Evaluation of Comprehensive Coelomic Fluid Analysis through Coelomic Pore Sampling as a Novel Diagnostic Tool in Elasmobranchs
Abstract
The objectives of this study were to describe a minimally invasive coelomic fluid sampling technique in elasmobranchs, to characterize the coelomic fluid composition in clinically normal and abnormal animals, and to compare findings from wild and managed populations. Fluid was collected via the coelomic pore in 89 individuals from 16 species spanning clinically normal and abnormal patients within a managed population (n = 54), a semi-managed open-lagoon population (n = 18), and a wild population (n = 17). Biochemical and cytological fluid analyses were performed on all samples, and bacterial and fungal culture, protein electrophoresis, and cholesterol electrophoresis were performed on a subset of samples. The presence of a variable volume of colorless to white and clear to slightly turbid coelomic fluid was consistent with a normal finding; however, the cytological and chemical makeup of coelomic fluid was found to provide additional clinically relevant information. The coelomic fluid from some of the abnormal samples (n = 37) contained white blood cells (n = 15) and concurrent bacteria (n = 7), the latter suggestive of bacterial coelomitis. Yolk was identified in both clinically normal and abnormal females. Of the biochemical parameters tested, calcium, chloride, cholesterol, osmolality, phosphorus, salinity, sodium, specific gravity, total protein, and urea nitrogen have clinical utility. Abnormal samples were mostly associated with reproductive disease, but to a lesser extent with coelomitis and hemocoelom. The wild and semi-managed groups had biochemical differences presumably reflective of the higher salinity of ocean water compared with that in the managed habitat. Aerobic bacteria were identified in normal (n = 7) and abnormal (n = 11) animals. Positive bacterial culture without inflammation may be normal. This study contributes to a further understanding of elasmobranch coelomic fluid analysis and its use as a diagnostic modality for the evaluation of elasmobranch health.
Elasmobranchs contain a perivisceral, intracoelomic fluid that is similar to the intra-abdominal fluid of mammals. In healthy mammals, intra-abdominal fluid serves as a transudate that lubricates the viscera (Andrews and Porter 1973). The coelomic fluid of elasmobranchs is more variable in volume and has a more complex composition. Earlier work focused on electrolytes, pH, total protein, and osmolality (Dakin 1908; Smith 1929; Bernard et al. 1966; Thorson et al. 1973; Thorson 1976). In light of its unique chemical composition and variable volume, the coelomic fluid of elasmobranchs should be considered for further diagnostic investigation. This is all the more important because the previous literature on this subject is dated (with some papers going back to 1908) and additional biochemical, cytological, and microbiological evaluation has the potential to provide ancillary diagnostic information. The objectives of this study were to describe a minimally invasive coelomic fluid sampling technique in elasmobranchs, to characterize the composition of coelomic fluid in clinically normal and abnormal individuals, and to compare findings from wild and managed populations.
Methods
Study animals included managed animals of multiple species (further described below) that were housed at The Seas with Nemo and Friends at the Epcot Center at Walt Disney World in Orlando, Florida; semi-managed Southern Stingrays Hypanus americanus that were housed in an open lagoon at Disney's Castaway Cay, The Bahamas; and wild Southern Stingrays sampled in Bimini, The Bahamas, during wildlife health assessments.
Samples were collected from a total of 89 individuals across all three populations (managed, semi-managed, and wild). Managed and semi-managed animals were sampled during regularly scheduled examinations conducted on a semiannual to annual basis depending on their clinical conditions. Wild animals were sampled in September 2016 and semi-managed animals in May 2016; the examinations of managed animals took place year-round. Full physical examinations were performed, including coelomic ultrasounds and venipunctures. Of the managed animals, 138 total coelomic fluid samples were collected from 56 individuals across 16 species, spanning normal and abnormal patients. Species included Spotted Eagle Rays Aetobatus narinari (n = 9), Whitespotted Bamboo Sharks Chiloscyllium plagiosum (n = 3), Reticulate Whiprays Himantura uarnak (n = 1), Honeycomb Whiprays Himantura undulata (n = 2), Southern Stingrays (n = 15), Lesser Devil Rays Mobula hypostoma (n = 2), Cowtail Stingrays Pastinachus sephen (n = 1), Bowmouth Guitarfish Rhina ancylostoma (n = 2), Cownose Rays Rhinoptera bonasus (n = 11), Scalloped Hammerheads Sphyrna lewini (n = 2), Bonnetheads Sphyrna tiburo (n = 1), Bluespotted Ribbontail Rays Taeniura lymma (n = 2), Round Ribbontail Rays Taeniurops meyeni (n = 2), Leopard Sharks Triakis semifasciata (n = 1), Yellow Stingrays Urobatis jamaicensis (n = 1), and Porcupine Rays Urogymnus asperrimus (n = 1). Coelomic fluid was collected from 17 wild Southern Stingrays from Bimini and 18 semi-managed Southern Stingrays from Castaway Cay.
Normal animals were defined as healthy based on the results of the clinical examination and the absence of any clinicopathological derangements revealed by the concurrent hematological and plasma biochemical analysis. Abnormal animals were defined as animals with abnormal behavior (e.g., decreased activity level and appetite), abnormal physical examination findings, and/or abnormal hematological and plasma biochemical data. Physical examinations were performed on all animals, so that even in wild animals strength, body condition, and hematological and biochemical data could be evaluated. Semi-managed animals were individually identified so that caretakers were able to routinely observe behavioral outliers within the group. Managed animals were housed in a 5.7-million-gal (1 gal = 3.79 L) indoor saltwater aquarium with a natural photoperiod. Housing followed established husbandry and water quality protocols used in aquaria (Mohan and Aiken 2004). Semi-managed animals were fed a controlled daily diet consisting of shrimp Penaeus schmitti, squid Loligo opalescens, and a gel product (Aquatic gel 57W9; Mazuri Exotic Animal Nutrition, St. Louis, Missouri), although they also had access to natural prey items. Wild animals were sampled from a wild population in the shallow, mangrove-fringed waters of South Bimini.
