Regional motor cortex dysfunction in amyotrophic lateral sclerosis
Funding information
The authors gratefully acknowledge the funding support by the Beryl Bayley Fellowship grant from the Motor Neuron Disease Research Institute of Australia, Stanford Family MND Research Grant and the National Health and Medical Research Council of Australia [Project grant numbers 510233, GIA 1726, 1024915, 1055778]. This work was also supported by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical research Council of Australia Program Grant (#1037746).
Abstract
Objective
The pathophysiological processes underlying amyotrophic lateral sclerosis (ALS) need to be better understood, although cortical dysfunction has been implicated. Previous transcranial magnetic stimulation (TMS) studies have assessed cortical dysfunction from the hand. The aim of the present study was to determine whether cortical dysfunction was evident across representations of three body regions, and to relate these changes to clinical features of ALS.
Methods
In this cross-sectional study, threshold tracking TMS was undertaken in 60 sporadic ALS patients, with motor evoked potential (MEP) responses recorded over the hand (abductor pollicis brevis), lower limb (tibialis anterior), and bulbar (trapezius) regions. The cross-sectional findings were compared to 28 age- and gender-matched controls.
Results
Cortical dysfunction was evident across the representation of the three body regions, although the degree and nature of the dysfunction varied. Cortical hyperexcitability, as heralded by reduced short interval intracortical inhibition (SICI), was evident in all cortical regions (hand, P < 0.01; leg, P < 0.05; bulbar, P < 0.05) in ALS patients when compared with healthy control subjects. Importantly, features of cortical hyperexcitability seemed more prominent in clinically affected body regions and correlated with functional disability and muscle weakness. Cortical inexcitability was more prominent in the leg (P < 0.001) and bulbar regions (P < 0.01) when compared with controls.
Interpretation
The nature of cortical dysfunction varied across the body regions in ALS, with cortical hyperexcitability being more prominent in the upper limbs while cortical inexcitability was more evident in the lower limbs and bulbar regions. The findings suggest a heterogeneity of cortical pathophysiological processes in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive neurodegenerative disorder of the motor neurons potentially mediated by a multistep process incorporating a complex interplay of pathophysiological mechanisms1-3. Cortical hyperexcitability appears to be an important pathophysiological mechanism in ALS, preceding the onset of clinical disease and detectable lower motor neuron (LMN) dysfunction, while also correlating with biomarkers of motor neuronal degeneration4-8. A pathophysiological association between cortical hyperexcitability and spinal motor neuron degeneration was postulated to be mediated by an anterograde trans-synaptic glutamatergic mechanism, the “dying forward” hypothesis9. While cortical hyperexcitability appears to be an early feature in ALS, most pronounced in patients with profuse fasciculations, preserved muscle bulk, and hyper-reflexia10, inexcitability of the motor cortex evolves with disease progression in ALS11, 12, perhaps reflecting evolving neurodegeneration of the corticomotoneuronal tracts.
Regional variability in cortical hyperexcitability has been previously reported in ALS patients13-15. Notably, a greater degree of cortical hyperexcitability was reported in regions contributing to the split hand and split hand-plus phenomenon14, 15. In addition, greater degree of cortical dysfunction was reported in the dominant motor cortex, contralateral to the site of disease onset, in ALS patients and this finding was associated with disease evolution6, 13. Consequently, it seems likely that differences in cortical dysfunction may be evident across body regions in ALS patient and may be influenced by the site of disease onset.
A correlation between cortical hyperexcitability, MRI measures of reduced functional connectivity in frontal regions and a greater degree of functional disability16, further supported a pathogenic role for the neocortex in ALS pathogenesis17. At a physiological level, a greater degree of intracortical inhibition and less facilitation was reported in the distal upper and lower limb regions when compared with the bulbar region, represented by the trapezius muscle18. This study supported previous findings reporting differences in cortical excitability across body regions19, 20. These findings were related to variable functional organization of the corticomotoneuronal (CM) system, with stronger monosynaptic CM projections emanating from the intrinsic hand and lower limb muscle representations within the neocortex21-23.
Dissecting out regional excitability changes across different cortical body representations may provide unique insights into the role of the neocortex in ALS pathogenesis17. Most studies in ALS have been focussed on assessing cortical excitability changes from the intrinsic hand muscles24, although TMS studies assessing corticobulbar and corticospinal tracts to lower limbs have reported subclinical dysfunction in sporadic ALS patients25. Consequently, the present study utilized threshold tracking TMS to determine the cortical excitability changes across three different body region representations in ALS, including bulbar, upper, and lower limb regions, and related these changes to the site of disease onset.
