RNA ImmunoGenic Assay: Simple method for detecting immunogenicity of in vitro transcribed mRNA
Abstract
In vitro transcribed (IVT) mRNA therapies is a promising approach for the effective and safe treatment of various diseases. However, IVT-mRNA triggers immune responses due to recognition by human RNA sensors, but incorporation of chemically modified nucleosides have been shown to reduce such responses. Nonetheless, an assay reflecting the complexity of the human immune system is still needed. Here, we present a simple and fast ex vivo method called “RNA Immunogenic Assay” for measuring the immunogenicity of IVT-mRNA in human whole blood. Chemically modified and unmodified mRNA were complexed with a transfection reagent (TransIT), and co-incubated in human whole blood and specific cytokines were quantified (TNF-α, INF-α, IL-6, and IL-12p70) using ELISAs. The qPCR analysis was conducted to unveil the activation of specific immune pathway. Our findings demonstrated that the complete replacement of uridine with pseudouridine reduced TNF-α, IL-6, and IL-12p70 levels and did not elevate the expression of genes involved in immune response against RNA including IRF7/3, EIF2A, & RNASEL. In addition, we observed that the transcript length was not found to be a confounding factor in RNA immunogenicity generation and our assay provide the feasibility to dissect donor specific immune response against mRNA therapeutics.
1 INTRODUCTION
The synthetic mRNA therapeutics have enormous potential to revolutionize gene therapy.1 Despite the first clinical application in 1992, the immunogenicity and short half-life of these mRNAs have prevented its development as a therapeutic product.2, 3 The synthetic mRNA triggers immune responses due to its recognition by human RNA sensors including Toll-like receptors (TLRs), RIG-I like receptors (RLRs), and NOD-like receptors (NLRs). Though the ability of synthetic mRNA to elicit immune response is a great advantage for "vaccination therapy" against various pathogens and certain types of cancer, it is a major concern for the use as "supplementation therapy".4, 5 However, the technological developments including utilization of modified nucleosides during in vitro transcription (IVT), coding sequence optimization, and column purification have minimized the above-mentioned hurdles.6 Nevertheless, the evaluation of immunogenicity is critical to define the safety profile of mRNA therapeutics and their possible use in human.
During the last decades, few methods have been developed to study immunogenicity of IVT-mRNA and rely on immune cells such as peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs) isolated from human whole blood.7-9 These cells were in most cases co-incubated with IVT-mRNA for overnight and followed by cytokines measurements. Though these methods mimic individual human immune systems to an extent, they associated with laborious ficoll isolation and DCs enrichment.7, 8 In addition, the isolation technique was observed to activate some of the innate immune cells and lead to cytokine induction without any stimulus.10 The THP-1 assay was used to measure the immunogenicity of IVT-mRNA, where genetically alerted THP-1 monocyte cells secrete luciferase upon the interferon induction.11 Unlike earlier methods, the THP-1 assay is an artificial cell line system and it does not imitate the complexity of human immune cells. Alternatively, mouse models were utilized to assess the immunogenicity of IVT-mRNA, but considerable difference in recognizing Uridine-rich RNA molecules was observed between mice and humans.12-15 Using primate models to measure immunogenicity would be closer to the human immune system, but using primates in research is ethically and economically difficult. Therefore, a simple method that is comparable to the human immune system and provides quick immunogenicity results is desirable.
A large body of experimental work established that the human whole blood assay (WBA) is being used in clinical research since three decades to screen antigen reactivity, vaccine formulations, immunogenicity measurement, and several others.16, 17 In addition, WBA is been commercialized to predict Cytokine Release Syndrome upon specific monoclonal antibody therapeutics by ProImmune Ltd., (www.proimmune.com). Here, we present an adapted version of WBA as an “RNA ImmunoGenic Assay” by way of a simple and fast method to measure the immunogenicity of IVT-mRNA as the assay does not require any cell isolation procedure. The assay utilizes human whole blood and it provides the most physiologically relevant immune cell systems, especially platelets, and white and red blood cells (RBCs) in an easily accessible ex vivo context. Recent studies have explored that the RBCs and platelets are involved in cytokine production and regulation upon activation.18, 19 Thus, in addition to immune cells, other blood components could play a major role in IVT-mRNA mediated inflammation. Unlike other methods, the RNA ImmunoGenic Assay account for other cellular constituents and is more comparable to the innate human immune system.