Collection of coelomic fluid negligibly increased total handling and anesthetic time. Managed animals were routinely examined under general anesthesia using 55–75 mg/L of tricaine methanesulfonate (Syndel USA, Ferndale, Washington) or 15–75 mg/L of eugenol (AquaTactics Fish Health, Kirkland, Washington). Wild animals were visualized from a boat. Once one was identified, a long seine net was placed into the water surrounding it; the surface area was then slowly reduced until the individual could be gently netted and moved to a 150-gal holding tub on the boat or near shore for the examination. With the animals in dorsal recumbency, the coelomic pore was gently cleaned with sterile saline and gauze to remove biofilm, and a sterile red rubber catheter (3.5–8 French gauge) was gently introduced into the coelomic pore (Figure 1). As the catheter was inserted into the coelomic cavity, gentle aspiration was performed while it was slightly advanced and then pulled back. Occasionally, the animal was tilted with its tail down to help the fluid shift caudally for better collection. Only a slight amount of negative pressure was necessary for coelomic fluid aspiration when the catheter was in the correct location. If negative pressure was present, the catheter opening was likely abutting an organ, requiring readjustment. A volume of 5–10 mL of coelomic fluid was collected from each animal to complete a full analysis, although a volume of 2 mL was sufficient. Only adults were sampled during each examination. The volume collected was not based on the animal's weight since there was a sufficient amount of fluid and no negative effects were encountered from removing it. After the procedure, animals were released near the shore around the mangroves where they were found. Because Southern Stingrays are a prey species in this area, they were not anesthetized so that they could be released immediately following sampling.
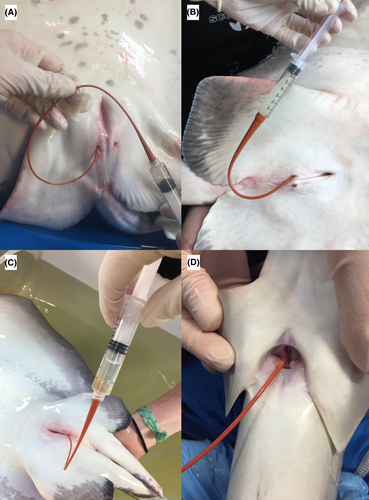
Following collection, the fluid was placed into sterile plain vacutainers and lithium heparin tubes, and the latter were thoroughly but gently mixed via accepted techniques (Weiser 2012). Samples were routinely sent unfrozen, but ones from Bimini were occasionally frozen immediately at −20°C due to field conditions and an anticipated delay in processing and then shipped on ice for biochemical analysis. In some animals, aerobic bacterial and fungal cultures were sampled immediately with a culturette (BD Culturette CultureSwab EZ Collection and Transport System; Becton, Dickinson and Co., Franklin Lakes, New Jersey) and performed at a commercial laboratory (IDEXX Laboratories, Westbrook, Maine) using standard methods for marine aquatic organisms (Buller 2004). Samples were collected for culture when feasible or based on clinical concern. These samples included all abnormal animals and a subset of normal animals to include approximately half (46%) of all the managed-animal samples submitted for culture.
Specific parameters of interest included fluid cytologic description, salinity, biochemical analytes, protein electrophoretograms, cholesterol (CHOL) electrophoretograms, specific gravity (SG), presence of bacterial and fungal isolates, and urine dipstick analytes. When possible, subjectively abnormal coelomic fluid samples (e.g., those with gross deviation of color or cellular makeup) were monitored through repeated sampling from the same individual and as logistically feasible (e.g., up to 11 times per individual at 1-month intervals during this study period). Normal individuals were not repeatedly sampled in this data set.
Fluid analysis entailed a gross description, including color and transparency, and a cytological review. Slides were stained with Wright Giemsa for routine evaluation, and periodic acid-Schiff (PAS) was used to confirm the presence of yolk when applicable (Ortiz-Delgado et al. 2008). Measured biochemical analytes included albumin (ALB), alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), calcium (Ca), chloride (Cl-), CHOL, conjugated bilirubin (CBili), creatine kinase (CK), creatinine, gamma-glutamyl transferase (GGT), globulins (GLOB), glucose (GLC), lactate dehydrogenase (LDH), phosphorus (P), potassium (K), sodium (Na), SG, total bilirubin (TBili), total carbon dioxide (TCO2), total protein (TP), total solids (TS), triglycerides (TRIG), uric acid (UA), and urea nitrogen (UN). Osmolality was measured by freezing point depression osmometry at the University of California–Davis Veterinary Medical Teaching Hospital or in-house via a 5010 OSMETTE III osmometer (Precision Systems, Natick, Massachusetts). Salinity was recorded with an in-house TS Meter-D digital refractometer (Reichert Technologies, Buffalo, New York) using routine protocols for evaluation as well as standard water quality parameters (Mohan and Aiken 2004).
Protein and CHOL electrophoreses were analyzed at the University of Miami's Comparative Pathology Laboratory. Protein components analyzed were ALB, TP, ALB/GLOB ratio, pre-ALB, and alpha-1, alpha-2, beta, and gamma GLOBs. The lipoprotein components analyzed included CHOL, high-density lipoprotein (HDL), lipoprotein(a)-cholesterol (LP(a)-C), very-low-density lipoprotein (VLDL), and low-density lipoprotein (LDL). Due to the low concentrations of protein and CHOL in coelomic fluid samples, 45–50 mg/dL of CHOL was used as a placeholder to quantify the lipid components. Protein electrophoresis was performed using SPEP-II agarose gels and the Beckman paragon electrophoresis system (Beckman-Coulter, Brea, California) with quantification of TP by the biuret method using the Kodak 750 XR system (Ortho Clinical Diagnostics, Rochester, New York). The gels were run as described previously (Cray and Tatum 1998). The percentage of protein fractions was quantified by laser densitometry, and then each fraction value was calculated by multiplying the percentage of the fraction by the TP concentration. CHOL electrophoresis was performed as described in Cray et al. (2015).
Coelomic fluid was tested using a commercially available urine dipstick (Siemens Healthcare Point of Care Diagnostics, Norwood, Massachusetts); parameters (GLC, hemoprotein, ketones, leukocytes, Multistix protein, nitrites, and pH) were measured according to the manufacturer's instructions.
