Double-hit and double-expressor B-cell lymphomas: Current treatment strategies and impact of hematopoietic cell transplantation
Abstract
High-grade B-cell lymphoma with rearrangement of MYC and BCL2 and/or BCL6 (double-hit lymphoma: DHL) was newly categorized as a subtype in the 2016 revision of the WHO classification of lymphoid neoplasms. DHL is a rare entity accounting for <10% of DLBCL and clinical data of DHL cases are still limited. Standard rituximab-incorporated chemotherapy was reported to be underpowered for this intractable disease, and some promising results with intensified regimen including dose-adjusted EPOCH-R (rituximab, etoposide, vincristine, adriamycin, cyclophosphamide, and prednisone) have been emerging. The benefit of intensified regimen for DHL patients should be determined in randomized trials. The role of consolidative autologous (auto) hematopoietic cell transplantation (HCT) for newly diagnosed cases has been also undetermined. In regards to salvage chemotherapy followed by auto-HCT for chemotherapy-sensitive relapsed cases, the prognosis seems to be unsatisfactory in patients with DHL, and novel treatment strategies to incorporate effective salvage, auto-HCT and maintenance treatment after auto-HCT are warranted. Clinical application of allogeneic (allo)-HCT has not been established in newly diagnosed and refractory/relapsed (ref/rel) cases. Recently, favorable survival data of allo-HCT for ref/rel DHL was reported. To clarify the indication of various treatment strategies, larger-scaled studies or new prognostic models for DHL are required. As another topic, clinical investigation of several novel agents such as BCL2 inhibitor is conducted along with DLBCL. Here, we summarize the data relating to DHL focusing on the application of HCT, and also discuss about the combination therapy using novel agents in the setting of HCT.
1 INTRODUCTION
The WHO classification of lymphoid neoplasms was revised in 2016, and a new subtype “high-grade B-cell lymphoma with rearrangement of MYC and BCL2 and/or BCL6” was established.1 This subtype was termed as double-hit lymphoma (DHL) and was often handled similarly to diffuse large B-cell lymphoma (DLBCL). Due to a rarity of DHL, clinical data of DHL cases are limited.2 In addition, clinical efficacy of standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is unsatisfactory in patients with DHL. Thus, the development of new treatment strategies including intensified protocol is crucial to overcome the poor prognostic impact of DHL.3-6
Hematopoietic cell transplantation (HCT) has been chiefly applied in the setting of consolidation after salvage chemotherapy for relapsed DLBCL cases.7-10 However, clinical efficacy of HCT for DHL has not been fully determined. Thanks to recent advances in basic research, several molecular-targeted agents have been introduced into clinical practice for aggressive lymphoma.11 As the next step, developing combination therapy of HCT and novel agents is attractive to improve the cure rate of DHL. In this article, we summarize the latest evidence about treatment of DHL with a focus on HCT and discuss prospects of HCT in the era of novel agents.
2 DHL AS A NEW SUBTYPE IN REVISED 2016 WHO CLASSIFICATION
