Important aspects of T-cell collection by apheresis for manufacturing chimeric antigen receptor T cells
Abstract
Chimeric antigen receptor (CAR)-T cells have proven to be an effective cancer therapy for CD19-expressing neoplasms. Efforts to optimize the manufacturing process can help to ensure the efficacy and safety of the therapy. Peripheral blood T cells, which serve as the source material, are collected by apheresis. However, the majority of apheresis collection protocols do not selectively collect T cells, but rather isolate the mononuclear cell (MNC) layer, which also contains other mononuclear leukocytes present in the peripheral blood. Since currently CAR-T cells are autologous, the patient's clinical condition is a major factor in the planning, coordination, and execution of the apheresis procedure to subsequently manufacture the product successfully. Efforts have been made to identify predictors of a successful collection (ie, precollection peripheral blood CD3+ count). The characteristics of the source material influences the manufacturing process directly, and therefore affects the quantity, viability, immune cell composition, and T cell phenotypic makeup of the final product. Here we review the CAR-T cell manufacturing process from the apheresis perspective, highlighting considerations before, during, and after collection that could potentially alter the outcome of the manufacturing process and ultimately, the safety and efficacy of the therapy for the patient.
1 INTRODUCTION
Chimeric antigen receptor (CAR)-T cells are genetically modified T cells manufactured to express a molecule that combines the antigen specificity of B cells with the cytotoxic function of T cells. The chimeric antigen receptor contains a single chain variable fragment directed toward a specific antigen, and the CD3 signaling and cosignaling domains of the T cell receptor complex. This combination in one cell surface protein bestows antigen-specific recognition function of an antibody paired with the killing function of a cytotoxic T lymphocyte to any T cell into which it is introduced.1 The clinical success and recent FDA approval of two CAR-T cell products have propelled the field of tumor adoptive immunotherapy forward. However, optimization of the CAR-T cell manufacturing process is ongoing.
Upstream to the manufacturing process is the acquisition of source material, peripheral blood T cells, which is accomplished by apheresis. Given CAR-T cells are autologous, the patient's clinical condition is a major factor in the collection. This review highlights considerations before, during, and after apheresis collection that could potentially impact the success of the manufacturing process and ultimately, the safety and efficacy of CAR-T therapy for the patient (Table 1).
| Timing | General considerations | Specific considerations |
|---|---|---|
| Pre-apheresis | Patient general health | Patient tolerance of apheresis collection |
| Ability to tolerate turnaround time for CAR-T cell manufacturing | ||
| Patient diagnosis | Clinical indication for CAR-T cells | |
| Predetermined requirements | FDA-approved therapy or clinical study criteria | |
| Patient access | Peripheral or central venous access | |
| Laboratory parameters | Precollection CD3+ count may predict if collection will be adequate/volume to process | |
| CBC to determine need for transfusion/device priming | ||
| Active peripheral disease may compromise collection | ||
| Prior therapies | Time of last chemotherapy/immunotherapy should be considered for scheduling collection | |
| Scheduling | Patient geography | |
| Time slot availability for CAR-T cell manufacturers and apheresis collections | ||
| Peri-apheresis | Total blood volume | Processing volume determination |
| Extracorporeal volume | Determination of need for priming apheresis machine | |
| Collection goals | Affects processing volume and time spent on apheresis machine | |
| Anticoagulant used | ACD-A or heparin | |
| Anticipated hypocalcemia | Calcium supplementation | |
| Collection efficiency | Specific to institution, apheresis machine, software, operator | |
| Post-apheresis | Product contents | Determination of need for T-cell selection or lymphocyte enrichment by elutriation |
| Cellular composition | Potential to affect manufacturing success | |
| CAR genetic material | Potential to affect transduction efficiency | |
| Manufacturing techniques | Media used, system used (ie, automation) | |
| Manufacturing times | Potential to affect individual turnaround time and overall manufacturing throughput |
2 CONSIDERATIONS BEFORE LEUKAPHERESIS
2.1 Clinical indications