The Shapiro–Wilk normality analysis was performed to examine whether study variables conformed to a normal distribution. Descriptive statistics (median, first and third quartiles, mean, and SD) were calculated for chemistry analytes across species and based on normal or abnormal health status. Samples from the combined normal animals (managed, semi-managed, and wild groups) were compared with samples from abnormal animals. To control for environment and husbandry, managed abnormal animals (pooled data) were compared with managed normal animals using the t-test. The Southern Stingray was the representative species for all groups studied (normal–managed, abnormal–managed, semi-managed, and wild). Semi-managed and wild animals were deemed clinically normal. These groups were compared using ANOVA and a Tukey HSD post hoc test. Coelomic fluid color was compared with fluid calcium using multiple linear regression. Protein and CHOL electrophoretogram data from normal and abnormal animals were compared using the t-test. The 95% confidence limits for the predicted values were used to assess the significance of this experiment (P ≤ 0.05). JMP statistical software (SAS Institute, Cary, North Carolina; Version 13) was used to analyze the data.
Results
Gross Description
The color of coelomic fluid samples varied widely. The wild Southern Stingray population contained colorless and white samples, with clear to slightly cloudy turbidity. Samples from the managed population included colorless (n = 84), yellow (n = 17), white (n = 14), amber/tan (n = 2), pink/red (n = 2), green (n = 2), blue (n = 1), gray (n = 1), and green and red (n = 1). Normal samples were predominantly colorless and clear (48/57 samples); however, normal animals also produced white (n = 2) or yellow (n = 5) samples. The presence of yolk was identified in all sample colors at least once (colorless [4/84 samples], white [7/14 samples], yellow [7/17 samples], amber [1/2 samples], red/pink [1/3 samples], and green [1/2 samples]), so colorless samples do not preclude its presence. There was no association between sample color (colorless or white) and fluid calcium (colorless, P = 0.30; white, P = 0.33).
Cytology
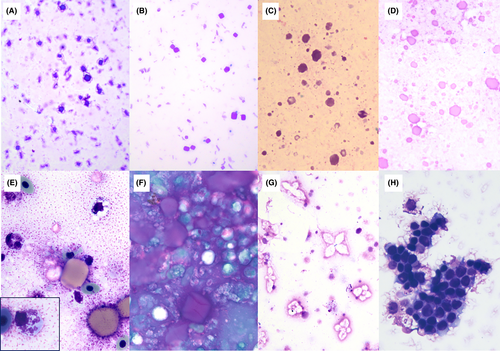
Cytological findings of coelomic fluid in 13 wild Southern Stingrays included crystals with morphological features suggestive of salt origin (n = 8), followed by well-differentiated mesothelial cells (n = 6), red blood cells (RBC; n = 4), and pleomorphic variably sized round or square globular material suggestive of yolk (n = 1). No bacteria or white blood cells (WBC) were identified in this wild population. Cytological evaluation performed on 18 Southern Stingrays in the semi-managed environment resulted in identification of crystals suggestive of salt (n = 13), RBC (n = 5), material suggestive of yolk (n = 4), and mesothelial cells (n = 3). No bacteria or WBC were identified in the semi-managed population. Cytology performed on seven normal–managed Southern Stingrays identified crystals suggestive of salt (n = 1), globular material suggestive of yolk (n = 1), RBC (n = 1), and WBC (n = 1). Bacteria and mesothelial cells were absent in samples from the normal–managed population. Cytology from 37 samples of seven abnormal–managed Southern Stingrays was summarized for analysis. The most common findings were RBC (n = 19), WBC (n = 15), mesothelial cells (n = 12), material suggestive of yolk (n = 10), bacteria (n = 7), and crystals suggestive of salt origin (n = 3). Coccidia were absent in all samples. See Figure 2 for examples of various cytological findings.
Clinical Chemistry
A summary of descriptive statistics (medians, quartiles, means, and SDs) for coelomic fluid chemistry analytes is listed in Table 1 for those species for which over five clinically normal and abnormal individuals were available and sampled. Results for species with less than five individuals are listed in their entirety for the species. Descriptive statistics for all independent normal samples and all pooled abnormal samples across species are listed. Significant differences in chemistry analytes were observed between normal (managed, semi-managed, and wild) and abnormal animals. For all elasmobranchs, the following analytes were near or below the detectable limits of measurement: ALB, ALP, ALT, AST, CBili, CK, creatinine, LDH, GGT, GLC, TBili, TCO2, triglycerides, and UA.