2 MATERIALS AND METHODS
2.1 Ethics statement
Human blood samples were collected from healthy volunteers and two adult cystic fibrosis patients. Written informed consent was obtained from all participants. Ethical approval was obtained from institutional review board, Children's University Hospital Tuebingen, Germany (No: 349/2013BO2 and 829/2016BO2).
2.2 IVT mRNA production
The open reading frame of DsRed, hCFTR, SpCas9 were amplified from their original plasmids and sub-cloned into pVAX1.A120 vector (www.lifetechnologies.com). The plasmids were linearized with XhoI and served as the template for IVT-mRNA using T7 RNA polymerase in MEGAscript T7 kit (www.ambion.com). All mRNA were produced with an anti-reverse CAP analog (ARCA; [m7G(5’)G]) in the 5′ end (www.trilink.com). The IVT-mRNA were made with following chemical modifications in the indicated ratios: 100% Pseudo-UTP, 100% N1-methylpseudo-UTP, 25% s2-thio-UTP/5-methyl-CTP, and 100% N1-methylpseudo-UTP/100% 5-methyl-CTP (www.trilink.com). All IVT mRNAs were purified using the MEGAclear kit (www.ambion.com) and analyzed for quality and concentration using an Agilent 2100 Bioanalyzer (www.agilent.com).
2.3 Preparation of bacterial total RNA
E. coli bacterial cell pellet was obtained from overnight culture and homogenized in a Precellys Evolution Homogenizer at 5000 rpm for 20 seconds using 0.5 mm glass beads. Total RNA was isolated using the Precellys Bacterial/Fungal RNA Kit following manufacturer instructions.
2.4 Blood collection and ex vivo stimulation
Peripheral blood was collected from healthy adult volunteers in EDTA containing vacutainer tubes. All blood samples were processed immediately after collection without delay. Immunogenicity of IVT-mRNA was assessed in the undiluted whole blood. We utilized both chemically modified and unmodified mRNA to validate our assay. The 15 µg of IVT-mRNA with or without carrier molecule added to 2 mL of human whole blood in 12-well plate and were gently mixed by pipetting and incubated for 6-24 hour at 37°C in a humidified incubator containing 5% CO2. R848 (TLR 7/8 agonist) and total bacterial RNA were utilized for the positive ex vivo immune stimulation. The 15 µg of (un-)modified IVT-mRNA were complexed to 15 µL of TransIT and 15 µL of boost reagent (www.Mirus-Bio.com). The complete detail of chitosan-coated PLGA nanoparticles [Chitosan (83% deacetylated (Protasan UP CL 113) coated PLGA (poly-D,L-lactide-co-glycolide 75:25 (Resomer RG 752H)) nanoparticles can be found in our previous works.20 We complexed 15 µg of IVT-mRNA with CS-PLGA nanopartilces. Post 6-hour, suspension was gently mixed and 1 mL of whole blood removed from each well. The samples were centrifuged at 2000 RPM for 10 minutes and blood plasma was collected. After 24 hours of incubation, plasma was collected from remaining samples by centrifugation. During the establishment step, we tried four different concentrations (15 µg) of IVT-mRNA and five different (0, 1, 3, 6, and 24 hour) incubation times.
2.5 Measurement of cytokines by ELISA
The blood plasma levels of TNF-α, INF-α, INF-γ, IL-6, and IL-12p70 were measured using commercially available ELISA kit (www.eBiosciences.com) following the manufacturer‘s instructions. Cytokine levels were measured by calculating unknown values from standard curve using the Michaelis–Menten model in the Graphpad Prism v.6.0d software (www.graphpad.com).