Clinical CAR-T cell trials have mostly focused on CD19-expressing neoplasms, a cell surface antigen expressed on B-cell precursors, mature B cells, and various B-cell malignancies,2-12 with impressive responses in up to 90% of patients with relapsed/refractory acute lymphoblastic leukemia (B-ALL) reported in some studies.7, 8 Despite potential serious adverse events, such as cytokine release syndrome and potential deficit in humoral immunity, the remarkable response rates have led to FDA approval of two CD19-targeted CAR-T cell products, Tisagenlecleucel (KYMRIAH) for B-ALL, and later for diffuse large B-cell lymphoma (DLBCL), and Axicabtagene ciloleucel (YESCARTA, Kite Pharma) for DLBCL.13-16 Although expensive and lacking in long-term data, with base prices of approximately $475 000 and $373 000, respectively, both Tisagenlecleucel and Axicabtagene ciloleucel would be considered cost-effective long-term therapies, if successful.17, 18
CAR-T cell therapies have not yet produced the same levels of success in patients with solid tumors. On-target/off-tumor toxicities, such as those observed in clinical trials for renal cell carcinoma (targeting CAIX),19, 20 colorectal cancer (targeting ERBB2),21 and gastrointestinal tumors (targeting CEACAM5),22 demonstrate the difficulty in identifying unique tumor antigens that are either not present on healthy tissue or if present, do not result in serious adverse events.23 In the case of CAR-T cells for B-ALL, on-target/off-tumor toxicity manifests as B-cell aplasia, which is medically manageable. Antigenic targets that displayed a better toxicity profile, such as HER-2 in sarcoma,24 mesothelin in pancreatic carcinoma,25-27 CEA in adenocarcinoma gastrointestinal origin and metastatic to liver,28 EGFRvIII in glioblastoma multiforme,29 and α-folate receptor in ovarian carcinoma,30 have thus far resulted in limited therapeutic benefit. Targeting and penetration of CAR-T cells into solid tumors, as well as their persistence and expansion in vivo remain hurdles that need to be overcome for successful therapy.31
2.2 T-cell collection by leukapheresis
The first step of CAR-T cell production is leukapheresis for collection of peripheral blood MNCs, from which T cells are isolated. The collected MNCs are prepared and, in most cases, transported to a facility for genetic modification and cell expansion. Then, the CAR-T cell product is returned to the clinical facility for infusion into the patient. Precise coordination by a multidisciplinary team, before, during, and after product administration, is essential. For development and implementation of CAR-T cell therapies various manufacturing and logistical aspects warrant considerations, in addition to clinical challenges. Specifically, the nonuniform nature of source material, partially due to the unique disease and treatment history of each patient, variability in apheresis machines/operation and institutional policies, and delivery of cellular products to and from manufacturing sites are some of the potential difficulties that could be encountered.
2.3 Clinical considerations
CAR-T cell collections are not a standardized process. The optimal pre-apheresis parameters both for maximizing overall cell collection efficiency (CE) and obtaining the optimal product composition are not clearly defined. CAR T-manufacturing failure rates, largely due to not reaching the target T-cell dose, have been reported to range from 1% to 7% in chronic lymphocytic leukemia (CLL)10 and up to 14% in lymphomas.32 The correlation between precollection peripheral blood CD3+ T-cell counts and manufacturing success has led some centers to set absolute lymphocyte count (ALC) thresholds for collection. Variation in approaches has been reported, including minimum ALC of 100 to 500 cells/µL, and in some cases an additional minimum CD3+ count of 150 cells/µL, prior to collection.33, 34
In addition to T-cell number, the immune cell composition of the starting material as well as the T-cell repertoire can affect final product characteristics. Allen et al35 reported that starting product variability influenced CAR-T cell product manufacturing outcomes in patients with solid and hematologic malignancies. MNC collection does not exclude other “contaminants” (ie, blasts, immune cells with suppressive functions) in the layer collected that could be detrimental to the manufacturing process. Myeloid cell contamination has been reported to inhibit CD19- and GD2-CAR-T cell expansion, while monocyte depletion by plastic adherence has been demonstrated to rescue poor T-cell expansion,36 suggesting that optimizing processing pathways based on starting material cellular composition may be essential in obtaining adequate product yields. The T-cell repertoire in the starting material also correlates with manufacturing success. Singh et al37 showed that enrichment for early T cell lineages may rescue T cell expansion capability.