| Species | n | Statistics | UN (mg/dL) | Na (mmol/L) | Cl- (mmol/L) | P (mg/dL) | Salinity (ppt) | Osmolality (mOsm/kg) | TP (g/dL) | GLOB (g/dL) | CHOL (mg/dL) | Ca (mg/dL) | K (mmol/L) | pH | SG refract | TS (g/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal individuals | ||||||||||||||||
| Spotted Eagle Rays | 7 | Median | 1,188 | 223 | 320 | 0.3 | 42 | 1,003 | 0.2 | 0.2 | At or below limit of detection | 2.1 | 10.5 | 6 | 1.020 | 2.6 |
| Q1 | 1,165 | 215 | 318 | 0.0 | 42 | 968 | 0.1 | 0.1 | 1.2 | 9.6 | 5 | 1.020 | 2.6 | |||
| Q3 | 1,213 | 230 | 324 | 0.4 | 42 | 1,019 | 0.2 | 2.0 | 2.8 | 11.1 | 6 | 1.020 | 2.5 | |||
| Mean | 1,195 | 223 | 320 | 0.3 | 42 | 974 | 0.2 | 0.2 | 2.2 | 10.2 | 5.6 | 1.020 | 2.6 | |||
| SD | 33 | 9.8 | 6.9 | 0.31 | 0.57 | 79 | 0.1 | 0.1 | 1.1 | 1.1 | 0.53 | 0.003 | 0.06 | |||
| Managed Southern Stingrays | 9 | Median | 1,005 | 233 | 279 | 0.1 | 39 | 909 | 0.2 | 0.2 | 2 | 2.9 | 7.5 | 6 | 1.017 | 2.1 |
| Q1 | 930 | 223.5 | 274.5 | 0.1 | 38 | 907 | 0.1 | 0.1 | 1.25 | 2.4 | 7.4 | 6 | 1.016 | 2.05 | ||
| Q3 | 1,046 | 258 | 292.5 | 0.25 | 40 | 924 | 0.2 | 0.2 | 3.75 | 4.2 | 9.6 | 6 | 1.018 | 2.3 | ||
| Mean | 989 | 242.7 | 284.9 | 0.15 | 38.9 | 936 | 0.17 | 0.17 | 2.4 | 5.6 | 9.1 | 5.8 | 1.018 | 2.19 | ||
| SD | 98.6 | 23.6 | 19.4 | 0.09 | 1 | 66 | 0.07 | 0.07 | 1.2 | 7.8 | 3.3 | 0.35 | 0.004 | 0.22 | ||
| Wild Southern Stingrays | 17 | Median | 1,240 | 307.5 | 330 | 0.15 | 49.5 | 1,105 | 0.3 | 0.3 | 4.5 | 6.65 | 10.3 | NP | NP | NP |
| Q1 | 1,217.5 | 286.25 | 316.25 | 0.1 | 39 | 1,093 | 0.25 | 0.25 | 3.25 | 4.4 | 9.5 | |||||
| Q3 | 1,365 | 320 | 350 | 0.275 | 50 | 1,109 | 0.3 | 0.3 | 5.75 | 12.6 | 11.95 | |||||
| Mean | 1,262 | 301.67 | 328.33 | 0.175 | 44.33 | 1,094 | 0.28 | 0.28 | 4.67 | 7.88 | 11.07 | |||||
| SD | 106 | 25.97 | 26.23 | 0.087 | 10.26 | 44 | 0.05 | 0.05 | 2.19 | 4.48 | 2.19 | |||||
| Semi-managed Southern Stingrays | 18 | Median | 1,390 | 295 | 320 | 0.3 | 45 | 1,083 | 0.3 | 0.3 | 6 | 16.1 | 10 | 5.75 | 1.023 | 2.6 |
| Q1 | 1,360 | 275 | 320 | 0.1 | 44.75 | 1,076 | 0.3 | 0.3 | 5 | 10.1 | 8.4 | 5.5 | 1.020 | 2.6 | ||
| Q3 | 1,430 | 300 | 325 | 0.3 | 46 | 1,097 | 0.4 | 0.4 | 9 | 20.9 | 12.5 | 6 | 1.025 | 2.7 | ||
| Mean | 1,399 | 287 | 322.67 | 0.22 | 45 | 1,089 | 0.33 | 0.33 | 6.67 | 16.35 | 10.43 | 5.77 | 1.023 | 2.61 | ||
| SD | 43.1 | 16.67 | 4.95 | 0.1 | 0.87 | 27 | 0.1 | 0.09 | 2.44 | 7.21 | 2.48 | 0.3 | 0.003 | 0.1 | ||
| Cownose Rays | 8 | Median | 1,110.5 | 253.5 | 312.5 | 0.7 | 42 | 957 | 0.2 | 0.2 | 1.5 | 6.4 | 12.25 | |||
| Q1 | 1,060 | 223.75 | 308 | 0.575 | 41.25 | 949 | 0.175 | 0.175 | 1 | 5.7 | 11.08 | 5.5 | 1.017 | 1.65 | ||
| Q3 | 1,181.25 | 277.5 | 320 | 1.15 | 45.25 | 971 | 0.325 | 0.325 | 7.25 | 7.7 | 14.63 | 6 | 1.02 | 2.4 | ||
| Mean | 1,117.7 | 252 | 312.8 | 0.82 | 42.4 | 903 | 0.23 | 0.23 | 4.7 | 6.9 | 13.2 | 5.8 | 1.018 | 2 | ||
| SD | 58.4 | 26 | 6.9 | 0.31 | 5.3 | 203 | 0.1 | 0.1 | 7.55 | 1.9 | 3.2 | 0.4 | 0.001 | 0.35 | ||
| Honeycomb Whiprays | 1 | 990 | 310 | 315 | <0.1 | NP | 922 | 0.2 | 0.2 | 14 | 2.2 | 6.3 | 6 | 1.018 | 2.3 | |
| Cowtail Stingrays | 1 | 920 | 295 | 325 | 0.3 | 43 | 922 | 0.3 | 0.3 | 2 | 6 | 6.5 | 6 | 1.033 | 1.8 | |
| Bowmouth Guitarfish | 2 | 657 | 380 | 375 | <0.1 | NP | 1,009 | <0.1 | 0.1 | <1 | 9.10 | 6.7 | 6 | 1.016 | 2 | |
| 547 | 351 | 348 | <0.1 | NP | 925 | 0 | 0 | <1 | 6 | 7 | 6 | 1.015 | <2.0 | |||
| Bonnetheads | 1 | 814 | 256 | 329 | <0.1 | 37 | 861 | 0 | 0 | 1 | 15 | 5 | 6 | 1.017 | 2.1 | |
| Bluespotted Ribbontail Rays | 2 | 983 | 241 | 300 | 0.5 | 40 | 929 | 0.2 | 0.2 | 1 | 6.4 | 8.7 | 6 | 1.022 | NP | |
| 982 | 248 | 310 | 0.6 | 45 | 902 | 0.2 | 0.2 | 1 | 5.6 | 7.9 | 5 | 1.02 | NP | |||
| Combined normal animals | 63 (73 for osmolality) | Median | 1,208 | 278 | 320 | 0.2 | 44 | 1,012 | 0.25 | 0.25 | 3.5 | 6.1 | 10 | 6 | 1.020 | 2.1 |
| Q1 | 1,038 | 243 | 310 | 0.1 | 41 | 924 | 0.2 | 0.2 | 1 | 3.8 | 8 | 5.5 | 1.020 | 2 | ||
| Q3 | 1,370 | 300 | 330 | 0.3 | 46 | 1,088 | 0.3 | 0.3 | 6 | 13.7 | 11.5 | 6 | 1.020 | 2.5 | ||
| Mean | 1,184 | 274 | 318 | 0.25 | 43 | 1,000 | 0.25 | 0.25 | 4 | 8.9 | 10.2 | 5.8 | 1.021 | 2.2 | ||
| SD | 210 | 38 | 23 | 0.24 | 6 | 120 | 0.1 | 0.09 | 3.9 | 7.2 | 2.8 | 0.4 | 0.000 | 0.3 | ||
| Abnormal individuals | ||||||||||||||||