2.5.1 Real-time RT-PCR
The 15 µg of IVT-mRNA with carrier molecule (Trans IT) were added to 2 mL of human whole blood in 12-well plate and were incubated for 24 h at 37°C in a humidified incubator containing 5% CO2. The Total RNA were isolated at 24 hour by PAXgene Blood RNA kit (www. Qiagen.com). Reverse transcription of 200 ng RNA was carried out using an iScript cDNA synthesis kit (www.bio-rad.com). The cDNA was diluted 1:20 dilution for further experiment. Detection of mRNADsRED was performed by SYBR-Green based quantitative Real-time PCR in 15 μl reactions on a ViiA7 (www.lifetechnologies.com). In all involved procedures MIQE protocols was followed for RealTime experiments.21 Reactions were incubated for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 2 min at 50°C (annealing and extension), followed by standard melting curve analysis. The following primer pairs were used: DsRed fwd 5′-GCCCCGTAATGCAGAAGAAG-3′, rev 5′- GTGTAGTCCTCGTTGTGGGA-3′; IRF7 fwd 5′- CCACGCTATACCATCTACCTGG −3′, rev 5′-GCTGCTATCCAGGGAAGACACA −3′; IRF3 fwd 5′-TCTGCCCTCAACCGCAAAGAAG-3′, rev 5′-TACTGCCTCCACCATTGGTGTC-3′;EIF2A fwd 5′- CTGGACCTCATGCAGCTTTAGC-3′, rev 5′-CTCCATAGTAGGAAGCTCCTGTC-3′; RNASEL fwd 5′-AAGGCTGTTCAAGAACTACACTTG-3′, rev 5′-TGGATCTCCAGCCCACTTGATG-3′; IL-6 fwd 5′-AGACAGCCACTCACCTCTTCAG-3′, rev 5′-TTCTGCCAGTGCCTCTTTGCTG-3′; TNF-alpha fwd 5′-AGACAGCCACTCACCTCTTCAG-3; rev 5′-TTCTGCCAGTGCCTCTTTGCTG-3′
18S rRNA fwd 5′-GGGAGCCTGAGAAACGGC-3′, rev 5′-GACTTGCCCTCCAATGGATCC-3′.
2.5.2 Flow cytometry analyses
All flow cytometry analyses were performed using a BD LSR Fortessa X-20 (www.bdbioscience.com). For detection of DsRed protein, the whole blood was treated with 15 µg DsRed-mRNA and 15 µl TransIT carrier. The blood had been collected after 24 hours and subsequently treated with erythrocyte lysis buffer (www.c.c.pro GmbH) to remove red blood cells. At the minimum 50,000 gated cells per tube were counted. Data were analyzed with BD FACSDivaTM software.
2.6 Statistics
Mann-Whitney U rank sum tests and Kruskal-Wallis (one-way ANOVA) were applied to analyze differences in cytokine levels between the IVT-mRNA modifications. The similarity in several cytokine expressions in same experimental set-up was tested by the correlation analyses between different cytokines levels by non-parametric Spearman ́s rank coefficient tested as implemented in Graphpad Prism v.6.0d (www.graphpad.com).
3 RESULTS
3.1 Optimization of “RNA ImmunoGenic Assay”
As a first step to establish the RNA ImmunoGenic Assay, we tested the immune stimulation of 10 µg of non-complexed IVT-mRNA and complexed IVT-mRNA with either chitosan-coated PLGA nanoparticles or TransIT (a transfection reagent). The human whole blood, derived from three different healthy donors, was co-incubated with above specified settings where bacterial total RNA and R848 were used as a positive control. Blood incubated with just PLGA nanoparticle or TransIT were used as mock control along with untreated blood as negative control. After 24 hours of incubation, the blood plasma was collected and analyzed for TNF-α, IFN-α, and, IL-12p70. The induction of TNF-α response was observed against bacterial total RNA and R848 in all the three-tested transfection methods (Figure 1A). Significant difference between IVT-mRNAs were observed only in TransIT group for TNF-α and IL-12p70 expression between unmodified DsRed mRNA (mRNADsRed) and DsRed mRNA modified with 100% Ψ-pseudo uridine (cmRNADsRed Ψ1.0; P < .05; Figure 1A and B). However, no difference was noted in the non-complexed naked IVT-mRNA treated group and IVT-mRNA complexed to PLGA nanoparticles. A very low level of IFN-α response against unmodified DsRed mRNA with higher donor-variability was observed. No stimulation was detected with bacterial total RNA but R848 produced a high expression of IFN-α (Figure S1A). No detectable IFN-γ was determined (data not shown). The correlation analysis of TNF-α with IL-12p70 resulted in positive correlation (Spearman's rho coefficient: ρ = 0.49, P = .04; Figure S1B).