As for the definite diagnosis of DHL, detecting genetic rearrangement of MYC and BCL2 and/or BCL6 by fluorescent in situ hybridization (FISH) is indispensable.1 While DHL with MYC and BCL2 rearrangement (MYC/BCL2-DHL) is a major type, DHL with MYC and BCL6 rearrangement (MYC/BCL6-DHL) or THL with these three rearrangements are less common. According to the Mitelman database for 326 patients with DHL/THL, 62% of the cases belonged to MYC/BCL2-DHL, 8% MYC/BCL6-DHL, and 16% THL, respectively.12 Pathomorphological feature in each DHL case is various. B-cell lymphoma unclassifiable with features intermediate between DLBCL and Burkitt lymphoma (BCL-U) or DLBCL are common types, whereas some cases have unique images such as Burkitt lymphoma, follicular lymphoma, or lymphoblastic lymphoma.5, 13-17 Recently revealed mutational profiling separated morphological, immunophenotypic and cytogenetic 108 cases of Burkitt lymphoma, DLBCL or BCL-U into three groups: the first group harboring mutations in Burkitt lymphoma-associated genes (ID3, TCF3, CCND3, and CMYC); the second group harboring mutations in DLBCL-associated genes (BCL2, EZH2, CREBBP1, EP300, SGK1, and MEF2B); the third group sharing these two mutation patterns.18
Double-expressor lymphoma (DEL) is not presented as an independent subtype in the 2016 WHO classification, and described as DLBCL with concurrent MYC and BCL2 expression by immunohistochemistry.1 According to the previous studies, 21-44% of DLBCL cases belonged to DEL, and this percentage was much higher compared with 2%-11% in DHL.13, 19-25 Here, it should be noted that cutoff values of positivity varied in the range 40%-50% for MYC and 30%-70% for BCL2 in these studies. The 2016 WHO classification recommends cutoff values of 40% for MYC and over 50% for BCL2.1 The discrepancy of incidence between DHL and DEL indicates that not all DLBCL with concurrent MYC and BCL2 expression has MYC and BCL2 genetic rearrangement. MYC overexpression is explained by not only MYC rearrangement but also other mechanisms such as copy number variation or transcriptional upregulation.26 While BCL2 overexpression is associated with BCL-2 rearrangement in germinal center B-cell (GCB)-type DLBCL, the overexpression is caused by NF-κB activation or BCL2 gene locus (18q21) amplification in activated B-cell (ABC)-type DLBCL.27 Immunohistochemical and cytogenetic analysis for 125 DLBCL series showed that 14% of cases had discordant results between MYC expression and MYC rearrangement. MYC expression had a sensitivity of 53% and a specificity of 96%, and the positive and negative predictive value for MYC rearrangement was 80% and 87%, respectively.28 In terms of cell of origin classification, it is known that the prevalence is different between DHL and DEL. In the pathological analysis by Green et al., the percentage of GCB-type was higher in DHL (91% in DHL vs 27% in DEL), and non-GCB type was prevalent in DEL (9% in DHL vs 73% in DEL).19 Collectively, these results suggest that DHL and DEL are entities with different biological features.
Although exploring genetic rearrangement is a gold standard for the diagnosis of DHL, it is disadvantageous in terms of cost, labor, and the need for fresh-frozen tissue compared with immunohistochemistry.29 On the other hand, immunohistochemical and cytogenetic analysis for 117 DLBCL series showed that 6% of the cases were DHL without MYC/BCL-2 co-expression, which suggests immunohistochemical screening might overlook a part of DHL.30 Therefore, some researchers insist that genetic rearrangement of MYC, BCL2, and BCL6, and protein expression of MYC and BCL2 should be assessed for all cases.31 National Comprehensive Cancer Network Guidelines for DLBCL recommend cost-effective FISH analysis of MYC, BCL2, and BCL6 for only GCB-DLBCL.32 Sesques et al. also describes further selective FISH for only GCB-DLBCL exhibiting concurrent MYC/BCL-2, reducing the number of subjects by more than 90%.33 While FISH testing for all newly diagnosed DHL seems practically difficult, the analysis for relapsed DLBCL cases might give clinicians some helpful information to choose the type of HCT: auto-HCT or allo-HCT. This topic is still under debate and future standardization of diagnostic procedure is needed.