| Managed Southern Stingrays | 7 (with 42 total samples) | Median | 1,065 | 264.5 | 293 | 0.2 | 43 | 954 | 0.2 | 0.2 | 6 | 13.6 | 9.3 | 6 | 1.019 | 2.5 |
| Q1 | 1,046 | 236.8 | 268.5 | 0 | 40 | 927 | 0.1 | 0.1 | 2.5 | 5.5 | 7.7 | 6 | 1.018 | 2.3 | ||
| Q3 | 1,119 | 275 | 304.8 | 2.95 | 45 | 968 | 0.4 | 0.35 | 11 | 16.25 | 17.8 | 6 | 1.020 | 2.6 | ||
| Mean | 1,082.7 | 257.7 | 287.6 | 1.1 | 43.2 | 955 | 0.46 | 0.41 | 17.6 | 13.6 | 12.1 | 5.9 | 1.021 | 2.5 | ||
| SD | 47.7 | 30.3 | 21.3 | 1.56 | 3.1 | 11 | 0.84 | 0.74 | 39.8 | 12.9 | 6.4 | 0.59 | 0.005 | 0.4 | ||
| Spotted Eagle Rays | 2 | 1,113 | 261 | 287 | 1.1 | NP | 976 | 0.3 | 0.3 | 5 | 4.3 | 10 | 6 | 1.018 | NP | |
| 1,176 | 195 | 302 | 0.7 | 42 | 993 | 0.3 | 0.3 | 2 | 2.9 | 9.00 | 6 | 1.018 | NP | |||
| Lesser Devil Rays | 1 | 71 | 369 | 417 | 0.2 | NP | 878 | 0.1 | 0.1 | 1 | 36.8 | 9.4 | NP | NP | NP | |
| Round Ribbontail Rays | 1 | 830 | 305 | 320 | 0.3 | 37 | 932 | 0.1 | 0.1 | 2 | 4.3 | 12.5 | 5 | 1.016 | 2 | |
| Honeycomb Whiprays | 1 | 990 | 310 | 315 | 0.0 | NP | 922 | 0.2 | 0.2 | 14 | 2.2 | 6.3 | 6 | 1.018 | 2.3 | |
| Scalloped Hammerheads | 1 | 801 | 279 | 322 | 0.1 | 43 | 920 | 0.4 | 0.4 | 5 | 17.9 | 16.6 | 6 | 1.019 | 2.5 | |
| Combined abnormal animals | 18 (with 51 total samples) | Median | 1,057 | 275 | 304 | 0.2 | 43 | 946 | 0.2 | 0.2 | 3 | 6.7 | 9.4 | 6 | 1.020 | 2.5 |
| Q1 | 875 | 208 | 287 | 0.0 | 40 | 913 | 0.1 | 0.1 | 2 | 4.3 | 7 | 6 | 1.020 | 2.3 | ||
| Q3 | 1,112 | 305 | 321 | 0.6 | 45 | 963 | 0.3 | 0.3 | 8 | 16.8 | 16.6 | 6 | 1.020 | 2.6 | ||
| Mean | 975 | 277 | 308 | 0.7 | 43 | 927 | 0.2 | 0.2 | 5 | 12 | 11.6 | 5.9 | 1.021 | 2.4 | ||
| SD | 233 | 47 | 34 | 1.2 | 3 | 100 | 0.16 | 0.1 | 4.7 | 12 | 5.1 | 0.5 | 0.003 | 0.4 | ||
When the results of coelomic fluid were combined for normal animals, osmolality (P = 0.0038) and UN (P = 0.0002) were significantly higher than in abnormal animals. Abnormal animals had significantly higher TS (P = 0.0098). No significant differences were observed in Ca, CHOL, Cl-, GLOB, K, Na, P, pH, salinity, SG, or TP across groups.
When comparing only normal–managed and abnormal–managed animals to control for environmental factors, even more significant differences were observed. Abnormal samples had significantly higher Ca (P = 0.0002), CHOL (P = 0.0297), P (P = 0.0207), salinity (P = 0.0007), SG (P = 0.0069), and TS (P = 0.0013) than samples from normal–managed animals. No significant differences in Cl-, GLOB, osmolality, K, Na, TP, or UN were identified. Refer to Table 1 for the list of species.
Across the four Southern Stingray populations (normal–managed, abnormal–managed, semi-managed, and wild), ANOVA yielded significant differences in Ca, Cl-, Na, osmolality, P, salinity, and UN. A Tukey post hoc test showed that wild and semi-managed populations had significantly (P < 0.05) higher Cl-, Na, and UN than the two managed populations (normal and abnormal). The abnormal population of managed animals had significantly higher P than the other three groups. The salinity of the managed–normal group was significantly lower than that of the semi-managed group. Both managed populations had a significantly lower osmolality than the semi-managed and wild populations. The normal–managed and semi-managed groups had significantly higher Ca than the other groups. No significant differences were observed in ALB, CHOL, GLOB, K, P, TP, or TS between groups.
Protein and CHOL Electrophoresis
Thirteen samples from 9 individuals across 5 species (Spotted Eagle Rays, Southern Stingrays, Bowmouth Guitarfish, Cownose Rays, and Porcupine Rays) underwent protein electrophoresis, including 10 samples from 6 abnormal individuals and 3 samples from 3 normal individuals. Sixteen samples from 12 individuals across 7 species were processed for CHOL electrophoresis. Eleven samples from 7 abnormal individuals from 3 species and 5 samples from 5 normal individuals across 5 species were analyzed. Low concentrations of protein and CHOL resulted in negligible fractionation, and peaks within the electrophoretograms were difficult to visualize. Descriptive statistics are listed in Tables 2 and 3. There were no significant differences in the protein and CHOL fractions between normal and abnormal samples.