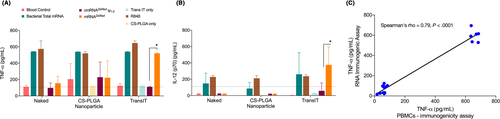
Since TransIT complexed IVT-mRNA yielded measurable immunogenicity outcomes for unmodified IVT-mRNA, we performed a dose escalation study to finalize the concentration IVT-mRNA needed for RNA ImmunoGenic Assay. We have co-incubated four increasing concentrations of TransIT-complexed unmodified DsRed mRNA (10 µg, 15 µg, 20 µg and 25 µg) with the human whole blood of three healthy donors. The concentration of 15 µg of unmodified IVT-mRNA had been determined to sufficiently induce the immune response similar to that of bacterial total RNA (Figure S1C). Next, we compared the RNA ImmunoGenic Assay with PBMCs based immunogenicity assay from the same donors. As we decided to reduce the incubation time, we measured the TNF-α response to IVT-mRNA at 6 hours’ time point in addition to 24 hours for both assays. The induction of TNF-α to mRNADsRed (unmodified) was seen in both assays. However, the concentration of TNF-α were relatively higher for mRNADsRed in PBMCs compared to the RNA ImmunoGenic Assay at post 6-hour incubation, but not significantly higher (P = .1; Figure S2). Nonetheless, the observation was not seen at 24-hour measurement (Figure S2). The comparison of IVT-mRNA mediated immunogenicity between the RNA ImmunoGenic Assay vs PBMCs based immunogenicity assay revealed better correlation (Spearman's rho, ρ = 0.79, P < .0001; Figure 1C) without significant difference (P = .1) and suggested the reliability of the RNA Immunogenic Assay.
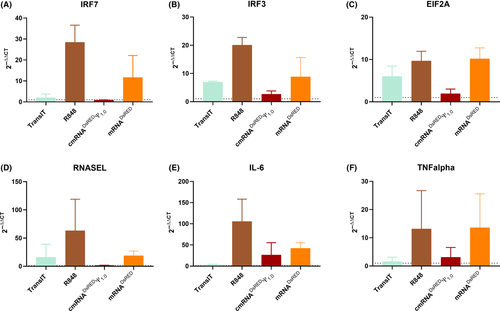
As our results were relatively similar between 6-hour and 24-hour time points, we aimed to test other lower time points, as it might aid to reduce the total duration of the RNA immunogenic Assay. Therefore, we performed an experiment to measure kinetics of cytokine production where human whole blood was stimulated with TransIT R848, mRNADsRed, and cmRNADsRed Ψ1.0. Blood plasma was collected at 0-hour, 1-hour, 3-hour, 6-hour, and 24-hour intervals. Both TNF-α and IL-6 were measured at all time points. Our data demonstrates that a minimum of 6 hours of incubation is essential to observe reliable immunogenicity outcomes for TNF-α (Figure S3). However, for IL-6 a measurement at 24 hours was observed to be optimal (Figure S3). As we noted RNA immunogenicity in our assay, we sought to explore specific immune signaling pathways activated due to IVT-mRNA(s). To accomplish this objective we selected important reported genes to represent different immune signaling pathways which activated by mRNA therapeutics.1 As interferon response is the central part of immune response of against RNA, we performed qPCR analysis on relevant targets. We observed that the expression of interferon stimulated genes including both Interferon regulatory factor 7 (IRF7) and Interferon regulatory factor 3 (IRF3) was elevated in unmodified mRNA compared to modified and TransIT only control (Figure 2A and B). Likewise, our experiments on eukaryotic initiation factor 2A (eIF2A) which is responsible for stalled mRNA translation showed minimal expression for cmRNADsRed Ψ1.0 compared to mRNADsRED, and R848 (Figure 2C). Similar results were noted for other genes involved in RNA degradation and inflammation such as RNASEL, IL-6 and TNF-α where modified mRNA exhibited reduced expression compared to unmodified and R848 counterparts (Figure 2D and F).
