Massive cortical reorganization is reversible following bilateral transplants of the hands: evidence from the first successful bilateral pediatric hand transplant patient
Abstract
In this repeated measures case study, we show that sensory deafferentation after limb amputation leads to changes in cortical somatotopic maps which are reversible after restoration of sensory input. Using magnetoencephalography (MEG), we observed in a child with bilateral hand transplants large-scale shifts in somatosensory lip cortical representation from anatomic hand area to anatomic face region. After recovery of tactile sensation in the digits, responses to finger stimulation were localized to orthotopic sensory cortex, but with atypical electrophysiologic features (amplitude and frequencies).
Introduction
Loss of peripheral sensory inputs leads to functional reorganization of primary somatosensory cortex.1-3 Following deafferentation of the upper limb in adult primates, the area of somatosensory cortex deprived of afferent signals begins to respond to tactile stimulation of the face, a massive cortical reorganization (MCR) mediated subcortically through the cuneate nucleus.4 Similar demonstrations of MCR have also been reported in human adults with unilateral upper limb amputations using MEG.5-7 MCR has not been demonstrated in children previously, and the cortical adaptation to restoration of sensory inputs in children is unknown. We utilized the spatial and temporal resolution of MEG measurements of tactile sensory responses to provide insight into the dynamics of sensory reorganization and to ask, can massive reorganization of primary somatosensory cortex after early-life limb loss revert to a typical somatotopic map after hand transplantation in a child?
MEG somatosensory responses and index finger touch sensitivity (using monofilament testing) were recorded over the year following transplantation at visits: 1 (6 weeks), 2 (18 weeks), 3 (33 weeks), and 4 (54 weeks). We sought to determine whether (1) MCR of face to hand area was present during the prerecovery period, (2) MCR would revert to typical orthotopic somatosensory locations with recovery of sensory function of the hands, and (3) somatosensory responses from tactile stimulation of transplanted fingers differ from age-matched controls.
Methods
The vascularized composite tissue allotransplantation (VCA) occurred at the Children's Hospital of Philadelphia (CHOP), six years after bilateral hand and foot amputation at age two resulting from septic shock.8 Renal transplantation had occurred previously. VCA resulted in reafferentation of the medial, ulnar, and radial nerves serving hand somatosensation and motor function. Consultation with the institutional review board determined that this single case report of data collected in the course of clinical care of this patient did not require separate IRB oversight. Consent for publication of these data was, however, obtained from the patient's parent. Age-typical control data were also collected from five children (mean age 10.2 years; range 9.3 years to 11.4 years) who provided written informed consent for participation in accordance with standards approved by our institutional review board (IRB) ethics committee.
Tactile sensation measures
At each visit, tactile sensation was measured using Semmes-Weinstein monofilament testing with the patient blindfolded. The threshold of sensitivity was defined as the thinnest monofilament which the patient affirmed feeling in at least three of five trials (with no responses on false trials) over each of several locations in the ulnar, median, and radial distributions of each hand. Monofilament scores correspond to the monofilament size (lower score indicates greater sensitivity). To assess gnosis, digit identification with eyes open and closed was also assessed at visits 2, 3, and 4.
Magnetoencephalography
Neuromagnetic activity to tactile stimulation was recorded using a whole-head 275-channel CTF MEG system (VSM MedTech Ltd) located inside a magnetically shielded room. MEG signals were recorded continuously (1200 samples/s; 0 to 200 Hz). Three fiducial localization coils were used for coregistration of MEG data to the patient's brain MRI. Head position was recorded continuously to ensure that head movements did not exceed 1 cm over the duration of the MEG recordings.
Somatosensory stimuli were presented to the right lower lip (visits 1–4) as well as right and left index fingers (visits 3 and 4) using pneumatic pulses of compressed air (30 p.s.i.) delivered via clip-on balloon diaphragms (pulse duration 35 msec; 0.5sec and 0.7sec random ISI). MEG data were collected in epochs of −0.1 to 0.3 sec for a total of 500 trials and filtered using 1–40 Hz band-pass filter. The tactile “P50m” averaged evoked response was identified by source latency and orientation and used for source localization.