| Samples | n | Statistic | ALB (g/dL) | TP (g/dL) | A/G (g/dL) | Pre-ALB (g/dL) | Alpha-1 GLOB (g/dL) | Alpha-2 GLOB (g/dL) | Beta GLOB (g/dL) | Gamma GLOB (g/dL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | 3 | Q1 | 0.36 | 1.4 | 0.21 | 0 | 0.12 | 0.19 | 0.4 | 0.09 |
| Median | 0.37 | 2.2 | 0.35 | 0 | 0.12 | 0.23 | 1.45 | 0.17 | ||
| Q3 | 1.76 | 3.8 | 0.87 | 0.01 | 0.14 | 0.24 | 1.48 | 0.32 | ||
| Mean | 0.83 | 2.46 | 0.48 | 0.003 | 0.1 | 0.22 | 1.11 | 0.19 | ||
| SD | 0.8 | 1.22 | 0.35 | 0.006 | 0.04 | 0.03 | 0.61 | 0.12 | ||
| Abnormal | 10 | Q1 | 0.19 | 1.75 | 0.14 | 0 | 0.04 | 0.12 | 0.64 | 0.13 |
| Median | 0.35 | 1.8 | 0.24 | 0.03 | 0.08 | 0.21 | 1.01 | 0.08 | ||
| Q3 | 0.52 | 2 | 0.54 | 0.05 | 0.12 | 0.27 | 1.14 | 0.22 | ||
| Mean | 0.38 | 1.8 | 0.35 | 0.04 | 0.07 | 0.21 | 0.94 | 0.16 | ||
| SD | 0.23 | 0.19 | 0.26 | 0.05 | 0.04 | 0.1 | 0.23 | 0.09 |
| Samples | n | Statistic | CHOL (mg/dL) | HDL (mg/dL) | LP(a)-C (mg/dL) | VLDL (mg/dL) | LDL (mg/dL) |
|---|---|---|---|---|---|---|---|
| Normal | 5 | Mean | 49 | 10.9 | 6.36 | 14.5 | 17.3 |
| SD | 2.2 | 6.4 | 2.6 | 8.1 | 7 | ||
| Abnormal | 11 | Mean | 48.6 | 14.5 | 7.7 | 13 | 13.4 |
| SD | 2.3 | 5.7 | 3.1 | 5.4 | 7.5 |
Microbiology
Aerobic bacterial and fungal organisms were isolated from both normal– and abnormal–managed animals. Sixty-six total samples were cultured for aerobic bacteria from 33 individuals across 11 species. Nineteen samples from normal–managed animals were cultured from 17 individuals across 8 species. Of these normal animals, 7 of 19 samples from 7 individuals were positive for aerobic bacterial growth. Of abnormal animals, 47 samples were cultured from 17 individuals across 10 species. There were 23 of 47 samples from 11 individuals that were positive for aerobic bacterial growth. Bacterial species isolated include Acinetobacter junii (n = 1), Citrobacter sp. (5 separate cultures at different time points from 2 individuals), Enterobacter aerogenes (2 cultures from 1 individual), Enterococcus spp. (5 cultures from 4 individuals), Lactococcus spp. (2 cultures from 1 individual), Morganella morganii (n = 1), Photobacterium damselae (12 repeat cultures from 8 individuals), Pseudomonas putida (n = 1), Serratia marcescens (n = 1), Staphylococcus spp. (2 cultures from 2 individuals), Streptococcus spp. (3 cultures from 2 individuals), Vibrio spp. (5 cultures from 4 individuals), Vagococcus fluvialis (n = 1), and a nonenteric gram-negative rod (n = 1).
Forty-three samples from 18 individuals across 7 species were cultured for fungus. Eight samples from 8 individuals belonging to 5 species of normal–managed animals were cultured for fungus. No growth was observed. Of abnormal–managed animals, 36 samples from 12 individuals across 6 species were cultured. Seven of these samples from 3 individuals (25%) were positive for fungal growth. Paecilomyces spp. were cultured from one Southern Stingray. Five repeat samples from 1 abnormal Lesser Devil Ray were positive for Candida glabrata. One sample from an abnormal Porcupine Ray was positive for several genera of environmental molds (Cladosporium spp., Alternaria spp., and Curvurlaria spp.), which were presumed to be environmental and/or skin contaminants. All positive samples were also positive for aerobic bacterial growth.
Urine Dipstick
Coelomic fluid was analyzed with a urine dipstick to evaluate its practical use in a clinical setting. One hundred and two total samples were analyzed across 15 species. Nitrites, ketones, and glucose were negative in all samples. Three samples (2.5%) were positive for leukocytes; however, only two of these were confirmed cytologically. Protein readings ranged the full spectrum from negative to “+++,” which is inconsistent with the generally low total protein measured using protein electrophoresis (interquartile range: 1.4–3.8 g/dL), likely due to alkalinity interference with the test pad. The pH ranged from 5 to 8, with the medians and quartiles listed by species in Table 1. There was no significant difference in pH across normal and abnormal animals. Hemoprotein readings also ranged across the full spectrum from negative to “+++,” often secondary to noted RBC that were intact or hemolyzed. These results were generally supported by cytological observation of RBCs.
Examples of Using the Coelomic Fluid as a Diagnostic Tool over Time
The following case summaries describe repeated coelomic fluid sampling over time with changes in color and cytologic composition until clinical improvement. For each case the fluid color and/or turbidity was initially considered abnormal but changed to colorless or white at the time of clinical resolution (unless lost to follow-up). These examples highlight how changes in color, when used in conjunction with cytology, can be helpful in monitoring individual patients.
Case 1
In a managed adult female Whitespotted Bamboo Shark (C. plagosium), bacterial yolk coelomitis was identified by cytology and tracked over the course of 4 months before the fluid was considered cytologically normal. The color of the fluid was diagnostically useful, starting at brown–green and cloudy before changing to yellow–amber and cloudy near the time of resolution of the coelomitis, and finally yellow–white and slightly cloudy once resolved as determined by cytologic evaluation (e.g., absence of inflammation and bacteria).
Case 2
In a managed female Lesser Devil Ray, bacterial coelomitis secondary to gastrointestinal perforation was identified via coelomic fluid analysis. Over the course of 3 weeks the turbid fluid changed from yellow to pink, back to yellow, and then to green. Granulocytes with phagocytized bacteria admixed with material suggestive of ingesta were initially observed, followed by the presence of degenerating granulocytes and a noticeable reduction in the number of bacteria and yeast organisms over the course of 4 weeks, implying improvement. This animal was lost to follow-up.