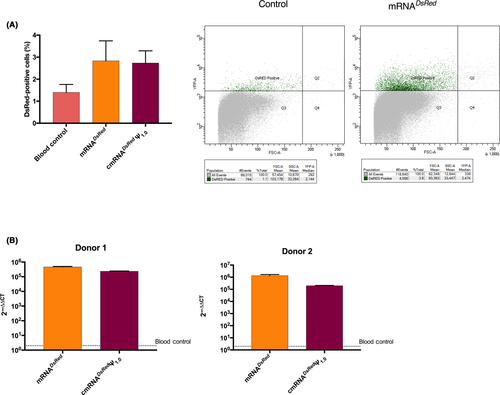
Next, we aimed to assess transfection ability of TransIT to the cells in whole blood matrix as the environment known to be rich in nucleases that potentially degrade mRNA rapidly. Therefore, we isolated PBMC after erythrocyte lysis and performed flow cytometry analysis and found that only 3%-4% of the total PBMCs exhibited DsRed expression (Figure 3A). However, our qPCR analysis in two different donors showed that compared to the mock control, both unmodified and modified mRNA complexed with TransIT lead to up to million fold higher presence of DsRed RNA transcripts and signify the accumulation of DsRed mRNA in blood immune cells and transfectability of TransIT (Figure 3B).
3.2 “RNA ImmunoGenic Assay”: the effect of transcript length and modifications
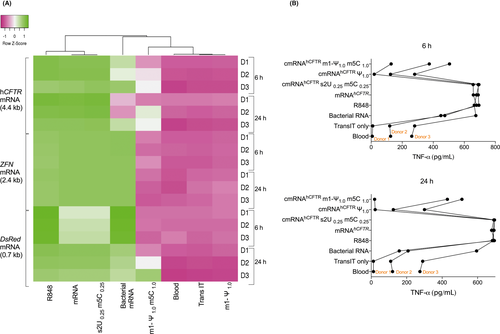
Since the Uridine base of RNA sequence is known to activate most of the RNA sensors including TLRs, NLRs, and RLRs, we tested the immunogenicity of three different transcript length RNA molecules with and without U-base nucleoside modifications [100% Ψ-pseudouridine (Ψ1.0), 100% N1-Methyl-Ψ-pseudouridine/5-Methylcytidine (m1-Ψ1.0/m5C1.0) and 25% 2-thio Uridine/5-Methylcytidine (s2U0.25/m5C0.25)]. In an independent experiment using whole blood from three different donors, we utilized DsRed mRNA (0.7kb), zinc finger nuclease (ZFN) mRNA (2.4kb), human cystic fibrosis transmembrane, and conductance regulator (hCFTR) mRNA (4.4kb) to assess the immune response. In all these experiments, we used Resiquimod (R848; TLR7/8 agonist) as a positive control in addition to bacterial total RNA to induce immune responses similar to IVT-mRNA.
Similar to R848/bacterial total RNA, all the unmodified mRNA transcripts induced strong TNF-α secretion the same as (Figure 4A). Likewise, Ψ1.0 modifications completely abrogate the immune response in terms of TNF-α expression in all three three-tested mRNA molecules (Figure 4A). We observed that m1-Ψ1.0/m5C1.0 significantly reduced the immunogenicity compared to its unmodified counterparts, however, in the case of hCFTR mRNA we observed increased immune response in two of the three donors (6-hour and 24-hour, respectively; P < .05). Hierarchical cluster analysis of TNF-α induction revealed two distinct clusters for the investigated IVT-mRNAs. The TNF-α induction of modified IVT-mRNA with Ψ1.0, and m1-Ψ1.0/m5C1.0 clustered together with blood/TransIT controls due to their low immunogenicity. However, unmodified and s2U0.25/m5C0.25 IVT-mRNA clustered together with R848 due to the high level of immunogenicity. Conversely, the partially modified IVT-mRNA with s2U0.25/m5C0.25 stimulated moderate to high level of immune response. We observed similar pattern for other investigated cytokines IL-6 and IL-12p70 (data not shown). Interestingly, we observed donor specific immunogenicity in terms of TNF-α expression against hCFTR mRNA and positive controls (Figure 4B). Of note, we observed that the same donor exhibited similar immunogenicity profiles for IVT-mRNA when analyzed on different days (data not shown).