Materials and Methods
Subjects
In total, 78 participants with progressive muscle weakness were prospectively and consecutively recruited from the multidisciplinary ALS clinics at Sydney Health Partners (Westmead Hospital and Brain Mind Centre, University of Sydney) between January 2015 and December 2017 to undergo transcranial magnetic stimulation (TMS). The diagnosis of ALS was made in accordance with the Awaji Shima diagnostic criteria26 and confirmed in 62 participants with 60 participants consenting to undergo TMS testing. All participants recruited in this study had sporadic ALS. Genetic testing for the C9Orf72 and SOD1 gene mutations was negative in the younger participants (<50 years, N = 7) recruited. All participants provided informed consent, approved by the Western Sydney Local Health District Ethics Committee, prior to enrollment into the study. Handedness was determined by the Edinburg handedness inventory27 with leg dominance being recorded separate to hand dominance. A single operator performed all the studies to avoid the possibility of inter-operator variability. Exclusion criteria for ALS patients and controls were as follows: (i) in situ pacemakers or other ferromagnetic devices; (ii) use of psychotropic or other essential medications described to alter TMS parameters; (iii) Acute migraine headaches in the month prior to TMS assessment; (iv) history of surgical brain procedures and brain trauma; (v) Presence of concurrent neurological disorders including epilepsy, movement disorders, or strokes. Twenty-eight healthy control participants were screened to exclude neurological disease and underwent cortical excitability testing over the three target muscles on the dominant or nondominant side. The neurological examination was normal in all controls, incorporating strength and deep tendon reflex assessment, and none of the controls were receiving any medications which could alter cortical excitability. No participant was found to have dysfunction of the median, peroneal, or spinal accessory nerves.
Clinical measurements
Participants were assessed for clinical signs of upper and lower motor neuron dysfunction with measurement of muscle strength by the medical research council (MRC) criteria28 from the following muscle groups in the upper and lower limbs: shoulder abduction, elbow flexion and extension, wrist extension, finger abduction, thumb abduction, hip flexion, knee extension, and foot dorsiflexion. Maximum muscle strength was 60 in the upper limbs and 30 in the lower limbs resulting in an MRC sum score of 90. Neck muscle strength was tested in flexion, extension and lateral rotation along with trapezius muscle strength and no weakness of the trapezius muscle was noted in any patient. Deep tendon reflexes were elicited at the biceps, triceps, brachioradialis, knee, and ankle bilaterally along with bilateral plantar responses and the jaw jerk in order to obtain a UMN score29 with a maximum number of 15. Functional assessment was undertaken using the revised ALS functional rating scale (ALSFRS-R)30. TMS testing was undertaken at initial assessment and all follow-up was clinical.
Peripheral neurophysiology measurements
Prior to undertaking TMS testing, the maximal peak to peak CMAP amplitude was recorded for the abductor pollicis brevis (APB) muscle with median nerve stimulation at the wrist, tibialis anterior (TA) muscle with peroneal nerve stimulation at the fibular head and trapezius muscle following stimulation of the spinal accessory nerve in the neck.
Cortical excitability
Motor evoked potentials (MEP) were recorded from the APB (hand region) and TA (leg region). While MEP recordings from the oropharyngeal muscles would have been desirable, threshold tracking TMS is difficult to perform reliably on the oropharyngeal muscles. Rather, the upper trapezius muscle was selected as a “bulbar” muscle as recent studies have reported the upper trapezius muscle to be a sensitive indicator of clinical and subclinical bulbar neuron dysfunction31, 32. All three muscles were selected from the side of disease onset in participants with lateralized disease or the dominant side in patients with non-lateralized disease. For APB recordings, a 90-mm circular coil, connected to a BiStim device (Magstim Co., Whitlands, South West Wales, UK), was utilized to record cortical excitability, with the coil positioned to induce a current in the posterior-to-anterior direction33. A 110-mm double cone coil was used to record MEP responses from the TA and upper trapezius muscle according to a previously reported technique18.
Short interval intracortical inhibition and intracortical facilitation (ICF) were recorded by using the paired-pulse threshold tracking TMS technique as previously described18, 33, 34. Briefly, the conditioning stimulus was set to 70% RMT and SICI was recorded over the following interstimulus intervals (ISIs) for each muscle: 1, 1.5, 2, 2.5, 3, 3.5, 4, 5 and 7 msec. ICF was recorded at 10, 15, 20, 25 and 30 msec ISIs for each muscle. SICI and ICF were calculated off-line using the previously reported formula33. Inhibition was calculated off-line as follows:
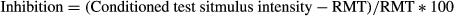
Facilitation was measured as the decrease in the conditioned test stimulus intensity required to elicit a target MEP.