3 CLINICAL IMPACTS OF DHL AND DEL
As shown in Table 1, retrospective studies demonstrated poor prognosis of DHL patients under various initial regimens. Johnson et al. reviewed 54 patients with MYC/BCL2-DHL and reported that 59% of them died within 6 months after the diagnosis and only 11% survived in remission at a median follow-up of 5.3 years.13 52 MYC/BCL2-DHL series by Li et al. showed the median overall survival (OS) of 18.6 months.34 According to the analysis of 49 patients with MYC-rearranged aggressive B-cell non-Hodgkin lymphoma (NHL) by Cohen et al., 59% of the cases had concurrent BCL2 rearrangement, and the median progression-free survival (PFS) and OS in patients with MYC/BCL2-DHL was 8.0 months and 12.5 months, compared with 16.6 months and 37.7 months in all MYC-rearranged cases.35 Green et al. also conducted immunohistochemical and cytogenetic analysis for 193 DLBCL samples and demonstrated that MYC/BCL2 rearrangement detected in 11 cases was a significantly poor prognostic factor for PFS and OS (3-year PFS: 46% vs 65%, P = 0.01; 3-year OS: 46% vs 75%, P = 0.002).19
| Author | Study design | Number of patients | Treatments | CR rate | PFS | OS |
|---|---|---|---|---|---|---|
| Petrich et al.3 | Retrospective | 311 | R-CHOP (N = 100), R-HyperCVAD (N = 65), DA-EPOCH-R (N = 64),R-CODOX-M/IVAC (N = 42), Others (N = 40) | NA | Median 10.9 months (R-CHOP 7.8 months, Intensive regimen 21.6 months), 40% at 2-years | Median 21.9 months, 49% at 2-years |
| Oki et al.4 | Retrospective | 129 | R-CHOP (N = 57), R-HyperCVAD/MA (N = 34), DA-EPOCH-R (N = 28), Others (N = 10) | 55% (R-CHOP 40%, R-HyperCVAD/MA 68%, DA-EPOCH-R: 68%) | 33% at 2-years (R-CHOP 25%, DA-EPOCH-R 67%, R-HyperCVAD 32%) | 44% at 2-years (R-CHOP 41%, DA-EPOCH-R 76%, R-HyperCVAD 44%) |
| Landsburg et al.5 | Retrospective | 159 | R-CHOP (N = 35), R-HyperCVAD (N = 32), DA-EPOCH-R (N = 81), R-CODOX-M/IVAC (N = 11) | NA | 80% at 3-years (R-CHOP 56%, Intensive regimen 88%) | 87% at 3-years |
| Howlett et al.6 | Meta-analysis | 394 | R-CHOP (N = 180), DA-EPOCH-R (N = 91), Others (N = 123) | NA | Median R-CHOP 12.1 months, DA-EPOCH-R 22.2 months, Others 18.9 months | Median R-CHOP 21.4 months, DA-EPOCH-R 31.4 months, Others 25.2 months |
| Johnson et al.13 | Retrospective | 54 | CHOP (N = 23), R-CHOP (N = 11), High-dose chemotherapy with/without HCT (N = 6), Palliation (N = 14) | NA | NA | Median CHOP 4.8 months, R-CHOP 16.8 months |
| Gandhi et al.16 | Retrospective | 106 | R-CHOP (N = 36), DA-EPOCH-R (N = 33), R-HyperCVAD or R-CODOX-M/IVAC (N = 28) | 54% (R-CHOP 49%, DA-EPOCH-R 68%, R-HyperCVAD 33%, R-CODOX-M/IVAC 33%) | Median 8.8 months (R-CHOP 7.7 months, DA-EPOCH-R 9.2 months, R-HyperCVAD 8.0 months, R-CODOX-M/IVAC 4.0 months) | Median 12.0 months (R-CHOP 12.0 months, DA-EPOCH-R 11.6 months, R-HyperCVAD 11.0 months, R-CODOX-M/IVAC 6.4 months) |
| Green et al.19 | Retrospective | 11 | R-CHOP (N = 11) | 64% | 46% at 3-years | 46% at 3-years |
| Johnson et al.20 | Retrospective | 14 | R-CHOP (N = 14) | NA | 18% at 5-years | 27% at 5-years |
| Li et al.34 | Retrospective | 52 | R-CHOP (N = 19), R-Hyper-CVAD (N = 28) | NA | NA | Median 18.6 months, 58% at 1-year |
| Cohen et al.35 | Retrospective | 49 | R-CHOP (N = 17), DA-EPOCH-R (N = 17), Others (N = 15) | 59% | Median 16.6 months | Median 37.7 months |
| Abramson et al.36 | Retrospective | 34 | R-CHOP (N = 15), DA-EPOCH-R (N = 12), R-CODOX-M/IVAC (N = 6) | 64% | Median 8 months (R-CHOP 6 months, DA-EPOCH-R 21 months, R-CODOX-M/IVAC 6 months) | Median 11.0 months (R-CHOP 8 months, DA-EPOCH-R 34 months, R-CODOX-M/IVAC 7 months) |
| Dunleavy et al.40 | Prospective phase II | 52 | DA-EPOCH-R (N = 52) | NA | 79% at 14 months | 77% at 14 months |
- CR, complete response; DHL, double-hit lymphoma; HCT, hematopoietic cell transplantation; NA, not available; OS, overall survival; PFS, progression-free survival.
