Anti-tau antibody reduces insoluble tau and decreases brain atrophy
Abstract
Objective
We previously found a strong reduction in tau pathology and insoluble tau in P301S tau transgenic mice following intracerebroventricular infusion of the anti-tau antibody HJ8.5. We sought to determine the effects of HJ8.5 in the same model following peripheral administration.
Methods
The primary objective was to determine if HJ8.5 administered at a dose of 50 mg kg−1 week−1 by intraperitoneal (IP) injection to 6-month-old P301S mice for 3 months would influence phospho-tau (p-tau) accumulation, tau insolubility, and neurodegeneration.
Results
Treatment with HJ8.5 at 50 mg/kg showed a very strong decrease in detergent-insoluble tau. Importantly, HJ8.5 significantly reduced the loss of cortical and hippocampal tissue volumes compared to control treated mice. HJ8.5 treatment reduced hippocampal CA1 cellular layer staining with the p-tau antibody AT8 and thio-S-positive tau aggregates in piriform cortex and amygdala. Moreover, mice treated with HJ8.5 at 50 mg/kg showed a decrease in motor/sensorimotor deficits compared to vehicle-treated mice. Some effects of HJ8.5, including reduction in brain atrophy, and p-tau immunostaining were also seen with a dose of 10 mg kg−1 week−1. In BV2-microglial cells, we observed significantly higher uptake of P301S tau aggregates in the presence of HJ8.5. HJ8.5 treatment also resulted in a large dose-dependent increase of tau in the plasma.
Interpretation
Our results indicate that systemically administered anti-tau antibody HJ8.5 significantly decreases insoluble tau, decreases brain atrophy, and improves motor/sensorimotor function in a mouse model of tauopathy. These data further support the idea that anti-tau antibodies should be further assessed as a potential treatment for tauopathies.
Introduction
The accumulation of hyperphosphorylated, aggregated forms of the tau protein defines many neurodegenerative diseases termed tauopathies including Alzheimer's disease, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and certain forms of frontotemporal lobar degeneration (FTLD).1 The accumulation of tau aggregates correlates well with neurodegeneration in these disorders. Mutations in the microtubule-associated protein tau gene, MAPT, causes an autosomal dominant FTLD, a primary tauopathy.2, 3 Certain changes in MAPT are also recognized as a risk factor for PSP and Parkinson's disease and other disorders.4 Under normal conditions, tau is an abundant, soluble, intracellular protein and its main function is to bind to microtubules and promote microtubule stability.5, 6 Under pathological conditions, tau aggregates, becomes hyperphosphorylated, and forms neurofibrillary tangles inside neurons and dystrophic neurites.1 The amount of tau pathology correlates well with synaptic dysfunction, neuronal loss, and functional decline in human and transgenic mouse models.7-9 In addition to its location in the cytoplasm, monomeric tau is also normally present in the extracellular space of the central nervous system (CNS) in both the cerebrospinal fluid (CSF) and interstitial fluid (ISF).10 Under disease conditions, there is mounting evidence that oligomeric or fibrillar forms of tau can escape cells where it can be taken up by nearby and synaptically connected cells. It can then lead to seeding of monomeric tau and further tau aggregation and spread of pathology.11-13
Active vaccination studies in tau transgenic mice using different phospho-tau peptides has been shown to reduce tau pathology14, 15 and some studies have shown improvement in behavioral deficits.16-18 Passive immunization studies in tauopathy mice with anti-phospho tau and other types of anti-tau antibodies have reported reduced tau pathology19 and improvement in behavioral deficits.20-22
In our recent study, we initially hypothesized that if oligomeric/aggregated forms of tau reach the extracellular space and promote tau seeding and spreading of tau pathology and that anti-tau antibodies which block the propagation of tau aggregates from cell to cell would be most effective in treating tauopathies in vivo.23 We first screened anti-tau antibodies that block seeding of tau in cell culture, and then selected three anti-tau antibodies and tested them by assessing their effects following intracerebroventricular (ICV) infusion in P301S tau transgenic mice, a mouse model of tauopathy.24 All three antibodies, which bound to different nonphosphorylated epitopes on tau, reduced p-tau staining, insoluble tau accumulation, or both to varying degrees. The antibody with the most potent effects was HJ8.5. As peripheral administration of humanized antibodies used commonly is more practical than ICV administration, we set out to assess the effects of peripheral treatment with HJ8.5. Herein, we peripherally administered HJ8.5 to assess its effect on tau pathology, biochemistry, and behavior in P301S tau transgenic mice. Our results suggest that this treatment reduces tau pathology, improves certain motor functions, and reduces brain atrophy.
