discussion
The mechanisms of the visual improvement which we observed under mepivacaïne is currently unknown. Still this unexpected effect is conceivable considering the properties of mepivacaïne, an amino-amide commonly used as a locoregional anesthetic.
Nature of the deficit
One may ask whether the reversible right hemianopia actually consisted in hemispatial neglect, which may also be improved with pharmacological agents.4 However, this hypothesis receives little support in the present case. First, during off periods, the patient did not display any kind of asymmetry in his perceptual and motor behavior. Second, the topography of the right hemianopia was not consistent with neglect, as the spared region was located in the right periphery, while in neglect the right periphery would show the most severe deficit. Third, the lesion was restricted to low-level visual cortex, sparing all areas associated with spatial neglect.
Mechanism of action
Amino-amides primarily inhibit nerve conduction by binding to and inactivating sodium channels. However, they also modulate the activity of potassium and calcium channels, including NMDA receptors,5, 6 and their effects may indeed last longer than predicted from the kinetics of sodium channel blocking.7 They pass the blood-brain barrier, as illustrated by central neurological side effects, including drowsiness, tremor, and seizures, which however do not occur with normal doses.8, 9 Note that mepivacaine is among the amino-amides with the highest CNS concentration and free brain exposure.10
There is rich evidence that stroke lesions cause changes in long-range functional network connections.11 In the present case, PET-scan and resting-state fMRI showed that mepivacaine triggered some increase in perilesional metabolism, and the restoration of functional connections between the visual cortex and remote areas, subtending the visual improvement. This effect may be considered as the correction of a diaschisis, that is, of the deactivation of intact brain regions remote from but connected to the area of primary injury, including metabolic depression in visual cortices following lesions to the optic radiations.[12]
Effect duration
In the present case, before adjunction of paroxetine, the visual effect of mepivacaïne was longer than expected on the basis of its half-life of 4–5 h, as measured during regional anesthesia.13 However, such discordance between short half-life and long clinical effects is not unusual, and has been related to the complex kinetics of tissue distribution,14 and to the cellular mechanisms of action. Thus, nicotinic receptor agonists have enduring cognitive effects out of proportion with their short half-life, due to their induction of Long Term Potentiation phenomena.15 As to the shortening of the effect by physical and psychological stress, it fits with the multiple consequences of stress and effort on pharmacokinetics and brain function.16, 17
Role of paroxetine
Inhibitors of serotonine reuptake enhance motor recovery after stroke,18 an effect which seems independent from the doubling of the mean mepivacaine effect duration under paroxetine. This doubling likely results from the potent inhibition by paroxetine of P450 cytochromes, which degrade amido-amines.19 Indeed some drugs show a 3- or 5-fold increase in their half-life due to such interactions with inhibitors of serotonine reuptake.[20]
Potential clinical scope
Amido-amines won’t obviously alleviate the symptoms of any stroke, as best illustrated within the patient himself by the contrast between the impact on right as compared to left hemianopia. The left occipital lesion was noteworthy by the contrast between the severity of the hemianopia and the limited lesion on anatomical imaging. Accordingly, experimental infarcts in sensory or motor cortex yield long-lasting reduction in neural activity in volumes much larger than the lesion itself.21 Importantly, the bulk of the left-sided lesion was in white matter, while the visual cortex was largely intact and potentially arousable, as opposed to the right-sided lesion which destroyed the visual cortex, yielding an irreversible deficit. Finally, it is possible that in addition to lesion features, the effect may vary across patients depending on individual polymorphisms of the involved receptors. Indeed, in the past other single-case or small sample studies have reported behavioral benefits from CNS drugs that were not confirmed in large randomized trials.
We believe that the mechanisms underlying the present effect, the conditions for efficacy, and the scope of application deserve further investigation. Patients whose deficit results from a mechanism of disconnection may be the primary beneficiaries of this approach.
Acknowledgments
We thank the patient for his participation. This study was funded by the “Investissements d’avenir” program (ANR-10-IAIHU-06) to the Brain and Spine Institute.
Conflicts of Interest
Laurent Cohen, Amélie Ponchel, Aurélie Kas, Sébastian Ströer, Antoine Del Cul, Guillaume Guérineau de Lamérie, Randa El Hachem, Piirika Crépin, Alexandre Morin declares no conflict of interest.