Cases 3 and 4
In a managed male Southern Stingray, a hemocoelom was identified grossly by its red opaque fluid and confirmed microscopically by the presence of abundant erythrocytes. The hemocoelom was resolved at a recheck 1 month later, when the coelomic fluid was colorless, clear, and free of erythrocytes by cytology. A second case with hemocoelom was identified in another managed male Southern Stingray with green and red fluid, erythrocytes, and material suggestive of bile by cytology. Two months later the fluid was colorless with brown sediment and rare hematoidin crystals suggestive of a resolving hemorrhage.
Discussion
This study offers new information on the utility of comprehensive coelomic fluid analysis as a diagnostic tool in elasmobranch clinical assessment. Elasmobranchs contain a variable volume of perivisceral, intracoelomic fluid that this study supports as a normal finding. In addition to providing valuable insight into patient health, the use of coelomic fluid is appealing because collecting it through the coelomic pore is minimally invasive. The classical method of collecting intra-abdominal or intracoelomic fluid involves percutaneous aspiration, which often requires lavage and results in a diluted sample (Stamper et al. 1998); by contrast, the catheterization technique presented here yields a representative sample during every collection attempt and the specimens reflect in vivo conditions. The coelomic pore is a unique anatomical feature of elasmobranchs and provides a passage from the coelomic cavity to the environment (Bles 1898). In an adult elasmobranch, paired coelomic (‟abdominal”) pores are located within the cloacal opening on the ventral surface (Bles 1898). These pores are thought to be excretory in function, with a collapsed lumen compressed by the pressure of the body cavity preventing any afferent flow (Bles 1898).
Evaluation of sample color along with other results was considered useful. Using the wild Southern Stingray group as a normal control, colorless to white fluid with clear to slightly cloudy turbidity may be normal. The chemical difference between clear and white fluid is unknown, as the cellular components, lipid, and calcium content do not seem to correlate with the difference in color. In the managed population, the majority of normal samples were colorless; however, normal animals also produced colorful samples, suggesting that color alone is not a specific indicator of health status.
Common cytological findings in both normal and abnormal animals across all groups included crystals with morphology suggestive of salt, mesothelial cells, and RBC. The formation of crystals may be related to the environment (e.g., humidity in the air) and differences in temperature between in vivo coelomic fluid, sample collection devices, and microscope glass slides. Yolk was identified in both normal and abnormal animals. Since bacteria concurrent with WBC were absent in normal animals from all the wild and semi-managed groups, their cytological identification should be interpreted as abnormal, especially when observed in clinically abnormal individuals. Although there was no significant difference in pH between normal and abnormal animals as measured by the urine dipsticks, this test is not considered as precise as using a pH electrode (Kwong et al. 2013). Utilization of urine dipsticks generally did not provide information beyond that obtainable from the cytology and chemistry data, and the results for dipstick protein were inconsistent with those from the more accurate biuret method of protein measurement by a chemistry analyzer. The inconsistency presumably resulted from the interference of color-containing components or pH-altering bacteria with the test pad, as has been documented for the urine dipstick. Thus, dipstick evaluation is not recommended for acquiring additional information.
Previous studies report that coelomic fluid is more acidic than both serum and environmental seawater (Smith 1929; Bernard et al. 1966; Thorson 1976). The data presented here are similar, with the mean pH in all groups being between 5 and 6, compared with a previously reported serum pH between 7.3 and 7.5 (Bernard et al. 1966; Thorson 1976). These studies also reported a higher Cl- concentration in coelomic fluid than in serum and pericardial fluid (Smith 1929; Bernard et al. 1966; Thorson 1976). The Cl- content of the intracoelomic fluid reported here conforms to those findings. One measurement that did not follow previous trends was Ca. Previous studies reported lower intracoelomic Ca than serum Ca; however, in this study Ca was variable across species and health status, with significantly higher levels in the managed–abnormal Southern Stingray (Bernard et al. 1966; Thorson 1976). This study did not compare coelomic fluid with the sera of the individual at the time of sampling; however, comparisons of the data presented here reveal findings similar to those in the historical studies. These findings suggest that the distinct chemical composition of coelomic fluid is directly influenced by the body's own physiology, likely a consequence of the secretory abilities of the coelomic wall lining, or mesothelium (Smith 1929). Some association between open nephrostomes and the presence of coelomic pores also suggests that the coelomic membrane is involved in this excretory function (Bles 1898; Smith 1929). The coelomic pore allows for discharge of this fluid into the environment and was previously measured by monitoring discharges of Indian ink and suspensions of bacteria from intracoelomic injections from the coelomic pore (Dobbie 1988). Additionally, electron microscopy of the mesothelial lining of the teleost coelom revealed a pitted surface made up of micropinocytoic vesicles (Dobbie 1988). Taking these findings together, it may be prudent to consider the coelomic wall as an active part of the excretory system of these animals.
The limited detection or absence of ALP, ALT, AST, CBili, CK, creatinine, GGT, GLC, LDH, TBili, TCO2, TRIG, and UA suggests that the coelomic lining does not participate in the active excretion of these analytes. The limited ALB detection with both the chemistry analyzer and protein electrophoresis is consistent with previous studies that suggest that elasmobranchs do not produce this protein (Metcalf and Gemmell 2005; Hyatt et al. 2016). The discrepancy between the refractometer and the colorimetric TS measurements can be explained by interference with the refractive index by the refractometer (high Na, Cl-, UN, protein [i.e., from yolk proteins], or hemolysis). All of these potential types of interference can be present in elasmobranchs, resulting in artifactually increased TS readings.
The significant differences in chemistry between normal and abnormal animals in the managed population are suggestive of changes consistent with reproductive activity in females, e.g., vitellogenesis. Specifically, abnormal animals had significantly higher Ca, CHOL, P, and TS. This is further supported by the disproportion of sexes among the abnormal samples, which were composed of 16 females and 4 males. Reproductive disease in females was the most common clinical abnormality encountered in the managed population, as revealed by observations of ovaries with multiple retained, inspissated follicles and cysts in elasmobranchs under managed care. The data set is not robust enough to infer sex differences, though this relationship deserves further investigation.