Methods
Antibodies
HJ8.5 monoclonal antibody recognize only human tau at the N-terminal region (epitope at residues 25–30 aa). The antibody is of the IgG2b isotype.23 Mouse monoclonal BT2 antibody, biotinylated mouse monoclonal anti-tau BT-2 antibody, mouse monoclonal anti-human tau-specific biotinylated HT7 antibody, and biotinylated AT8 antibody were purchased from Thermo Scientific (Asheville, NC). Rat anti-mouse CD68 antibody was purchased from AbD Sero Tec (Raleigh, NC).
Animals
P301S male transgenic mice (Jackson Laboratories, Bar Harbor, ME) expressing P301S human tau T34 isoform (1N4R) under the control of PrP promoter were used.24 These mice are on the B6C3 background. Animal procedure and experimental protocols were approved by the Animal Studies Committee at Washington University School of Medicine.
Administration of anti-tau antibodies
At 6 months of age, male P301S mice were peripherally (IP) injected with two different dose of HJ8.5 (10 and 50 mg/kg) or vehicle (PBS – phosphate-buffered saline). All mice received single injection weekly for a period of 3 months. At 9 months of age, all the mice were sacrificed. Behavioral analysis was performed during the last 4 weeks of treatment.
Tau enzyme-linked immunosorbent assay
Total tau enzyme-linked immunosorbent assay (ELISA) was performed as described previously.23 Briefly, HJ8.7 antibody in carbonate buffer pH 9.6 was used to coat plates and incubated at 4°C, overnight on a shaker. ELISA plates were washed five times (BioTek ELx405 plate washer, BioTek, Winooski, VT) with PBS and blocked with 4% BSA (bovine serum albumin) in PBS for 1 h at 37°C. Sequentially extracted brain samples in reassembly buffer (RAB), radio immunoprecipitation assay buffer (RIPA), or 70% formic acid (FA) soluble tau brain tissue fractions were diluted in sample buffer (0.25% BSA in PBS, 300 nmol/L Tris pH 7.4 supplemented by protease inhibitor) at 4°C overnight on shaker. FA (70%) fractions were neutralized with 1 mol/L Tris pH 11.0 by 1:20 dilution and further dilutions were made in sample buffer. ELISA plates were washed eight times with PBS, followed by incubating the plates with biotinylated mouse monoclonal anti-tau antibody BT-2 antibody (0.3 μg/mL, Thermo Scientific) in 0.5% BSA in PBS for 1.5 h at 37°C; by addition of streptavidin-poly-horseradish peroxidase-40 (1:4000, Fitzgerald, Acton, MA) for 1.5 h in the dark at room temperature (RT); and then developed with Super Slow ELISA TMB (Sigma, St. Louis, MO) and absorbance read at 650 nm (BioTek Synergy 2 plate reader, BioTek, Winooski, VT). Recombinant human tau was used to develop standard curve. Negative control wells included omission of primary antibody in each plate. To determine the levels of human tau in 70% FA fractions, mouse monoclonal antibody Tau5 (20 μg/mL, gift from L. Binder) was used as coating antibody and mouse monoclonal anti-human tau-specific biotinylated HT7 antibody (0.2 μg/mL, Thermo Scientific) was used as detection antibody.
To measure the plasma tau, we utilized an ELISA in which plates were first coated with 1 μg/mL of BT2 (Thermo Scientific) antibody in PBS supplemented with 20% glycerol. The next day, plates were blocked with 2% BSA in PBS supplemented with Tween-20 0.05% and 20% glycerol for 1.5 h at RT. Plasma samples were diluted in sample buffer (0.25% BSA in PBS, 300 nmol/L Tris pH 7.4 supplemented by protease inhibitor) at 4°C overnight on a shaker. The next day, plates were washed 4 × with PBS followed by incubation with biotinylated mouse monoclonal anti-tau antibody HJ8.7 (1 μg/mL) in 1% BSA in PBS for 1.5 h at RT. Plates were washed 4 × with PBS followed by addition of streptavidin-poly-horseradish peroxidase-40 (1:6000, Fitzgerald). Plates were washed 4 × with PBS and then developed with Super Slow ELISA TMB (Sigma) and absorbance read at 650 nm. Recombinant human tau 1N4R (Tau-412, rPeptide, Bogart, GA) was used to develop the standard curve. Plasma from tau knockout mice was used to spike the standard curve to avoid matrix interference.