Single pulse TMS was used to record the resting motor threshold (RMT) for each muscle defined as TMS intensity required to produce and maintain a target MEP response of 0.2 mV ± 20%18, 33. The resting state of each muscle was confirmed by visual and auditory feedback. Patients with resting motor thresholds of >90% of the maximum stimulator output were considered inexcitable. The stimulus-response curve was generated with three stimuli at each of the following TMS intensities: 100, 110, 120, 130, 140% and 150% of RMT. The MEP amplitude expressed as a percentage of the maximal peak to peak compound muscle action potential (CMAP) amplitude was recoded as was the MEP latency (msec).
Statistical analysis
Prior to undertaking statistical analysis, normality of data was assessed using the Shapiro–Wilk test. TMS data were compared with 28 age and gender-matched controls (mean age 61.1 ± 2.1 years). Student t-test was used to compare single and paired-pulse TMS parameters and muscle CMAP between ALS and control groups. Chi-squared test for association was undertaken to assess the differences in cortical excitability between the ALS and controls groups for each of the three muscles and between the APB, TA and trapezius muscles in ALS patients. Pearson’s correlation coefficient was used to assess associations between variables. All results are expressed as mean ± standard error of the mean (SEM). A P value less than 0.05 was considered significant.
Results
Clinical characteristics
Cortical excitability testing was undertaken on 60 ALS patients (33 male and 27 female), with mean age being 63.3 ± 1.5 years (range 31–93 years). Limb onset disease was evident in 69% (53% dominant side; 47% nondominant side), bulbar onset in 26% and generalized onset in 5% of patients. The median disease duration, at the time of TMS assessment, was 11 months (range 6–18 months) with a median ALSFRS-R score of 42 (40–44), UMN score of 11 (6–12), and MRC sum score 83 (77–88), all indicative of mild functional impairment and early disease course. The early disease stage in the present cohort was further underscored by the fact that 30% of patients were classified as Awaji definite/probable and 70% as possible/negative at time of testing. Consequently 22% of participants were on riluzole therapy at the time of assessment, with the mean time on riluzole being 19.2 ± 5.0 months. The median survival from disease onset to last follow-up was 31 (24–42.3) months. Clinical features of frontotemporal dementia or frontal executive dysfunction were not reported by patient carers, although formal cognitive testing was not performed.
Lower motor neuron dysfunction across motor regions
Prior to undertaking cortical excitability studies, the degree of peripheral nerve dysfunction was determined in each region. The CMAP amplitude recorded over the APB (ALS 8.0 ± 0.4 mV; controls 10.9 ± 0.7 mV; P < 0.01) and TA (ALS 4.9 ± 0.4 mV; control 7.2 ± 0.4 mV; P < 0.001) muscles were significantly reduced when compared to controls, while CMAP amplitude of the trapezius muscle was comparable to controls (ALS 11.0 ± 0.4 mV; 11.7 ± 0.5 mV; P = 0.2, Table 1). Subgroup analysis disclosed a significant reduction in CMAP amplitude in limb onset patients when recording from the APB (ALS 8.2 ± 0.5; control 10.9 ± 0.7; P < 0.005), TA (ALS 4.5 ± 0.4 mV; control 7.2 ± 0.4 mV; P < 0.001) and trapezius (ALS 10.5 ± 0.5 mV; control 11.7 ± 0.5 mV; P < 0.05) muscles. In bulbar onset ALS patients, the CMAP amplitude was reduced when recorded over the APB (ALS 8.0 ± 0.8 mV; control 10.9 ± 0.7 mV; P < 0.05) and TA (ALS 5.6 ± 0.7 mV; control 7.2 ± 0.4 mV; P < 0.05) muscles but was comparable over the trapezius (ALS 12.6 ± 0.8 mV; control 11.7 ± 0.5 mV; P = 0.3, Table 1). It should be stressed that the mean values for CMAP amplitudes across the different muscles and for all participants were within our laboratory standards. CMAP amplitudes may be a relatively insensitive biomarker of LMN dysfunction in ALS35, representing a potential limitation of the present study.