Immunohistochemical studies for cases receiving R-CHOP revealed that DEL exhibited poor prognosis. Johnson et al. pathologically reviewed 167 cases of DLBCL and showed that MYC/BCL2 co-expression was associated with poor PFS and OS (P < 0.001), although MYC single expression without co-expressed BCL2 was not associated with a poor prognosis.20 In above-mentioned study by Green et al., co-expression of MYC/BCL2 as well as MYC/BCL2 rearrangement were poor prognostic factors for PFS and OS (3-year PFS: 39% vs 75%, P < 0.001; 3-year OS: 43% vs 86%, P < 0.001).19 Hu et al. also analyzed 157 DEL and 309 non-DEL cases and demonstrated shorter PFS and OS in patients with MYC/BCL2-DEL than non-DEL cases (5-year PFS: 27% vs 73%, P < 0.0001; 5-year OS: 30% vs 75%, P < 0.0001). Here, adverse impact of co-expression of MYC/BCL2 remained in both 241 GCB-DLBCL and 225 ABC-DLBCL cohort.21 According to 106 DLBCL series by Perry et al., MYC/BCL2-DEL cases had worse event-free survival (EFS) and OS in the entire cohort and in GCB-DLBCL cohort (P < 0.001, P < 0.001 for the entire cohort; P = 0.002, P < 0.001 for GCB cohort), although prognosis in non-GCB cohort was similar between DEL and non-DEL.22 These data consistently highlight the necessity to develop novel treatment strategies for DHL and DEL.
4 INTENSIFIED INDUCTION CHEMOTHERAPY FOR DHL
As discussed above, previous studies showed insufficient efficacy of R-CHOP regimen for DHL (Table 1). To overcome the poor prognostic impact of DHL, intensified induction chemotherapy is increasingly used in DHL patients. Petrich et al. reviewed 171 DHL patients receiving intensified regimens and 100 patients R-CHOP.3 They reported that complete response (CR) rate was higher with dose-adjusted (DA)-EPOCH-R (rituximab, etoposide, vincristine, doxorubicin, and prednisone) compared with R-CHOP or R-CODOX-M/IVAC (rituximab, cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate, alternating with ifosfamide, etoposide, and cytarabine (AraC)), and PFS was longer with intensive regimen than R-CHOP (median PFS: 21.6 months vs 7.8 months, P = 0.001). According to 129 cases of DHL cohort study in MD Anderson Cancer Center, CR rate was better with DA-EPOCH-R or R-Hyper-CVAD/MA (rituximab plus hyperfractionated cyclophosphamide, doxorubicin, vincristine, and dexamethasone alternating with high-dose methotrexate (MTX), and cytarabine) than R-CHOP (68% vs 40%, P = 0.02; 68% vs 40%, P = 0.01), respectively.4 While EFS and OS were similar between R-Hyper-CVAD/MA and R-CHOP, DA-EPOCH-R significantly improved EFS compared with R-CHOP (hazard ratio (HR): 0.37, P = 0.008). Landsburg et al. analyzed 159 cases with DHL achieving CR and reported that 3-year relapse-free survival (RFS) in intensive regimen group (R-Hyper-CVAD, DA-EPOCH-R and R-CODOX-M/IVAC) and R-CHOP group was 88% and 56%, respectively (P = 0.002).5 In 106 DHL case series by Gandhi et al., CR rate with DA-EPOCH-R was higher than that with R-CHOP (68% vs 49%, P = 0.01), and the percentage of primary refractory cases was lower in DA-EPOCH-R compared with R-CHOP (18% vs 46%, P = 0.005) or other intensive regimens (P = 0.03).16 Another report of 34 cases of DHL by Abramson et al. also found DA-EPOCH-R regimen as a favorable factor for OS (HR: 0.3), although it was not a significant prognostic factor for PFS.36 According to the meta-analysis for total 394 patients with DHL in 11 studies, median PFS in DA-EPOCH-R and R-CHOP group was 22.2 and 12.1 months, and DA-EPOCH-R significantly reduced the risk of progression (HR: 0.66, P = 0.03).6