3.3 “RNA ImmunoGenic Assay” for clinical use: analysis of cystic fibrosis patient's blood
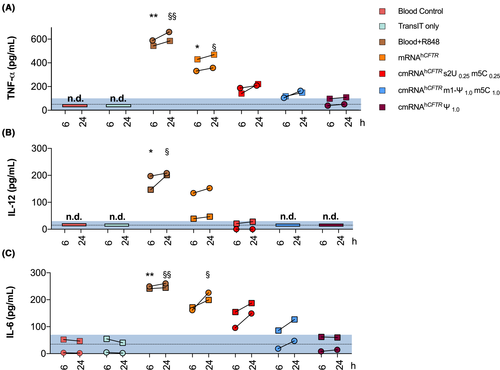
Next, we sought to validate our assay in clinical samples to show that the RNA ImmunoGenic Assay can be used in clinical settings. Therefore, we collected whole blood from two adult CF patients possessing the CFTR.262_263delTT mutation and co-incubated it together with Trans-IT complexed to hCFTR IVT-mRNA. We utilized three different chemical modification and compared them with unmodified hCFTR mRNA. Interestingly, the negative control groups (Blood-only and TransIT-only) were undetectable for TNF-α, and IL-12p70 (Figure 5), while IL-6 were already measurable in human blood untreated or treated only with Trans IT for one CF patient. The positive control (R848) primed a strong and significant production of cytokines (TNF-α, IL-12p70, and IL-6; 6 h and 24 h, respectively; P < .01 calculated by Kruskal-Wallis H test; Figure 5). Unmodified hCFTR mRNA caused a significant upsurge of TNF-α at 6 h and 24 (P < .05), and only a substantial increase in IL-6 at 24 hours (P < .05). No significant cytokine induction was observed in all the three examined hCFTR mRNA with modifications (s2U0.25/m5C0.25, Ψ1.0, and m1-Ψ1.0/m5C1.0). However, we observed increased IL-6 production for hCFTR mRNA with s2U0.25/m5C0.25 modification, but not statistically significant compared to the blood control (P = .07).
4 DISCUSSION
The mRNA-based therapeutics are proposed as a new class of drugs 1 which need to be evaluated for efficacy and safety. In the case of mRNA drugs, the immunogenicity is a major concern and was perfected through rational technological improvements.22 Moreover, a safety evaluation to choose immune-compatible IVT-mRNA is essential for their clinical translation. However, a method to estimate the unwanted immunogenicity of IVT-mRNA that closely resembles the in vivo situation still does not exists. The current in vitro methods and animal studies are laborious and cumbersome to perform as discussed earlier. Therefore, in this current study we aimed to establish a simple and fast method to detect immunogenicity of IVT-mRNA. First, we decided to overcome the hurdles associated with PBMCs isolation and DCs enrichment. Therefore, we opted to use whole blood as a sample to test immunogenicity of IVT-mRNA. Encouragingly, earlier studies had used whole blood to detect immunogenicity of therapeutic oligonucleotides, siRNA, and monoclonal antibodies.23-26 Hence, we co-incubated naked and complexed IVT-mRNA with and without modification in human whole blood and found that TransIT complexed IVT-mRNA stimulated immune response while naked IVT-mRNA and CS-PLGA NPs complexed IVT-mRNA did not. It is expected that naked IVT-mRNA failed to transfect the leucocytes and will not result in immune stimulation.