While investigation of specifications for leukapheresis products used for CAR-T cell manufacturing is underway (NCT03601442, clinicaltrials.gov), it is important to remain cognizant of collection and manufacturing variables for different CAR-T products, given that using universal minimum pre-apheresis ALC requirements or collection targets may not be feasible.
2.4 Other clinical care considerations
Various practical considerations prior to apheresis involve aligning multiple components of a complex clinical situation, including patient clinical condition, manufacturing slot availability and apheresis collection schedules, among others. Patients have oftentimes been through multiple rounds of oncologic therapies, which can contribute to deconditioning and immune compromise. Prior therapies may affect both the number as well as the function of T cells and therefore, the timing of prior treatments should be considered.38, 39 Challenges in standardizing the collection process because of varying underlying conditions can result in some donors collecting better than others. Furthermore, critically ill patients may experience more adverse events, prohibiting adequate collections.
Once patients are deemed suitable candidates for CAR-T therapy, appropriate venous access must be obtained. A single apheresis procedure can often be performed using peripheral venous access. However, these oncology patients have undergone numerous treatments potentially making peripheral venous access challenging and often necessitating placement of a central line. Of note, bulky lymphomatous disease in some patients may preclude central venous catheter placement. After successful collection, time for cell product manufacturing varies but can take up to 1 month, depending on the manufacturing facility availability.13, 14 Subject to manufacturing specifications, products can be collected and frozen, or sent fresh, which requires that collection and manufacturing times align with each other, as well as with the patient’s health status. CAR-T cell therapy and related patient care are not yet available everywhere, and considerable travel may be required. Consideration of all of these factors is critical to planning an optimal apheresis collection and subsequent manufacturing and infusion of CAR-T cells.
3 DURING LEUKAPHERESIS
CAR-T collection is performed by a standard leukapheresis procedure. Citrate is typically used as the anticoagulant (AC) during the procedure. However, if the patient is small in weight, a mixture of citrate and heparin can be used to reduce the risk of citrate toxicity if there is no contraindication for heparin and the specific product specifications allow it.33 Nonetheless, risk of bleeding and development of heparin-induced thrombocytopenia must be considered. Furthermore, calcium supplementation is generally provided throughout the procedure and if needed, intra-procedural ionized calcium can be measured to adjust the calcium supplementation rate.33 Overall, most patients tolerate apheresis procedures well with the majority of adverse events being minor.

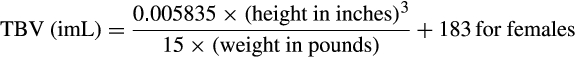
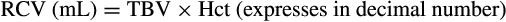
The extracorporeal volume (ECV) of the apheresis device is recommended to not exceed 15% of the patient’s TBV.41, 42 In order to ensure adequate oxygen carrying capacity throughout the procedure, the extracorporeal RCV of the apheresis device is also suggested to remain within ±10%-15% of the patient's RCV.41, 42 The ECV and the extracorporeal RCV is apheresis device-specific and usually noted in the operator manual. If the ECV is >10%-15% of the patient's TBV and/or the extracorporeal RCV is >10%-15% of the patient’s RCV, then priming the device with red blood cells (RBCs) is advisable. If only the ECV is >15% of the patient’s TBV, then priming with 5% albumin may be sufficient to address volume and avoid exposure to donor RBCs, which could result in alloimmunization of the recipient. These recommendations typically translate to performing a RBC prime if the patient weighs less than 25 kg, as in this case both the extracorporeal RCV and the ECV will exceed 10%-15%.33

Of note, CE is specific to the institution, apheresis device, and software, and sometimes operator dependent as well. Therefore, if the above formula is used, using historical data to determine the average/median CE is recommended. If the precollection CD3+ level is not available, Ceppi et al33 recommend to process at least three blood volumes or 9L, whichever is less, to achieve a collection target of 1 × 109 MNCs/kg.