Biochemical extraction of brain tissue
Brain tissue extraction was performed as described previously.10, 23
Histology
Preparation of tissue and staining was performed as described previously.23 Briefly, after 12 weeks of the treatment, P301S mice were anesthetized with IP pentobarbital 200 mg/kg, followed by perfusion with 3 U/mL heparin in cold Dulbecco's PBS. Brains were cut into two hemispheres, the left hemisphere was fixed in 4% paraformaldehyde for 24 h and then transferred to 30% sucrose in PBS. These fixed half brains were cut coronally into 50 μm sections with a freezing sliding microtome from the front to the back of the brain. All sections were stored in 24-well plates with cryoprotectant (0.2 mol/L PBS, 30% sucrose, 30% ethylene glycol) at −20°C until use. The cortex and hippocampus were dissected from the right hemisphere of freshly perfused brains and stored at −80°C until use. The stained tissues were scanned using a NanoZoomer digital pathology system (Hamamatsu Photonics, Middlesex, NJ).
Volumetric analysis of P301S brain sections
Every sixth coronal brain section beginning rostrally at the crossing of the corpus callosum to the dorsal end of the hippocampus was mounted (300 μm between sections). These sections corresponding to bregma coordinates 1.5 to −3.9 mm in the mouse brain atlas25 were used for volumetric analysis. All these mounted tissue sections were stained with PHF1 antibody (gift from Peter Davies). The stained tissues were scanned using a NanoZoomer digital pathology system (Hamamatsu Photonics).
Immunohistochemistry
Immunohistochemistry was performed as described in Yanamandra et al.23 Briefly, to assess abnormally phosphorylated tau in the brain, we utilized three 50-μm thick coronal brain sections spaced 300 μm apart in all treated mice. The brain sections were washed three times with Tris-buffered saline (TBS) followed by incubation in 0.3% hydrogen peroxide in TBS for 10 min at RT. Sections from all treatment groups (vehicle [n = 15], HJ8.5 – 10 mg/kg [n = 13], and HJ8.5 – 50 mg/kg [n = 12]) were blocked with 3% milk in TBS with 0.25% (vol/vol) Triton-X followed by incubation at 4°C overnight with the biotinylated AT8 antibody (Thermo Scientific, 1:500). AT8 antibody recognizes tau phosphorylated at Ser202 and Thr205.26 Three brain sections from each mouse separated by 300 μm, approximately corresponding to sections at bregma coordinates −1.4, −1.7, and −2.0 mm in the mouse brain atlas25 were used for AT8 staining. Stained sections were scanned by using NanoZoomer digital pathology system.
Thioflavin-S staining
Thioflavin-S (Thio-S) staining was performed as described previously.23 To assess thio-S staining, three brain sections from each mouse separated by 300 μm from all the treated groups were stained in thio-S in 50% ethanol (0.25 mg/mL) for 3 min, followed by washing in 50% ethanol and distilled water. Thio-S staining was quantified in the piriform cortex and amygdala by a blinded rater who gave a score from 1 (no staining) to 5 (maximum staining) in all control and anti-tau antibody-treated mice. Three sections at Bregma coordinates −2.3, −2.6, and −2.9 mm were utilized.
Immunoprecipitation of P301S tau aggregates and labeling
Nine-month-old male P301S mice brain tissues were homogenized in TBS supplemented with protease and phosphatase inhibitors (Roche, Indianapolis, IN) as described previously.23 Protein A/G agarose beads (150 μL) were coupled with 100 μg of HJ8.5 antibody incubated at 4°C for 24 h. HJ8.5 was then cross-linked to the beads using 0.05 mmol/L DSS (disuccinimidyl suberate) in coupling buffer. The cross-linking reaction lasted for 60 min at RT. Brain-homogenized TBS soluble fractions (ca. 300 μL) were then incubated in HJ8.5 cross-linked to protein-G-agarose beads (as per kit recommendations, Pierce Crosslink Immunoprecipitation kit, Thermo Scientific, Rockford, IL) at 4°C with end-over-end rotation for 24 h. Then the column was incubated with 50 μg of Alexa-Fluor-647 dye (Life Technologies) overnight at 4°C. The column was then washed three times with 1 × TBS buffer to remove the unbound dye. The column was then eluted to collect the labeled P301S tau-647 aggregates. For the Alexa-Fluor-647 dye-only sample condition, the antibody cross-linked column was incubated with 1 × TBS buffer, which was then followed by column incubation with Alexa-Fluor-647 dye overnight at 4°°C.