In terms of prospective data, Cancer and Leukemia Group B (CALGB) or the Spanish PETHEMA Group conducted phase II study of DA-EPOCH-R in untreated DLBCL patients, reporting CR rate of 84% (CALGB) and 80% (PETHEMA), respectively.37, 38 CALGB successively designed phase III randomized trial of R-CHOP vs DA-EPOCH-R in 524 patients with newly diagnosed DLBCL (CALGB/Alliance 50303).39 Preliminary analysis of EFS and OS in the entire cohort demonstrated no significant difference between R-CHOP and DA-EPOCH-R group (HR: 1.02, P = 0.89 for EFS; HR: 1.19, P = 0.40 for OS). As for MYC-rearranged DLBCL, Dunleavy et al. performed phase II trial of DA-EPOCH-R in 52 patient cohort including 14 with MYC/BCL2-DHL and reported that PFS at a median follow-up of 14 months was 79% in the entire cohort and 87% in DHL.40 Collectively, it is reasonable to consider DA-EPOCH-R as the most promising regimen in current clinical practice. The results of prospective studies in DHL patients would determine the efficacy of DA-EPOCH-R. Central nervous system (CNS) involvement is frequently observed in DHL patients, and even DA-EPOCH-R regimen is ineffective in not penetrating the CNS fully.15, 34 Therefore, as Friedberg recommends, intrathecal therapy with MTX or high-dose MTX/AraC combination therapy should be additionally conducted for lowering the risk of CNS relapse.41
5 THE ROLE OF CONSOLIDATIVE HCT AFTER INDUCTION CHEMOTHERAPY FOR NEWLY DIAGNOSED DHL
Another topic for newly diagnosed DHL is the application of consolidative HCT. Previous data about consolidative HCT are summarized in Table 2. Sun et al. reviewed 32 MYC/BCL2-DHL cases including 19 receiving R-CODOX-M/IVAC followed by HCT and reported that 2-year PFS and OS was 41% and 53% in the entire cohort, and 60% and 82% in HCT cohort.42 According to another study for 16 patients with MYC/BCL2-DHL receiving DA-EPOCH-R followed by auto-HCT, 2-year PFS, and OS was both 91%.43 While these data indicate potentiality of consolidative HCT, this approach has failed to show survival benefit in the retrospective comparisons with non-HCT cohort. Landsburg et al. compared outcomes between 62 cases receiving auto-HCT and 97 without HCT and showed that relapse-free survival (RFS) and OS were similar between the 2 groups (3-year RFS: 89% in the HCT group vs 75% in the non-HCT group, P = 0.12; 3-year OS: 91% in the HCT group vs 85% in the non-HCT group, P = 0.74).5 Under different induction regimen and transplantation type (auto-HCT or allo-HCT), the studies conducted by Petrich et al., Oki et al. or Gandhi et al. also could not demonstrate survival benefit by consolidative HCT after achieving CR.3, 4, 16 As for DEL, subset analysis of randomized phase III SWOG S9704 trial showed patients receiving consolidative auto-HCT had better outcomes, although the difference was not significant (2-year OS: 60% in the HCT group vs 18% in the non-HCT group, P = 0.25).44 There is still controversy about the indication of consolidative auto-HCT in patients with DHL after intensified induction chemotherapy. As Staton et al. reviews, careful observation without consolidative HCT would be a practical option for cases maintaining CR, and application for selective patients with high-risk disease should be individually judged.45
| Author | Number of patients undergoing consolidative HCT | Type of HCT | Induction regimen | Conditioning regimen | PFS/EFS of HCT cohort | OS of HCT cohort |
|---|---|---|---|---|---|---|
| Petrich et al.3 | 53 | Auto (N = 39), Allo (N = 14) | NA | NA | NA | Median not reached |
| Oki et al.4 | 26 | Auto (N = 24), Allo (N = 2) | R-CHOP (N = 2), DA-EPOCH-R (N = 14), R-HyperCVAD/MA (N = 8), Others (N = 2) | NA | 68% (2-years) | 70% (2-years) |
| Landsburg et al.5 | 62 | Auto (N = 62) | R-CHOP (N = 8), Intensive regimen (N = 54) | NA | 89% (3-years) | 91% (3-years) |
| Gandhi et al.16 | 14 | Auto (N = 13). Allo (N = 1) | Intensive regimen (N = 14) | NA | NA | NA |
| Sun et al.42 | 19 | Auto (N = 11), Allo (N = 8) | R-CODOX-M/IVAC (N = 19) | Auto: BEAM, ETP, CY, TBI, Allo: CY/TBI | 60% (2-years) | 82% (2-years) |
| Chen et al.43 | 16 | Auto (N = 16) | DA-EPOCH-R (N = 14) | BEAM (N = 16) | 91% (2-years) | 91% (2-years) |
| Puvvada et al.44 | 5 | Auto (N = 5) | R-CHOP (N = 5) | NA | 60% (2-years) | 60% (2-years) |
- allo, allogeneic; auto, autologous; BEAM, bleomycin, etoposide, cytarabine, and melphalan; CY, cyclophosphamide; DEL, double-expressor lymphoma; DHL, double-hit lymphoma; EFS, event-free survival; ETP, etoposide; HCT, hematopoietic cell transplantation; NA, not available; OS, overall survival; PFS, progression-free survival; TBI, total body irradiation.