Our attempt to unveil the specific immune pathway activation due to IVT mRNA using qPCR analysis on selected targets showed a useful information.1 As the interferon response is primarily an immune response towards any RNA of exogenous origin, our investigation on both IRF3 and IRF7 lead to elevated level of transcripts in treatment with TLR agonist (R848) and unmodified mRNA whereas modified mRNA levels were low as reported earlier.1, 7, 11 IRF7 and IRF3 mediate distinctive responses by activating Interferon which is associated with inflammation and inhibition of mRNA replication.27 In our investigation on Protein kinase R (PKR) pathway to see if EIF2A inhibits the translation of mRNA, we detected elevated expressions of EIF2A by unmodified mRNA and control R848.28 Later, we wanted to identify if expressions of previously mentioned signaling molecules were interrelated to RNA degradation by the 2′-5′-oligoadenylate synthetase (OAS)/RNase L pathway.29 Here, we noticed that the RNASEL were expressed relatively higher in unmodified mRNA treated sample compared to modified version. This indicates that TransIT complexed cmRNADsRed Ψ1.0 does not activate the (OAS)/RNase L pathway. Next, we analyzed the IL-6 and TNF-α expression level and noticed a higher expression in mRNADsRed when compared with cmRNADsRed Ψ1.0. Moreover, it suggests that mRNADsRed compared to cmRNADsRed Ψ1.0 is amplifying inflammatory response through accumulating IL-6 and TNF-alpha which is strictly regulated by NF-κB pathway.30, 31 In brief, our data demonstrate that chemical modification of IVT-mRNA reduce the inflammation (IL-6 and TNF-α), avoid the inhibition of exogenous RNA translation (eIF2A) and reduces exogenous RNA degradation (RNASEL) enhance stability in various modes mentioned above.
Although our flow cytometry analysis showed low level of DsRed protein expression with TransIT complex, qPCR data clearly provide an indication that TransIT was capable of transporting mRNA into blood cells. We observed that both chemically modified or unmodified mRNA were accumulated in high abundance in two separate donors compare to the control samples. The mRNA entering the cell in this procedure make the whole blood assay a viable technique to check TLR (3,7 and 8), RIG-1, and MDA5 mediated immune responses.32 However, we did not clearly understand the mechanism of reduced DsRed protein expression despite of the presence of magnitude of mRNA transcripts in whole blood cells. We presume that the low level of DsRed mRNA expression in whole blood cells might be due to the abundance of antigen presenting cells (monocytes, dendritic cells, and B-cells) among the blood cells and its tendency of processing of foreign protein to antigen presentation rather than expression or variation in two different readouts (flow cytometry vs qPCR).
The effect of Ψ1.0 modification to reduce immune responses has been reported several times including in our own investigation with Cas9 mRNA using the RNA ImmunoGenic Assay.7, 11, 33 However, the induction of IFN-α was negligible in all settings even for bacterial total RNA. We assume that the effect is through monocyte-mediated inhibition of IFN-α in the presence of leukocytes as proposed.34 Though we observed the transfection of CS-PLGA NPs in murine lung cells,20, 35 we did not observe any immune stimulation in CS-PLGA NPs complexed to IVT-mRNA in our assay, and we speculate that it is possibly due to cell specific penetration of tested NPs. Unexpectedly, we observed moderate levels of immune response against CS-PLGA NPs. Thus, our RNA ImmunoGenic Assay can be extended to preselect immune compatible delivery molecules. Importantly, we performed a comparative study where the RNA ImmunoGenic Assay was compared head-to-head with a PBMCs based immunogenicity assay. Notably, correlation analysis showed that the results of both assays were similar with high coherence values implying the reliability of “RNA ImmunoGenic Assay” using whole blood at the same time without any cell isolation.