Uptake of P301S tau aggregates and flow cytometry
Microglial BV2 cells were utilized at 90,000 cells per well in a 24-well plate. Primary cortical neurons were made from 18-day timed pregnant C57BL/6J mouse (E18) and isolated in filtered sterilized 2 mg/mL papain (Worthington Biochemicals, Lakewood, NJ) in hibernate E-calcium/B27 medium supplemented with 0.1% DNase I (Invitrogen, Grand Island, NY). Neurons in neurobasal media (Gibco, Invitrogen, Grand Island, NY) containing serum-free B-27 (Invitrogen) and supplemented with 0.5 mmol/L Glutamax (Invitrogen) were plated in 24-well plates precoated with 10 μg/mL poly-d-lysine (Sigma Aldrich, St. Louis, MO) followed by washing (4 × ) with sterile distilled water.
To determine whether anti-tau antibody HJ8.5 will promote the uptake of P301S tau aggregates, we preincubated 13 nmol/L of HJ8.5 and HJ3.4 (control antibody) with labeled P301S tau-A647 aggregates (10 nmol/L) which was then added to the BV2 cells or primary cortical neurons. Seven days (DIV7) after plating 100,000 cells/well cortical neurons in 24-well plates, cells were treated with preincubated P301S tau aggregates with or without antibodies. Cells were harvested at various time points (BV2 cells at 1, 2, 4, and 7 h; cortical neurons at 2, 4, and 11 h) with 0.25% trypsin for 3 min and fixed with 4% paraformaldehyde for 10 min, then resuspended in Hank's balanced salt solution with 1% FBS and 1 mmol/L EDTA buffer before running on a flow cytometer MACSQuant VYB (Miltenyi Biotec, San Diego, CA).
Immunoprecipitation of antibodies from plasma
Nine-month-old P310S mice were injected IP with vehicle (PBS) or HJ8.5 of 10 or 50 mg/kg (n = 3/group). Forty-eight hours later, mice were sacrificed and plasma collected. A column was packed with 220 μL of protein A/G agarose resin (Thermo Scientific) and 50 μL plasma samples were 1:1 diluted with PBS and incubated on the column for 2.5 h at 4°C. The columns were then spun at 1000g for 1 min in a refrigerated microfuge 20R centrifuge (Beckman Coulter, Indianapolis, IN). The flow through contained free tau unbound to antibodies. The column was washed four times with PBS, then eluted with 0.1 mol/L glycine (pH 2.7), and eluates were neutralized with 1 mol/L Tris pH 9.0. Following elution, samples contained tau bound to antibodies. All the samples were stored at −80°C until the tau levels were measured by ELISA.
Ledge and inverted screen tests
Ledge test
Each mouse was timed for how long it could maintain its balance on an elevated 0.75 cm wide Plexiglas “ledge” (a rectangular piece of Plexiglas placed perpendicular to a table top) without falling (60 sec maximum). A score of 60 sec was assigned if the mouse traversed the entire length (51 cm) of the apparatus and returned to the starting place in less than 60 sec without falling.
Inverted screen test
Each mouse was placed in the middle of a screen (wire mesh grid - 16 squares per 10 cm) that was inclined at 60° with its head oriented downward. When the mouse was judged to be stable in gripping the screen, it was gently inverted to 180° and the mouse was timed for how long it could remain hanging upside down on the screen using a 60 sec maximum trial time. The apparatuses for both tests were elevated 47 cm above the testing surface. Greater details for the tests may be found in previous publications, for example, Wozniak et al.27
Conditioned fear test
Conditioned fear test was performed as described previously.23
Statistical analysis
Statistical analysis of behavior data was performed as described previously.23 All the data were presented as mean ± SEM. Statistics were performed using GraphPad Prism 6.05 for Windows (GraphPad Software Inc.). As the prespecified primary outcome of these studies was to compare HJ8.5 at 50 mg/kg to PBS-treated mice, an unpaired t-test was used to compare these two groups. One-way ANOVA followed by Tukey's post hoc test was used to compare all three groups of P301S mice (PBS, HJ8.5 10 mg/kg and HJ8.5 50 mg/kg).