6 CLINICAL APPLICATION OF HCT FOR REFRACTORY/RELAPSED DHL
6.1 Auto-HCT
Disease status at auto-HCT is one of the most important factors affecting prognosis after HCT.7, 8 The intergroup Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) study for patients with DLBCL in first relapse or refractory disease identified refractory disease/early relapse from initial therapy as one of poor prognostic factors.9 Clinical outcomes of auto-HCT for refractory/relapsed (ref/rel) cases are listed in Table 3. In the subset analysis of 161 ref/rel DLBCL patients enrolled in the CORAL study, 12 MYC-rearranged patients were treated by intensive regimen followed by auto-HCT, and the 4-year PFS and OS in these cases was only 14% and 23%, respectively.46 Herrera et al. also reviewed 117 chemosensitive DLBCL patients receiving auto-HCT including 12 DHL and 47 DEL patients.30 DHL cases had shorter PFS and OS compared with non-DHL cases (4-year PFS: 28% vs 57%, P = 0.01; 4-year OS: 25% vs 61%, P = 0.002), and DEL cases also had poorer PFS than no-DEL cases (4-year PFS rate: 48% vs 59%, P = 0.05), although the difference was not significant for OS. The prognosis of five cases fulfilling the criteria of both DHL and DEL was dismal (4-year PFS rate: 0%). Standard auto-HCT is not able to overcome intractable DHL/DEL, and new approaches incorporating efficient salvage and conditioning regimen, or maintenance treatment after auto-HCT should be investigated.47
| Author | Number of patients undergoing HCT | Type of HCT | Conditioning regimen | Donor Source | PFS of HCT cohort | OS of HCT cohort |
|---|---|---|---|---|---|---|
| Cuccuini et al.46 | 12 (MYC-rearranged DLBCL) | Auto (N = 12) | NA | Auto | 14% (4-years) | 23% (4-years) |
| Herrera et al.30 | 12 (DHL), 47 (DEL) | Auto (N = 59) | DHL: CBV (N = 9), R/Z-BEAM (N = 3), DEL: CBV (N = 37), R/Z-BEAM (N = 10) | Auto | DHL 28% (4-years), DEL 48% (4-years) | DHL 25% (4-years), DEL 56% (4-years) |
| Herrera et al.48 | 10 (DHL), 31 (DEL) | Allo (N = 41) | DHL: MAC (N = 2), RIC (N = 8), DEL: MAC (N = 7), RIC (N = 24) | DHL: PBSC (N = 8), UCB (N = 2), DEL: PBSC (N = 26), BM (N = 3), UCB (N = 2) | DHL 40% (4-years), DEL 30% (4-years) | DHL 50% (4-years), DEL 31% (4-years) |
| Kawashima et al.49 | 37 (DEL) | Allo (N = 37) | MAC (N = 2), RIC (N = 35) | HLA-matched related (N = 12), HLA-matched unrelated (N = 6), HLA-mismatched related and unrelated (N = 7), Haploidentical (N = 2), UCB (N = 10) | 20% (2-years) | 46% (2-years) |
- allo, allogeneic; auto, autologous; BEAM, carmustine. etoposide, cytarabine, melphalan; BM, bone marrow; CBV, carmustine, cyclophosphamide, and etoposide; DEL, double-expressor lymphoma; DHL, double-hit lymphoma; DLBCL, diffuse-large B-cell lymphoma; HCT, hematopoietic cell transplantation; MAC, myeloablative conditioning; OS, overall survival; PBSC, peripheral blood stem cell; PFS, progression-free survival; R/Z, rituximab or ibritumomab tiuxetan; RIC, reduced-intensity conditioning; UCB, umbilical cord blood.