| Muscle | CMAP (mV) | P value | CMAP Limb Onset (mV) | P value | CMAP Bulbar Onset (mV) | P value | |
|---|---|---|---|---|---|---|---|
| APB | ALS | 8.0 ± 0.4 | <0.01 | 8.2 ± 0.5 | <0.005 | 8.0 ± 0.8 | <0.05 |
| Control | 10.9 ± 0.7 | 10.9 ± 0.7 | 10.9 ± 0.7 | ||||
| TA | ALS | 4.9 ± 0.4 | <0.001 | 4.5 ± 0.4 | <0.001 | 5.6 ± 0.7 | <0.05 |
| Control | 7.2 ± 0.4 | 7.2 ± 0.4 | 7.2 ± 0.4 | ||||
| Trapezius | ALS | 11.0 ± 0.4 | 0.2 | 10.5 ± 0.5 | <0.05 | 12.6 ± 0.8 | 0.3 |
| Control | 11.7 ± 0.5 | 11.7 ± 0.5 | 11.7 ± 0.5 |
- Muscle responses from representative muscles from three body regions namely: the abductor pollicis brevis (APB) (cervical region); tibialis anterior (lumbosacral region) and trapezius (cranial region) revealed reduction in compound muscle action potential (CMAP) over the abductor pollicis brevis (APB) and tibialis anterior (TA) muscles alone in ALS patients when compared with controls. All muscles namely APB, TA, and Trapezius had reduction in CMAP in patients with limb onset disease while the trapezius was relatively spared in bulbar onset disease.
Cortical dysfunction across motor regions
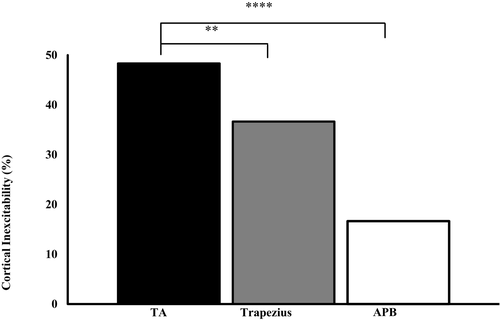
An inexcitable motor cortex was significantly greater in ALS patients when compared with controls when recording over the tibialis anterior (TA) (ALS 48.3%, controls 10.7%; P < 0.001) and trapezius muscles (ALS 36.7%, controls 0%; P < 0.001) but not over the abductor pollicis brevis (APB) muscle (ALS 16.7%, controls 7.1%; P = 0.23). The frequency of motor cortex inexcitability was significantly greater when recording from the lower limbs compared to APB (χ2 = 22.57, P < 0.001) and trapezius (χ2 = 8.07, P < 0.01) muscles in ALS patients (Fig. 3). In participants exhibiting motor cortex inexcitability in two regions, the mean survival was 49.5 ± 7.8 months and was significantly greater when compared to participants exhibiting a hyperexcitable motor cortex in all three studied regions (31.8 ± 5.4 months, P < 0.05).
In participants with recordable MEP responses, averaged SICI (between ISI 1–7 msec) was significantly reduced when recorded from the APB (ALS 3.1 ± 1.4%; controls 9.3 ± 1.6%, P < 0.01), tibialis anterior (ALS 7.4 ± 0.9%; controls 11.2 ± 1.0%, P < 0.05) and trapezius (ALS 4.2 ± 1.4%; control 9.4 ± 1.3%; P < 0.05, Fig. 1A) muscles. There was trend for the degree of SICI reduction to be greater from the APB (67.2 ± 14.7%) when compared to TA (33.7 ± 13.3%, P = 0.06) and trapezius (45.6 ± 20.2%, P = 0.19) muscles in ALS patients. In contrast, there was no significant difference in intracortical facilitation across the three cortical motor regions when compared to controls.

Single pulse TMS studies disclosed a significant increase in MEP amplitude when recording from the APB (ALS 36.8 ± 3.2; controls 19.3 ± 2.9; P < 0.01) and TA (ALS 40.7 ± 4.1; controls 16.1 ± 2.0; P < 0.01) muscles, but not the trapezius (ALS 13.9 ± 1.9; controls 15.1 ± 2.7; P = 0.74, Fig. 1B). Of further relevance, the CSP duration was significantly reduced when recorded from the APB muscle (ALS175.9 ± 4.5msec; controls 188.3 ± 4.0 msec; P < 0.05). In contrast, there was no significant reduction in CSP duration in ALS patients when recording from the TA (P = 0.11) and trapezius (P = 0.20) muscle.