Results
Anti-tau antibody treatment reduces detergent/insoluble tau
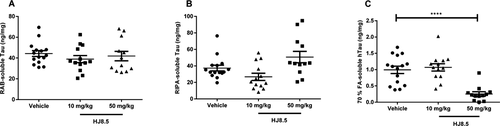
We previously reported that continuous ICV infusion of anti-tau antibody HJ8.5 into the lateral ventricle of P301S transgenic mice significantly reduces insoluble tau.23 To determine the effect of HJ8.5 on soluble and insoluble tau after IP administration, we decided to administer weekly IP injections with PBS or with HJ8.5. Before beginning this study, we decided that our prespecified primary outcome was to compare the effect of HJ8.5, given IP at 50 mg kg−1 week−1 on detergent insoluble tau, p-tau immunostaining, and specific behavioral tests. This was based on the fact that 2 days after IP administration of 50 mg/kg of HJ8.5, we were able to obtain CSF concentrations of HJ8.5 of ~1 μg/mL, similar to that obtained in our previous ICV injection study where HJ8.5 was effective at decreasing tau pathology. We also assessed a second dose of HJ8.5 at 10 mg kg−1 week−1. Treatment began in 6-month-old P301S tau transgenic mice, an age when tau accumulation becomes apparent by histopathology. After 3 months of treatment, the cortex was sequentially extracted with RAB (aqueous buffer), RIPA (detergent buffer), and finally 70% FA to solubilize the detergent insoluble final pellet. As the anti-tau antibody HJ8.5 binds only to soluble and aggregated forms of human but not mouse tau,23 we assessed the human tau in the brain lysates. P301S mice over express human tau under the control of the prion promoter at levels 5 × higher than mouse tau.24 Utilizing a quantitative human tau-specific ELISA described previously,23 we observed no difference in soluble human tau in either RAB (Fig. 1A) or RIPA (Fig. 1B) fractions.23 We further analyzed the detergent-insoluble human tau load that was solubilized by 70% FA. We found that at a dose of 50 mg/kg, HJ8.5 treatment markedly reduced detergent insoluble tau by ~75% compared to vehicle-treated mice (Fig. 1C). However, treatment with HJ8.5 at 10 mg/kg resulted in no difference in insoluble human tau compared to the vehicle-treated group (Fig. 1C).
Anti-tau antibody treatment reduces abnormally phosphorylated tau
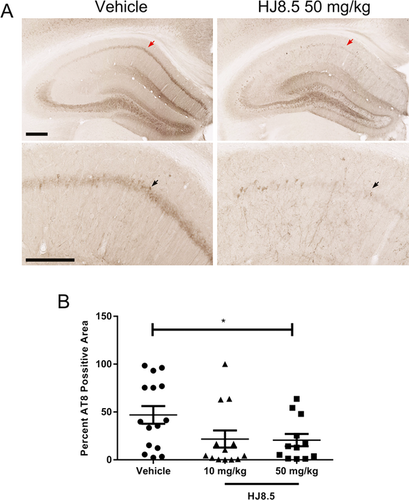
To assess the level of tau pathology in P301S mice after 3 months of treatment with HJ8.5 or vehicle, we stained brain sections with the AT8 antibody which detects the abnormally phosphorylated tau residues at Ser202 and Thr205.26 No staining of phosphorylated tau with the AT8 antibody was detected in 9-month-old wild-type mice in contrast to strong staining detected in the brain of P301S mice at 9 months of age (Fig. S1). Vehicle-treated P301S mice showed strong neuronal cell body and neuropil staining in the hippocampal CA1 cell layer (Fig. 2A). However, quantitative analysis of this region showed that treatment with HJ8.5 at 50 mg/kg reduced AT8 staining by >55% compared to the vehicle-treated group (P = 0.035) (Fig. 2A and B). Treatment with HJ8.5 at 10 mg/kg appeared to result in a similar reduction in AT8 staining but due to higher variability, the effect did not quite reach statistical significance (Fig. 2B).
Anti-tau antibody treatment reduced thio-S staining
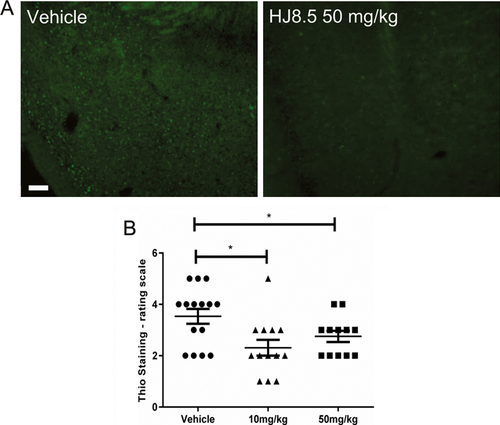
To test the effects of HJ8.5 on tau present in an amyloid conformation, we stained all the treated mice with thio-S. We semiquantitatively analyzed thio-S staining in two regions of brain of P301S mice where we could easily detect such deposits, in the piriform cortex and amygdala. Assessment of thio-S-positive staining was done in all treated animals by a blinded rater who gave a score from 1 (minimal staining) to 5 (maximal staining) as described previously.23 We found that HJ8.5-treated animals showed less thio-S-positive staining compared to vehicle-treated mice (Fig. 3A). By semiquantitative analysis of all treated mice, we observed there was a significant decrease in thio-S-positive staining in mice treated with HJ8.5 at both 10 and 50 mg/kg compared to vehicle-treated mice (Fig. 3B).