After successful establishment of RNA ImmunoGenic Assay, we examined the immunogenicity of U-base and the effect of its modifications along with three different transcript lengths. Our results suggesting that Ψ1.0 modification leads to complete absence of immune response while m1-Ψ1.0/m5C1.0 modified IVT-mRNA in most of the cases reduced immune responses except for hCFTR mRNA. However, earlier studies have bi-parted opinions about Pseudouridine modifications. Mouse models and mouse macrophage cell lines showed that Pseudouridine modification does not have any significant advantage in mitigating immunogenicity including TNF-α and IL-6 expression over unmodified mRNA.36, 37 On the other hand, various publication found Uridine depletion by sequence engineering or by chemical modification by Pseudouridine can reduce immunogenicity significantly.11, 38, 39 In fact, we observed similar results where Ψ1.0 modified Cas9 mRNA resulted in high interferon response in THP-1 assay but not in RNA ImmunoGenic Assay. We speculate that the presence of all blood cells in RNA ImmunoGenic Assay leads to different results than assay with monocytes alone (THP-1/RAW 264.7). However, with our assay we noticed the profound effect of Pseudouridine modification in alleviating immune response. Moreover, earlier we observed low levels of immune response against unmodified mRNA (Cas9 and hCFTR) in C57BL6/N mouse model,11, 20 but high levels of immune response in BALB/c mouse model.9 This demands deep investigation to understand why the immunogenicity levels vary in different mouse models. Unlike the mouse model, our assay exhibited similar immune modulatory profiles against specific IVT-mRNA advocated for the consistency of the RNA ImmunoGenic Assay. Therefore, we surmise that our observations within the ex vivo system is more reliable than information from mouse system.12, 13 Our results did not indicate any effect of transcript length against immunogenicity as all three variable sizes leads to similar levels of cytokine secretion. Interestingly, our cytokine kinetics study revealed that minimum 6 hours of incubation is essential for TNF-α whereas 24 hours is needed for a IL-6 mediated immunogenicity measurement. As we noted great correlation between TNF-α and IL-6 we propose that, we can use TNF-α expression as a surrogate for IL-6 induction. Therefore, the concept of reducing the incubation time to 6 hours can be achieved.
Unlike the DC’s based immunogenicity assay, the induction of IL-12p70 was not prominent in the whole blood based RNA ImmunoGenic Assay.38 Interestingly, we observed donor specific immune response against hCFTR mRNA. These data indicate that the RNA ImmunoGenic Assay can be used in personalized therapy where a patient's blood can be tested ex vivo as a preclinical safety measurement for immunogenicity prior to the administration of mRNA therapeutics. In this aspect, we tested the utilization of RNA ImmunoGenic Assay with the clinical samples from the cystic fibrosis (CF) patients. CF is the most common life-limiting genetic disease among Caucasians with limited treatment options. We earlier demonstrated that the transcript replacement therapy using hCFTR mRNA could restore the lung function in a CF-mouse model.20 As a preclinical proof-of-concept to test the safety, we co-incubated TransIT complexed modified and unmodified hCFTR mRNA and measured the immunogenicity in the two CF patients. Our results successfully demonstrated that the all the modified hCFTR mRNA resulted in reduced immune response compared to unmodified counterpart and presented the possible clinical compatibility of our established RNA ImmunoGenic Assay.
Together, the presented RNA ImmunoGenic Assay is easy to complete, fast to perform, and provides measurable immunogenicity results to be used in a clinical setting. Since the assay requires small volume of whole blood (<10 mL), it is suitable for pediatric patients and other patients with severe illnesses. As the assay uses only the blood plasma, the leukocytes can be collected for expression studies after RBCs lysis treatment. Nevertheless, our assay has some limitations as it failed to efficiently detect some key cytokines related with immunogenicity against IVT-mRNA including IFN-α and INF-γ.9 As neutrophils dominate the leucocytes in whole blood cells, the observed cytokine response might be skewed toward neutrophils.36 Neutrophils and neutrophil-mediated cytokine response was observed after the intravenous injection of IVT-mRNA-CS-PLGA in mice. We assume that the evaluation of our assay in blood of several donors and different disease patients would provide broader overview and subsequent adaptation to clinical use. To conclude, the present RNA ImmunoGenic Assay could offer simple and potential benefits to preselect immune compatible IVT-mRNA for clinical use.
ACKNOWLEDGMENTS
We thank voluntary blood donors for our study and Melanie Perreault for proof reading as a native English Speaker.
CONFLICT OF INTEREST
MSDK., holds a patent on RNA modification that licensed to the biopharmaceutical company, Ethris GmbH (EP2459231B1), and listed as main inventor on a patent application related to Nuclease encoding modified mRNA. MSDK., AH., and JSA hold a European patent on delivery of chemically modified mRNA complexed with nanoparticles to treat pulmonary diseases (EP3398963A1).
AUTHORS' CONTRIBUTIONS
Conceived and designed the experiments: JSA, and MSDK. Performed the experiments: JSA, AH, SB, and PW. Analyzed the data: JSA, and AH. Contributed reagents/materials/analysis tools: MSDK, MM, and RH. Wrote the paper: JSA, AH, and MSDK.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article [and its supplementary information files].