Subgroup analysis disclosed a significant reduction in SICI in bulbar onset ALS patients over the three body regions (Fig. 2A). The reduction in SICI was accompanied by an increase in MEP amplitude from the limb regions (Fig. 2B). In limb onset patients, SICI was significantly reduced in the upper limb region (Fig. 2C), while the MEP amplitude was increased over both the upper and lower limb representations (Fig. 2D). In the limb onset subgroup, 15 (34%) patients reported upper limb onset while 29 (66%) reported lower limb onset disease.
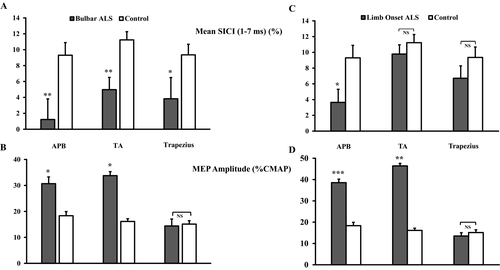
Correlation studies
There was a significant correlation between SICI recorded from the APB and the ALSFRS-R score (R = 0.25, P < 0.05). In addition, the MEP amplitude recorded from the APB muscle correlated significantly with the total MRC scores (R = −0.31, P < 0.05) and MRC score from the APB muscle (R = −0.26, P < 0.05). Furthermore, MEP amplitude recorded from the TA muscle was significantly correlated with the lower limb MRC score (R = −0.43, P < 0.05). For bulbar onset ALS patients, there was a significant correlation between SICI recorded from the trapezius muscle and the total MRC score (R = 0.41, P < 0.01). Interestingly, there was no significant correlation between SICI recorded in the APB muscle and ALSFR-R fine motor subscore, SICI TA and ALSFRS-R gross motor subscore; SICI trapezius and ALSFRS-R bulbar subscore.
Separately, SICI recorded in the upper limbs correlated with SICI recorded in the lower limb (R = 0.4, P < 0.05) and bulbar (R = 0.5, P < 0.01) regions, suggesting that the dysfunction of similar pathophysiological processes was operative. In contrast, there was no significant correlation between the TMS parameters and the upper motor neuron score, survival or disease duration.
Discussion
The human neocortex has been previously implicated in ALS pathogenesis through a dying forward hypothesis17. At a physiological level, cortical hyperexcitability is an early feature of ALS6, correlating with functional disability, lower motor neuron degeneration and adverse prognosis6, 36, 37. Patterns of disease spread13 and development of dissociated muscle involvement, a specific feature of ALS38-41, also appear to be associated with cortical hyperexcitability14, 15. These previous TMS studies have predominantly assessed cortical dysfunction from the hand region. In the present study, cortical dysfunction was documented across the three motor cortical body representation regions (upper limb, lower limb, and bulbar), with the nature of this cortical dysfunction being varied. Importantly, features of cortical hyperexcitability were most pronounced in the hand region and correlated with functional disability and muscle weakness. In contrast, inexcitability of the motor cortex was also documented, shown to be most pronounced in the lower limbs and associated with longer survival. Taken together, the findings suggest a disease-modulating effect of cortical dysfunction in ALS, potentially contributing to phenotypic heterogeneity and survival variability evident in ALS.
Cortical hyperexcitability and ALS
In the current ALS cohort, cortical hyperexcitability was heralded by a significant reduction in SICI, an observation evident across the three body regions. Neuropharmacological studies have established that SICI is mediated by short latency intracortical inhibitory GABAergic circuits acting via GABAA receptors42. Cortical modeling studies have provided additional evidence that interneuronal circuits, located in layers 2 and 3 of the motor cortex, were important for SICI generation43. The reduction in SICI, evident in the three different body regions, suggests that the dysfunction of inhibitory GABAergic intracortical circuits is a ubiquitous finding in the motor cortex of ALS patients, a notion supported by previous pathological studies44. In addition, the significant correlation between SICI values recorded across different body regions provides additional support for the fact that similar pathophysiological mechanisms (namely dysfunctions of cortical inhibitory circuits) were responsible for SICI reduction in ALS.
In addition to SICI reduction, the MEP amplitude was also significantly increased, albeit only when recording from the hand and lower limb regions. The MEP amplitude reflects the density of corticomotoneuronal projections onto motor neurons42 as well as the excitability of the local spinal cord circuitry45. The increase in MEP amplitude could be explained by cortical hyperexcitability, and is in keeping with previous studies in ALS patients13, 46, 47. Furthermore, the higher MEP amplitudes could also be mediated by hyperexcitability of the spinal motor neurons innervating the APB and TA muscles. Interestingly, the MEP amplitude was not significantly increased from the trapezius muscle, a finding that may be explained by differences in the functional organization of corticomotoneuronal inputs18. Specifically, the trapezius muscle receives a greater proportion of slower conducting corticomotoneuronal projections which indirectly synapse with motor neurons and are less prone to hyperexcitability48.