Anti-tau antibody reduces brain atrophy in P301S mice
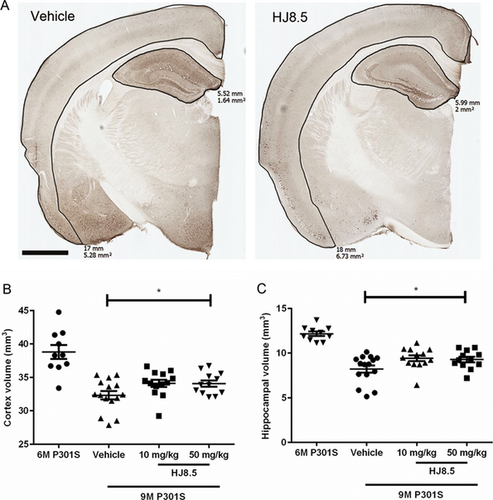
P301S mice develop not only tau aggregation but also prominent neurodegeneration. In prior characterization, it has been shown that there is significant and progressive loss of brain volume in these mice in both the hippocampus and neocortex at both 9 and 12 months of age. However, no significant difference in brain atrophy or neuronal loss was observed in these mice at 6 months of age compared to wild-type mice.24 This gave us an opportunity to determine effects of HJ8.5 not only on tau pathology but also to determine its effects on the brain atrophy observed in this model. To determine whether HJ8.5 treatment had any effect on brain volume loss, we performed a blinded stereological volumetric analysis of the cortex and hippocampus in P301S mice at 9 months of age following 3 months of treatment with HJ8.5 or PBS. In mice treated with HJ8.5 at 50 mg/kg, both the cortex and hippocampus were significantly larger than in PBS-treated animals (Fig. 4). The cortex was ~5% larger (Fig. 4B) and the hippocampus ~10% larger (Fig. 4C) in HJ8.5 versus PBS-treated mice. There was a strong trend of decreased atrophy with 10 mg/kg but it was not quite significant. To determine whether HJ8.5 treatment was preventing versus decreasing brain atrophy, we assessed the total hippocampal and cortical volumes in a separate cohort of 6-month-old P301S mice. Relative to 6-month-old P301S mice, the hippocampus was 33% and the cortex was 17% smaller in PBS-treated P301S mice at 9 months (Fig. 4). In contrast, the HJ8.5 treatment groups exhibited a ~23% smaller hippocampal and ~12% smaller cortical volume. This suggests that peripheral HJ8.5 treatment significantly attenuated but did not block brain atrophy.
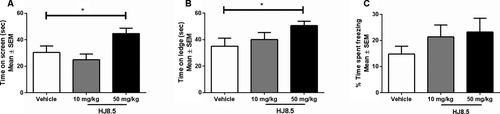
Anti-tau antibody improved motor/sensorimotor deficits in P301S mice
To determine whether HJ8.5 administration had any effect on function in P301S mice, we assessed motor coordination, balance, and strength since in some studies, motor/sensorimotor function has been shown to be impaired in these mice.24, 28 To evaluate these functions, we quantified the performance of all mice on both the inverted screen and ledge tests. Results from the inverted screen test, which requires both strength and coordination, showed that the mice treated with HJ8.5 at 50 mg/kg exhibited improved performance relative to vehicle-treated controls as evidenced by the HJ8.5-treated mice being able to hold onto the inverted screen for significantly longer periods of time (Fig. 5A). We also assessed performance of all mice on the ledge test, which requires balance, grip strength, and coordination. This test involves quantifying the amount of time a mouse can remain on a narrow Plexiglas “ledge.” Analysis of the ledge test data showed again that the mice treated with HJ8.5 at 50 mg/kg demonstrated significantly improved performance compared to the PBS-treated group (Fig. 5B). The ability of the anti-tau antibody treatments to rescue cognitive deficits in P301S mice was evaluated by assessing the performance of the mice on the conditioned fear procedure. All three treatment groups of mice exhibited similar levels of baseline freezing during the first 2 min in the conditioning chamber and there were no significant group comparisons found regarding the freezing levels observed during the tone-shock training which involved three pairings of the conditioned stimulus–unconditioned stimulus (CS-US) on day 1 (data not shown). On day 2, mice were evaluated on the contextual fear test. On this test, mice treated with HJ8.5 at both 10 and 50 mg/kg displayed increased freezing time levels compared to vehicle controls, but the amount of increased freezing was not significant (Fig. 5C).