Data analysis was performed with Brainstorm (http://neuroimage.usc.edu/brainstorm)9). For the computation of the dSPM distributions, an estimate for the diagonal noise-covariance matrix was computed using a 100 msec baseline (−100 to 0 msec). Regularization consistent with the signal-to-noise ratio of whitened data was applied in the computation of the minimum norm estimates and conducted on noise-normalized dSPM using an unconstrained source model. The dSPM distributions were thresholded at 80% of the maximum, with peak locations used for estimating the time-varying activity of the average from peak locations.
Time–frequency analysis
Peak locations corresponding to the maximum amplitude of the “P50m” response were identified for finger and lip stimulation separately. The location of the peak response was identified in 3D and manually grown in volume using a set of 20 vertices corresponding to ~4 cm2 in SI. Single-trial source waveforms (−100 to 300 msec) were extracted from peak locations and transformed using Morlet wavelets, with a central frequency of 1 Hz and a time resolution of 3sec. Time–frequency responses were computed and displayed between 1 and 150 Hz over 40 logarithmic frequency bins. Power values per frequency bin were Z-score normalized using baseline levels (−100 to 0 msec) to obtain absolute magnitude changes with respect to baseline for each participant.
Magnetic resonance imaging (MRI)
Whole-brain MRI including diffusion tensor imaging (DTI) was conducted on visits 1, 3, and 4 with a 3T Siemens Verio scanner using a 32-channel head coil. We obtained a 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) scan in axial orientation, with field of view = 256x256x192 and matrix = 256x256x192 mm to yield 1 mm isotropic voxel resolution. DTI was performed using 30 diffusion gradient directions at b = 1000sec/mm2 and voxel resolution of 2 x 2 x 2.5 mm. DTI measures from 3D tract regions defined by tractography of somatosensory radiation (postcentral gyrus to the posterior limb of the internal capsule bilaterally) were performed using FSL's diffusion toolkit.
Results
At visits 1 and 2, index fingertips were insensitive to tactile stimulation with even the largest monofilaments. At visits 3 and 4, the patient was able to sense light touch on the fingertips (monofilament size for right index finger was 4.08 and 3.85, and for the left index finger was 4.17 and 4.08, respectively; normal for age is 2.96).10
Typically, tactile sensation of the lip activates the inferolateral portion of the postcentral gyrus (primary sensory cortex, S1). By contrast, in our patient at visits 1 and 2, prior to recovery of hand sensation, cortical responses to tactile lip stimulation appeared in superomedial S1, adjacent to the hand knob of motor cortex. Accompanying this massive spatial reorganization was abnormally prolonged latencies of the evoked responses to lip stimulation (~55 msec; age-typical ~30 msec). At visits 3 and 4, lip stimulation activated orthotopic lip area of S1, inferolateral to the previously activated hand area, with a shorter, more age-typical latency (see Fig. 1). The postrecovery reduction in peak latency was not likely due to structural changes in white matter as somatosensory tract DTI measures (fractional anisotropy and mean diffusivity) did not change significantly between pre- and postrecovery time periods.