Separately, inexcitability of the motor cortex was significantly more frequent in the leg and bulbar regions when compared to the hand region in the current ALS cohort. Motor thresholds reflect the density of corticomotoneuronal (CM) projections onto spinal and bulbar motor neurons, being lowest in intrinsic hand muscles due to a higher density of CM projections20, 49, 50. The higher frequency of motor cortex inexcitability from leg and bulbar regions could relate to differences in the CM innervation across the regions. Namely, dysfunction of the smaller CM pool projections to the lower limb and bulbar regions may reach a threshold at which the motor cortex is inexcitable at an earlier stage of the disease process when compared to CM projections to the intrinsic hand muscles.
From a pathophysiological perspective, the presence of cortical hyperexcitability in all three regions was associated with a significantly shorter survival. This is in keeping with previous studies reporting that cortical hyperexcitability was an adverse prognostic biomarker in ALS37, a finding potentially explained by corticomotoneuronal-mediated degeneration of motor neurons via trans-synaptic glutamatergic mechanisms17. This notion is supported by clinical studies reporting the concordance between upper and lower motor neuron dysfunction in the clinically affected body regions both at outset and during disease progression51. Therefore, it could be hypothesized that hyperexcitability of specific corticomotoneuronal pathways mediates the development of clinical features within specific body regions, a notion somewhat supported by the present findings. Intriguingly, there was no correlation between cortical hyperexcitability and ALSFRS-R subscores, which may argue against any pathological association. However, a likely explanation for this lack of correlation may relate to the relative insensitivity of the ALSFRS-R subscores as a biomarker of functional impairment. Longitudinal studies assessing physiological dysfunction of the CM projections to the three different regions could help clarify the pathophysiological implication of our findings. However, classical ALS with rapid disease progression can result in technical limitation of TMS study due to the rapid development of muscle wasting or cortical inexcitability limiting the recording of TMS motor evoked responses.
It should be acknowledged that the present study has some limitations. Notably, cortical dysfunction was assessed unilaterally, thereby precluding any firm conclusions about the role of cortical dysfunction in disease evolution in ALS, particularly the contiguous-horizontal pattern of disease spread. In addition, the trapezius muscle was used a surrogate bulbar muscle, given that recording of cortical excitability from more traditional bulbar muscles (such as genioglossus) is difficult with the threshold tracking TMS technique. While demonstration of lower motor neuron dysfunction in the trapezius muscle was reported as a reliable biomarker of bulbar dysfunction in ALS37, the cervical innervation to the trapezius muscle could have impacted the findings. In addition, genetic testing (c9or72 and SOD-1) was only performed in participants exhibiting younger onset disease (<50 years, N = 7) and as such the presence of de novo genetic mutations in the older cohort (>50 years) could have exerted a confounding effect. Similarly, the presence of subtle cognitive dysfunction, which was not formally assessed in the current ALS cohort, could have also exerted a confounding effect.
In conclusion, the present study has established variations in cortical dysfunction across different body region representations. Importantly, features of cortical hyperexcitability were more prominent in the clinically affected body regions and were correlated with functional disability, muscle weakness, and adverse prognosis. While the present study suggests a role for cortical dysfunction in mediating the clinical features within a specific body region, longitudinal studies in ALS patients incorporating multiple body regions may conclusively confirm this possibility with implications for disease pathogenesis.
Acknowledgments
The authors gratefully acknowledge the funding support by the Beryl Bayley Fellowship grant from the Motor Neuron Disease Research Institute of Australia, Stanford Family MND Research Grant and the National Health and Medical Research Council of Australia [Project grant numbers 510233, GIA 1726, 1024915, 1055778]. This work was also supported by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical research Council of Australia Program Grant (#1037746).
Author Contributions
PM contributed to conception and design of the study, acquisition and analysis of data and drafting a significant portion of the manuscript or figures. CY contributed to conception and design of the study and acquisition of data. MK contributed to conception and design of the study and editing the manuscript. SV contributed to conception and design of the study, analysis of data and drafting a significant portion of the manuscript.
Conflict of Interest
Authors have no conflict of interest to report.