Anti-tau antibody promotes tau aggregates uptake in a microglial cell line as well as increasing tau levels in plasma
Following peripheral administration of IgG antibodies such as HJ8.5, ~0.1–0.2% of the concentration of the antibody in the plasma is present in the extracellular space of the brain (i.e., CSF).23 Once present in the extracellular space of the CNS, such an antibody can bind to and sequester both monomeric and aggregated forms of tau which might be present. Once HJ8.5 is bound to tau, it could potentially neutralize any potential toxicity of extracellular tau that is present. It could also lead to local clearance by cells or transport out of the brain. We wanted to test potential mechanisms of how such an antibody might be promoting the clearance of different forms of tau. Previous studies with anti-Aβ and anti-α-synuclein antibodies have shown that certain antibodies are able to trigger microglial cells to clear aggregates through Fc receptor-mediated phagocytosis and subsequent peptide degradation.29-32 To examine the potential ability of anti-tau antibody HJ8.5 to initiate cellular clearance of tau aggregates, we labeled P301S tau aggregates with Alexa-Fluor-647. Labeled tau aggregates were preincubated with HJ8.5 or control antibody (HJ3.4, anti-Aβ antibody). Preincubated tau aggregates with or without antibodies were applied to primary cortical neurons as well as the microglial cell line, BV2. We observed no significant difference in tau aggregate uptake in the presence or absence of HJ8.5 versus control antibody HJ3.4 using primary cortical neurons (Fig. 6A). However, in the presence of microglial-like BV2 cells, there was an increase in tau aggregate uptake over time under all conditions with HJ8.5, resulting in significant increased uptake compared to control conditions (Fig. 6B). These results suggest that HJ8.5 can promote the clearance of extracellular tau aggregates into microglial-like cells via an Fcγ-mediated clearance mechanism.
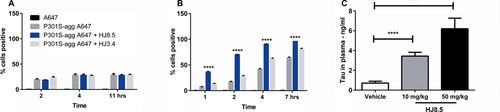
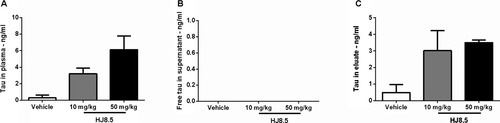
Previous studies using anti-Aβ antibodies have shown that following peripheral administration of certain antibodies, there is a large increase in plasma Aβ.33-35 To test whether an anti-tau antibody elevates plasma tau, we measured the levels of plasma tau in all treated mice by quantitative ELISA. We observed a strong, dose-dependent increase in plasma tau levels in HJ8.5-treated mice (Fig. 6C). To determine whether this increase in plasma tau is free or bound to antibody, we injected HJ8.5 or vehicle in a separate group of P301S mice. Forty-eight hours following a single peripheral IP injection of HJ8.5 (10 or 50 mg/kg) or vehicle, mice were sacrificed and their plasma was collected. We observed that the large increase in plasma tau following HJ8.5 administration is antibody bound (Fig. 7A and C). Free tau was not detected in the plasma of HJ8.5-treated mice (Fig. 7B).
Discussion
We previously found that ICV injection of certain anti-tau antibodies for a period of 3 months into P301S mice strongly reduced pathological forms of tau as assessed biochemically and by phospho-tau staining.23 Herein, we sought to determine if the antibody that was found to be most potent in the prior study would provide benefits when given peripherally. HJ8.5 was found to strongly reduce detergent insoluble tau and phospho-tau staining in the CA1 region of the hippocampus. Importantly, and to the best of our knowledge not previously reported, we found that an anti-tau antibody treatment resulted in significantly reduced brain atrophy. Motor/sensorimotor function was also improved with treatment. While the detailed mechanism of action of the antibody is not completely clear, HJ8.5 treatment resulted in a large increase in plasma tau. This increase may represent both stabilization of tau once it enters the plasma by the antibody but also enhanced brain to blood clearance. These data further support the idea that anti-tau antibodies should be further assessed as a potential treatment for tauopathies.