The Southern Stingray was the only species that could be compared across all four groups. The results show that some parameters may be more influenced by the environment; for example, the wild and semi-managed groups had significantly higher mean Cl-, Na, osmolality, and UN than the two managed populations. This may be a consequence of the elasmobranch's ability to osmoconform, i.e., to use urea and methylamines as solutes to maintain an osmolality similar to that of the environment (Browning 1978). Ocean water has a salinity of approximately 36–37 ppt, and the tank water of the managed animals was approximately 30–32 ppt, supporting the difference in osmolality between groups. Utilizing population-specific reference ranges or comparing the results of biochemical tests with environmental osmolality is recommended for interpretation.
Protein and CHOL electrophoresis are techniques used to evaluate protein and lipid fractions in body fluids. These techniques are commonly used in mammals, birds, and reptiles to characterize inflammation, infection, stress, or neoplasia (Cray and Tatum 1998; Cray 2012; Moore et al. 2014). Within the subclass Elasmobranchii, plasma protein and CHOL electrophoresis baseline reference intervals were established in Cownose Rays (Cray et al. 2015). Serum lipoprotein analysis is primarily used for evaluation of dyslipidemia and related diseases in humans (Yamauchi 2014); however, its use in oviparous animals may be of particular interest given the dramatic change in blood lipids that accompanies body fat mobilization during vitellogenesis. In the data presented here, the concentrations of lipid and protein were generally too low to yield useful results. A placeholder of 45–50 mg/dL of CHOL was used in CHOL electrophoresis to quantify the lipid fractions (e.g., HDL and LDL). These fractions were difficult to visualize in the electrophoretograms, making this test unacceptably subjective. For these reasons, neither protein nor CHOL electrophoresis are recommended for further evaluation of elasmobranch coelomic fluid. The low CHOL and lipid fractions are somewhat surprising, as many of the abnormal cases included were clinically diagnosed with chronic reproductive disease and yolk coelomitis. It is unclear why the yolk identified cytologically does not result in adequate levels for quantification, but this may be due to variations in chemical lipoprotein composition.
The normal microfauna in the bodies of elasmobranchs may indicate bacterial infection in other species. Mylniczenko et al. (2007) reported that healthy managed and wild elasmobranchs harbor a normal population of microfauna in their blood. Other studies have identified bacteria in various tissues from healthy sharks, including the intestine, liver, spleen, kidneys, and pancreas (Grimes et al. 1985). Aerobic bacteria were isolated from both normal and abnormal animals, though most samples were negative. The results for the organisms cultured here may reflect contamination during sample collection, handling, or processing in the laboratory; however, they may also reflect true growth, since the collection techniques were similar to those in previous studies with the exception of shipment to a laboratory with at least a 24-h delay prior to plating. In animals in which these organisms were not also identified cytologically or without evidence of inflammation, they may reflect a normal commensal microbiome. Culture alone is not an adequate method for identifying the microbiological makeup of specimens from elasmobranchs since routine culture techniques are inadequate for many species of bacteria, particularly aquatic microorganisms. A more comprehensive approach would require molecular techniques such as 16S rRNA PCR or high-throughput sequencing.
Another pathologic presentation that may benefit from the use of coelomic fluid analysis is coccidian disease. Large numbers of Eimeria southwelli were previously reported in the coelomic fluid of wild and aquarium-managed Cownose Rays and one Spotted Eagle Ray (Boulard 1977; Stamper et al. 1998). Intracoelomic coccidia were not observed in any cytological sample here, which suggests that previous reports of E. southwelli are localized to specific populations, i.e., by habitat and/or response to stress (Stamper et al. 1998).
This study provides a comprehensive evaluation of coelomic fluid in elasmobranchs, which will serve as a baseline for future studies with a focus on samples from additional normal and abnormal individuals. In our study, the sample size was impossible to control owing to the different number of animals managed on site at certain time points as well as the timing of routine examinations. The sample size in this study did not allow for a robust analysis of species or sex differences. Therefore, additional research is warranted. As the requirements of the American Society for Veterinary Clinical Pathology for the establishment of reference intervals require 120 individuals (Friedrichs et al. 2012), this study is descriptive in nature and does not report formal reference intervals.
In summary, the chemical and cellular composition of elasmobranch coelomic fluid suggests an excretory function of the coelomic wall. Sampling fluid via the coelomic pore proved to be a safe and effective technique. The presence of variable amounts of coelomic fluid is normal; however, the chemical and cytological makeup of this fluid can provide clinically useful information on the health of the patient. Based on the majority of results from normal animals, colorless to white and clear to slightly turbid coelomic fluid is normal when supported by other clinical findings. Cytology is useful in providing diagnostic information on the presence of yolk, coelomitis with and without bacterial infection, and hemocoelom. Yolk may be present in both normal and abnormal fluid samples from females. The presence of WBC paired with bacteria is supportive of coelomitis; however, coelomic microfauna without inflammatory cells may be considered normal. Samples from abnormal individuals had significantly higher P, CHOL, and Ca. Further work is needed to determine associations with reproductive disease. Repeated sampling to observe trends is considered useful. Neither protein and CHOL electrophoresis nor urinary dipstick analysis provided useful diagnostic information.
Acknowledgments
The authors wish to thank Ryan DeVoe, Carolyn Cray, and Hayley Bird for their technical assistance with this project. We give very special thanks to the animal hospital and husbandry staff at the Seas with Nemo and Friends at the Epcot Center and those at Castaway Cay and Disney's Animals, Science, and Environment for their excellent care of these animals. Additional thanks to the staff at the Bimini Biological Field Station for capturing wild rays. This study was supported in part by the Bahamas Department of Marine Resources, the National Institute of General Medical Sciences (grant P20GM104932), and Centers of Biomedical Research Excellence, Center of Research Excellence in Natural Products Neuroscience, Chemistry Research Core. This study was approved by the Disney Animal Care and Welfare Committee and the University of Florida's Institutional Animal Care and Use Committee under study number 201709748. There is no conflict of interest declared in this article.