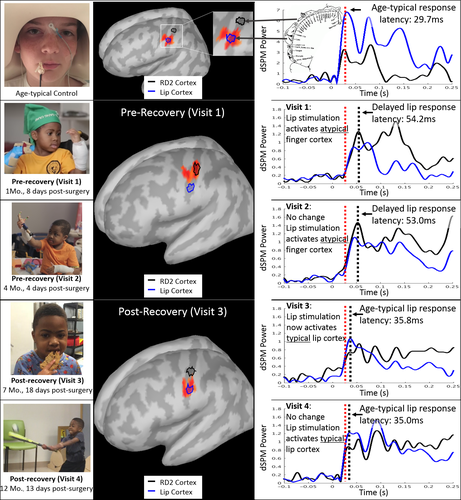
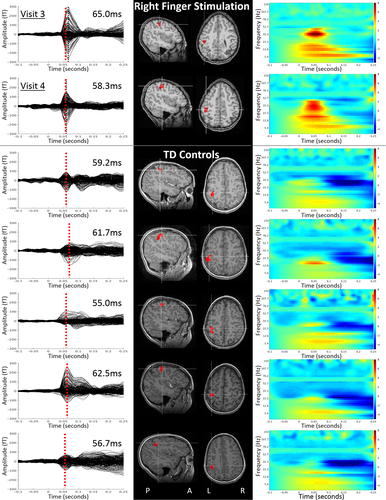
Right and left index finger stimulation at visit 3 produced abnormally strong cortical responses with age-typical latencies (mean age-typical peak latency ~59 msec),11 localized as expected to the contralateral S1 adjacent to the anatomic hand knob of motor cortex. Responses at visit 4 were weaker compared to visit 3, possible indicating a trend toward normalized responses. MEG testing did not include digit stimulation during visits 1 and 2 because of the absence of sensation on clinical examination.
Time–frequency analysis to reveal frequency constituents of the elicited brain activity showed that after recovery of sensation, cortical somatosensory responses are not only larger but show atypically strong event-related synchrony (ERS) at characteristic beta band (~15–30 Hz) frequencies (Fig. 2, upper panels). Time–frequency analysis also revealed absence of the poststimulus beta band event-related desynchrony (ERD) typically observed following tactile stimulation.12 While speculative, these atypical oscillatory dynamics may reflect limited higher order processing of the somatosensory stimuli, as the MEG response measures at time point 4 occurred at a stage of recovery where tactile stimuli were perceived but finger gnosis remained poor.
Discussion
These results show that MCR occurs after childhood limb loss, in a pattern similar to observations in adult nonhuman primates,13 and is reversible after sensory input is restored with limb transplantation. This novel observation of MCR in a child using MEG also revealed novel observations about the temporal features of cortical responses to tactile stimulation. In the absence of hand sensation, lip responses mapped to hand sensory cortex with a ~20 msec delay, and after return of hand sensation after transplantation, lip responses returned to lip cortex with a typical latency. These data suggest potential anatomic mechanisms by which the lip sensory pathway governed by the trigeminal nerve comes to activate the delayed cortical response in the hand area – a cortical region governed by a separate dorsal column pathway via the cuneate nucleus.4 Of note, no residual (i.e., parallel) cortical activity was observed from the finger somatosensory area for either of the postrecovery stimulations presented to the lip.
Atypically large somatosensory responses to tactile digit stimulation have been reported previously in adults who experienced unilateral upper limb replantation following amputation.14 The authors speculated that the large-amplitude responses might be related to the experience of pain, commonly reported in this adult amputee population. In our patient, atypically large somatosensory responses from transplanted fingers were observed in the absence of reported spontaneous pain sensations. Future research with pediatric and adult amputees may help characterize specific structural and neural correlates of MCR that likely differ with age and may ultimately predict both the emergence of pain and the optimal conditions for the successful resumption of somatosensation following transplantation.
Acknowledgments
The authors would like to thank Neeraj Jain PhD and Niranjan Kambi PhD for valuable discussions during the writing of this manuscript. This study was supported in part by the Intellectual and Developmental Disabilities Research Center (IDDRC – NIH U54 HD086984) at the Children's Hospital of Philadelphia and a grant from the National Institutes of Health NIH-R01DC008871-07 (TPLR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The listed funding sources had no role in the study design, collection, analysis, or interpretation of the data presented in this manuscript. TPLR would additionally like to acknowledge the Oberkircher family for the Oberkircher Family Chair in Pediatric Radiology at CHOP.
Conflict of Interests
TPLR serves as a consultant on the medical advisory board of CTF MEG International Services LP.