Notably, even apheresis collections >2 × 109 CD3+ T cells had a 5% failure rate to achieve the therapeutic target dose of 0.3-3 × 106 cells/kg. When <2 × 109 cells were collected, 31% of collections did not meet therapeutic goal.35 Interestingly, while there was no association between gender or diagnosis with below-target yields, patients who failed to meet target yields had significantly lower proportions and absolute numbers of circulating lymphocytes and CD3+ T lymphocytes and higher proportions of circulating blasts and NK cells than those who achieved the collection goal.35 Data in this study were gathered with the COBE Spectra (Terumo BCT), which is no longer supported by the manufacturer for clinical use. It remains to be seen whether the data can be reproduced on the currently used Spectra Optia (Terumo BCT).
From a technical perspective, while leukapheresis performed for CAR-T cells is similar to collections for both hematopoietic progenitor cells (HPCs) and donor lymphocyte infusions (DLIs), there are notable differences. Although similar to collections for DLIs in that donors are not mobilized, the typical autologous CAR-T donations are from heavily pretreated ill patients, unlike allogeneic DLI donations which are from generally healthy donors.43 Nonmobilization of donors can result in difficulty setting the cellular interface during collection, potentially resulting in lower CE. The Spectra Optia apheresis machine can be programmed to collect the mature lymphocyte fraction for DLI efficiently with minimal contaminating RBCs, granulocytes, and platelets.43-45 However, given that mature lymphocytes can be smaller and denser than HPCs and immature lymphocytes, RBC separation can still be more problematic than in the case of HPC collection.43 Therefore, while experience with the more “routine” HPC and DLI collections is certainly helpful, not all the aspects are directly translated to T-cell collections. Although most collections are adequate, multiple challenges with CAR-T cell apheresis collections remain which could benefit from further study.
4 AFTER LEUKAPHERESIS
4.1 Before manufacturing
Once collected by apheresis into a suitable receptacle, the product is processed, prepared for transport, and shipped to the manufacturing facility. The product generally contains MNCs (sometimes including malignant cells), RBCs, platelets, plasma, and additive solutions (mainly saline and AC used during apheresis). These components can vary widely based on many factors, including, but not limited to, the patient's overall health, disease status, previous therapies, AC used, volume of blood processed, procedural duration, and apheresis machine used.
The AC used during extracorporeal processing of blood could potentially affect blood cells. Studies using hemodialysis as the extracorporeal blood processing model have found that AC could have modulatory effects on monocytes, neutrophils, and platelets.46 Heparin and citrate are commonly used during apheresis, and adjustment of anticoagulation infusion rates during apheresis collections is not uncommon and leads to varying citrate levels in the final product used for production.47 Studies to assess the effects of citrate concentration (if any) on T-cell activation with respect to apheresis collections could be useful.
4.2 During manufacturing
Apheresis product cellular composition could also affect the downstream manufacturing process. Interestingly, apheresis and elutriation to separate lymphocytes for clinical use stemmed from myeloid cell enrichment and reduction of the T-cell content of HPC transplant products, performed for the purpose of reducing the risk of graft-versus-host disease. The reverse principle is applied to T-cell enrichment for CAR-T cell manufacturing.48
Stroncek et al36 studied two different CAR-T cells, CD19 for B-cell acute leukemias and GD2 for osteosarcoma and neuroblastoma, and reported that GD2-CAR-T cells had fewer transduced T cells than CD19 CAR-T cells, potentially due to more monocytes in the peripheral blood producing GD-2 CAR-T cells. They found that the CD19 CAR-T cell products deemed manufacturing failures had up to twice the number of monocytes and increased granulocytes when compared to successfully manufactured products. Monocyte depletion prior to a second manufacturing attempt resulted in success in two patients. They investigated elutriation prior to manufacturing to enrich for lymphocytes and remove nonlymphoid cells that could be potentially detrimental to the manufacturing process.49 Interestingly, they reported that yields differed depending on the CAR-T cell being produced and speculated that the greater increase in manufacturing efficiency in the GD2 CAR-T cells could be due to more successful myeloid cell removal. Knowledge of the product cellular composition could help guide process improvement and optimize cellular content during apheresis. The recent report of a malignant clone recurrence that acquired the CAR transgene during manufacturing and subsequently caused relapse, although as of yet the only example, underscores the importance of product content and refining cellular composition at all possible steps in the process, including apheresis.50 These studies highlight the importance of continued investigation of the interplay of the different cell types in the product and how it can affect the CAR-T cell manufacturing process.