6.2 Allo-HCT
The role of allo-HCT has not been fully established for DHL. Clinical data of allo-HCT for ref/rel cases are also listed in Table 3. Recently, Herrera et al. published an interesting data of allo-HCT for patients with ref/rel DHL/DEL.48 Of 78 patients with aggressive B-cell NHL, 58% of the cases received previous auto-HCT and 49% were refractory to initial regimen. There were no significant differences in PFS and OS between 10 DHL and 68 non-DHL cohort (4-year PFS: 40% vs 34%, P = 0.62; 4-year OS: 50% vs 38%, P = 0.46), or between 37 DEL and 41 non-DEL cohort (4-year PFS: 30% vs 39%, P = 0.24; 4-year OS: 31% vs 49%, P = 0.17), respectively. In contrast, Kawashima et al. analyzed 37 DEL and 23 non-DEL patients undergoing allo-HCT and showed that the 2-year PFS and OS were significantly worse in DEL cohort compared with non-DEL cohort (2-year PFS: 20% vs 78%, P < 0.001; 2-year OS: 46% vs 77%, P = 0.02).49 In their study, 32% of the cases had a history of auto-HCT and 35% were chemoresistant at allo-HCT. Considering retrospective nature and relatively small sample size, the discordance in two studies might be explained by the difference of patient backgrounds. Favorable survival data by Herrera et al. might indicate the possibility that allo-HCT could eliminate adverse impact by DHL/DEL. Considering the dismal outcome of auto-HCT in the setting of salvage therapy, it would be reasonable to choose allo-HCT in this setting. Larger-scaled studies are warranted to verify their results.
When clinical application of HCT is discussed, inherent biases in the selection of patients undergoing HCT are unavoidable.50 In other words, only young and fit cases usually proceed to HCT, which makes the direct comparison between patients who received HCT and those who received another treatment more difficult. HCT-specific comorbidity index or pretransplant assessment of mortality score are widely used for evaluating comorbidities of allo-HCT in advance.51, 52 In selecting DLBCL patients who are feasible for allo-HCT after auto-HCT failure, the Center for International Blood and Marrow Transplant Research working group proposed a prognostic model composed of four factors (Karnofsky performance status < 80, chemoresistance, interval from auto-HCT to allo-HCT shorter than 1-year and myeloablative conditioning).53 From the point of limited number of DHL patients proceeding to allo-HCT, constructing prognostic models for DHL might be difficult. Klyuchnikov et al. proposed algorithm for application of HCT in DLBCL based on chemosensitivity, risk for failure of auto-HCT and risk for non-relapse mortality (NRM) with allo-HCT.47 It should be validated whether the same approach is also applicable to DHL. As another point, suitable conditioning regimen or donor source for DHL is unknown, and clinical practice is generally founded on approaches for DLBCL. Because of increased NRM, the superiority of myeloablative conditioning over reduced-intensity conditioning was not shown in DLBCL patients.54, 55 On the other hand, the association between graft-vs-host disease and decreased relapse rate was not evident in DLBCL, which suggests anti-lymphoma effect might not be robust for DLBCL.56-58 Further studies of DHL in the setting of allo-HCT are warranted.