A number of studies have now reported that peripherally administered monoclonal anti-tau antibodies to tau can have beneficial effects. When administered to transgenic mouse models that overexpress different forms of human tau that lead to autosomal dominant forms of MAPT-related frontotemporal dementia, some of these antibodies have resulted in improved behavior20, 22 and reduced phospho-tau staining.19 Only a few have shown a reduction in detergent insoluble tau.19, 20, 22 To the best of our knowledge, the effect of HJ8.5 on reducing detergent insoluble tau by 75% is the largest effect on this parameter reported to date. We are also unaware of other reports showing that an anti-tau antibody can reduce brain atrophy. This finding is potentially of significant importance since brain atrophy in human tauopathies correlates strongly with tau accumulation and dysfunction in the brain regions affected and is a parameter that can be quantitatively assessed in clinical trials.
Our prior studies and other studies suggest that the efficacy of an anti-tau antibody might be related to the ability of such antibodies to bind to capture aggregated forms of tau in the extracellular space and block spread of tau pathology back into the same cell, adjacent cells, or even synaptically connected cells.23, 36, 37 Once an anti-tau antibody enters the brain, it could potentially affect the fate of extracellular forms of monomeric or aggregated forms of tau in different ways. We assessed two different potential fates herein.
First, we found that if aggregated tau binds to HJ8.5, it was more readily taken up by microglial-like cells in vitro. Fc-mediated uptake of anti-tau antibody-tau complexes may be one mechanism by which such a treatment could clear extracellular aggregates and decrease their toxicity or spreading. Further experiments using antibodies that lack a functioning Fc domain in vivo would be required to determine the importance of this mechanism.
Second, we found that peripheral treatment with HJ8.5 resulted in a large increase in plasma tau by ~10-fold over baseline levels. We found that most if not all of the increase in plasma tau was antibody bound. A previous study has shown an increase in plasma tau following passive immunization with an anti-oligomer-specific tau antibody.21 Why plasma tau is increased following HJ8.5 treatment is not entirely clear. There are very low amounts of tau present in human plasma38 and also low levels present in P301S mouse plasma. P301S mice over express tau predominantly in the brain so it is likely a substantial amount of plasma tau in these mice is derived from the CNS. Further studies will be required to determine the origin of plasma tau both in this mouse model and in humans. The half-life of tau in the CNS is ~10–12 days,39 however, the half-life of tau once it enters the plasma is not known. The plasma half-life of antibodies in mice is usually 1–2 weeks. It is possible that once tau enters the plasma and binds an antibody such as HJ8.5, plasma tau levels increase due to the antibody preventing rapid tau degradation as the half-life of the HJ8.5-tau antibody complex is long. Even if this is one explanation for the increase in plasma tau, it seems likely that part of this increase is due to the HJ8.5 that enters the brain, captures tau, and then exits the CNS. In fact, following injection of HJ8.5 into the CNS, we found a large amount of tau bound to HJ8.5 in the periphery.23 Since the concentration of an IgG in the CNS extracellular space is 0.1–0.2% of the concentration of IgG in plasma and IgG is constantly moving in and out of the brain, it is likely that once HJ8.5 enters the brain extracellular space, it binds to whatever tau is present (monomers and aggregates) and these complexes take on the fate of the antibody, a component of which is cleared into blood via glymphatic flow.40 It will be interesting to determine whether the concentration of plasma tau following peripheral anti-tau antibody in some way reflects CNS tau concentrations as well as the amount of tau pathology. Clearly, further experiments need to be done to sort out the origins of tau in plasma in the presence and absence of anti-tau antibodies.
While the greatest effects of HJ8.5, including effects on detergent insoluble tau, phospho-tau staining, atrophy, and behavior were seen at a dose of 50 mg/kg, some effects were also seen at 10 mg/kg. The fact that the 10 mg/kg dose had these effects without decreasing detergent insoluble tau is interesting and suggests the possibility that at somewhat lower concentrations, the antibody is able to bind to some sort of toxic extracellular species of tau and neutralize its negative functional effects. This type of mechanism was suggested by a recent study.21 While there is as yet no direct evidence for this possibility, if future methods allow for detection of extracellular species of tau oligomers in vivo, this hypothesis may be testable.
Acknowledgment
This work was funded by grants from the Tau Consortium (D. M. H. and M. I. D.), C2N Diagnostics (D. M. H. and M. I. D.), Cure Alzheimer's Fund (D. M. H.), and the JPB Foundation (D. M. H.).
Conflict of Interest
D. M. H. cofounded and is on the scientific advisory board of C2N Diagnostics. D. M. H., M. I. D., and H. J. are inventors on a submitted patent “Antibodies to Tau” that is licensed by Washington University. D. M. H. consults for Genentech, AstraZeneca, Neurophage, and Eli Lilly. Washington University and UTSW receive grants that support laboratory research of D. M. H. and M. I. D. from Janssen. M. I. D. is a cofounder of ARTA Biosciences.