Manufacturing along with premanufacturing process optimization can dramatically improve efficiency and effectiveness of CAR-T therapy. Lu et al51 eliminated the need for human- or other animal-derived serum, developed functionally closed systems for manipulating products, enhanced transduction efficiency by addition of a retronectin treatment step, and shortened culture duration with the goals of developing a streamlined, standardized process with higher potential for scalability. Their process optimization resulted in a 6-day turnaround time for CAR-T cell manufacturing. Alnabhan et al52 showed that supplemental additives may further improve the manufacturing process but may also promote the development of different phenotypes. As manufacturing processes continue to develop, these considerations could be potentially applied to pre-manufacturing workflow, such as resuspension in media prior to shipping to help ensure cell viability.
Shorter CAR-T cell culture time also resulted in increased CAR-T cell efficacy.53 Furthermore, reduction from 9-14 to 3-5 days could change not only individual wait time, but drastically increase overall throughput. However, to maintain a pre-determined clinical dose based on FDA-approved parameters, higher lymphocyte count criteria for collection may be necessary, which could impact eligibility. Improvement upon apheresis collection procedures to increase CE would be useful. Addition of methods to optimize T-cell manufacturing, such as preparation of product collection bags treated with retronectin or supplemented with media that encourages cell survival, warrant further consideration and testing.
5 FUTURE DIRECTIONS FOR CAR-T CELL THERAPY
CAR-T cell development is rapidly evolving. Major efforts are underway to standardize, streamline, automate, and scale the manufacturing process, all of which have implications for cell collection by apheresis. Automation is a sought-after strategy for streamlining and standardizing the CAR-T cell manufacturing process, while simultaneously opening up opportunities for facilities without the resources for GMP facilities to manufacture CAR-T cells in a closed system on a small scale.54, 55 Lock et al56 demonstrated that the process yielded consistent results independent of starting material, operator, or device. Advantages include reproducibility and elimination of user variability inherent in manual processes. Furthermore, this system could provide a way to study starting material factors that could impact the end result in a system with many other variables controlled. Disadvantages include the limitations of the device itself, which is only capable of producing one product at a time.
Standardized production of “universal” allogeneic CAR-T cells is a goal of CAR-T cell evolution. CAR-T cells with the target transgene inserted, thereby disrupting expression of, the endogenous T-cell receptor gene, is one avenue being explored for potential clinical application.57 Successful translation to the clinical setting has far-reaching implications for scalability and application. Apheresis collection facilities could refine their processes to include healthy donors with higher lymphocyte counts and therefore less volume needed to process, thereby decreasing the number of overall collections required, potentially establishing a process somewhat similar to collection of other blood products.
6 CLINICAL IMPLICATIONS
Constant progress is being made in the field of CAR-T cell therapy; apheresis is no exception. Apheresis is the means for CAR-T cell source material collection. Understanding the aspects of patient care as it relates to apheresis for those receiving CAR-T cell therapy will lead to further process refinements. Categorizing these factors by patient (collection criteria), procedure (apheresis specifications), and processing (manufacturing criteria) emphasizes the importance of each in the final product.32 Consistent cell processing is an important goal in the continued efforts to manufacture T cells on a large scale.58 Variability in apheresis can occur from not only patients themselves, but also from the differences between apheresis machines and operators, institutions, health systems, and countries. Establishment of standardized protocols will be key. However, as different products become available to the larger population by virtue of FDA or equivalent approval, institutions offering these different treatments will have to maintain unique protocols, one for each product, until such time that standardization can apply to multiple products. Currently, apheresis collection is customized for each individual patient to ensure a safe and successful collection and manufacturing process. Involvement of apheresis physicians and providers familiar with the cellular collection process in specific CAR-T cell collection protocol development is an essential component of an interdisciplinary team approach that supports a successful manufacturing process and treatment course as well as contributing to the advancement of CAR-T cell therapy development.
7 ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest with regard to this manuscript.