7 DEVELOPMENTS OF NOVEL AGENTS FOR REFRACTORY/RELAPSED CASES
Considering controversial role of HCT for DHL and severe toxicities brought by HCT, patients with ref/rel DHL should be actively enrolled into clinical trials using novel agents. Owing to the nature of rarity, clinical developments for DHL are underway along with DLBCL as a whole. The onset of DHL is biologically explained by BCL2 rearrangement followed by MYC rearrangement, where MYC increases cell proliferation and BCL-2 inhibits apoptosis.2, 14 The approach targeting MYC and BCL-2 as driver genes is attractive, and clinical investigations for BCL-2 inhibitors or agents suppressing MYC are in progress.14 Preclinical models showed that MYC activated aurora kinases in B-cell lymphoma and these kinases were necessary for the maintenance of MYC-positive DLBCL, which theoretically explains the usefulness of aurora A kinase inhibitor.59 In the phase I studies of the first-generation BCL-2 inhibitor navitoclax or highly selective BCL-2 inhibitor venetoclax as a single agent, overall response rate (ORR) for ref/rel DLBCL patients was 0% and 18%, respectively.60, 61 According to the phase II trial of aurora A kinase inhibitor alisertib by Friedberg et al., ORR was 14% in DLBCL cohort.62 Efficacy of BCL-2 inhibitors and alisertib as a single agent (ORR less than 20%) is limited in aggressive DLBCL and combination therapies with cytotoxic agents should be tested to achieve sufficient disease control. Lenalidomide and ibrutinib are promising especially for patients with non-GCB type DLBCL. Phase I trial of lenalidomide plus DA-EPOCH-R regimen for 15 DHL/DEL patients in the University of Chicago showed ORR of 75%, and phase II study for the same population is in progress (NCT02213913).63 As for MYC-positive lymphomas, trials of oral proteasome inhibitor ixazomib plus DA-EPOCH-R (NCT02481310) or dual PI3 kinase/histone deacetylase inhibitor CUDC-907 alone (NCT02674750) are underway. Sauter et al. investigated ibrutinib plus R-ICE (rituximab, ifosfamide, carboplatin, and etoposide) regimen for transplant-eligible 21 ref/rel DLBCL patients.64 Of evaluable 20 patients, 18 (90%) achieved response and 16 (80%) proceeded to HCT. Remarkably, all of eight cases with non-GCB type achieved CR after completing the first cycle. Clinical trial of ibrutinib targeting DHL cases seemed to be finished in M.D. Anderson Cancer Center (NCT02272686). This trial assessed clinical effect of ibrutinib maintenance after auto-HCT, and the design is novel in incorporating a novel agent into auto-HCT and trying to intensify disease control after HCT. Besides molecular-targeted agents, antiprogrammed death-1 monoclonal antibody nivolumab or infusion of anti-CD19 chimeric antigen receptor T cells exhibited ORR of 36% and 67% for ref/rel DLBCL, and additional investigation for DHL is expected in the future.65, 66
8 CONCLUSION AND PROSPECTS FOR THE FUTURE
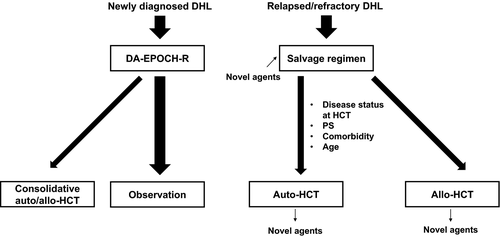
The revised WHO 2016 classification of lymphoid neoplasms highlights DHL as a rare and intractable subtype. As a brief summary, current discussion topics of DHL are described in Figure 1. In the first place, clinical data of DHL cases alone are limited. In addition, antitumor effect of standard R-CHOP regimen is insufficient for DHL, and promising intensive regimen including DA-EPOCH-R are currently examined as initial treatment. Standard auto-HCT for relapsed cases seems to be inefficient, requiring new strategies to overcome intractable DHL. Although it is still controversial, favorable survival data of allo-HCT have just been reported for ref/rel DHL cases, and larger scaled studies are expected to clarify the position of allo-HCT. In the era of novel agents, it is focused how to combine HCT with these agents aiming at better prognosis. Clinical trials of ibrutinib plus R-ICE salvage regimen or ibrutinib maintenance after auto-HCT are examples of this challenge, and further trials are strongly warranted. Our proposed treatment algorithm based on available data are shown in Figure 2. In the future, the clinical decision to choose auto-HCT or allo-HCT for DHL or feasibility of HCT for elderly patients should be discussed based on clinical trials.

CLINICAL IMPLICATIONS
While there have been no established treatment strategies for newly diagnosed DHL/DEL, initial intensive chemotherapy such as DA-EPOCH-R regimen without consolidative HCT is commonly used. In relapsed/refractory cases, salvage chemotherapy followed by auto-HCT or allo-HCT are practically applied.
CONFLICTS OF INTERESTS
The authors declare no competing financial interests.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a review article.