Chapter 3 Separations with a Liquid Stationary Phase: Countercurrent Chromatography or Centrifugal Partition Chromatography
Abstract
Ever since Yoichiro Ito reported the separation of blood plasma cells with a sealed helical tube in 1966, countercurrent chromatography (CCC) has been a fertile ground for instrumental and technical innovations. The key innovation of CCC was to use centrifugal forces to retain the stationary liquid phase in the column in such a way that it can interact dynamically with mobile phase without any solid support. The great diversity of countercurrent separation terminology reflects both the innovative spirit of the field and the global appeal of this technique. The selected biphasic liquid system is the core of the CCC technique. The CCC columns must be able to generate the centrifugal field needed to maintain the liquid stationary phase. They cannot be a simple tube with frits at both ends. Rotors, gears, spools, and rotating seals are very specific things that are not needed in classical LC with a solid stationary phase. This chapter describes the commercially available CCC columns and few practical examples. The special terminology used in CCC is explained sorting terms into three classes: the terms linked to the CCC instrumentation, the terms linked to CCC operation, and the terms coming from the biphasic liquid system.
1 Introduction
Chromatography is defined as a separation technique using a mobile and a stationary phase. Differences in the affinity of analytes to the stationary phase versus the mobile phase lead to different analyte mobility and, thus, separation yield. The nature of the mobile phase is used to classify chromatographic techniques into main groups such as gas chromatography (GC), liquid chromatography (LC), and supercritical fluid chromatography (SFC). As such countercurrent chromatography (CCC) belongs to the LC family as it uses a support-free liquid mobile phase.
CCC is a generic term covering all forms of liquid–liquid chromatography in which an immiscible (biphasic) liquid system is used without any solid support. The mobile phase is one phase of the biphasic liquid system; the stationary phase is the other. Despite the name, the two phases never flow countercurrent to one another, so the technique naming and acronym could be considered as inappropriate.
The term countercurrent chromatography and the triple C acronym have a strong historic background and were coined by Yoichiro Ito in the early 1970s [1]. The two different hydrostatic and hydrodynamic systems were already described both being named CCC. Today, the term and acronym CCC is used ambiguously, referring only to hydrodynamic systems or referring to all liquid–liquid separation techniques or subsets thereof. Given the impressive number of articles that appeared using the CCC acronym, it does not seem wise to propose a new term in this text. The second definition using CCC as a generic term will not be favored enthusiastically.
The liquid nature of the stationary phase imposes the design of specific “columns.” Centrifugal fields are always used to maintain the liquid phase stationary, while a liquid mobile phase is pushed through it. Two solutions were found viable with reliable commercial instruments being developed. The characteristics of the two kinds of CCC “columns” are described and compared.
The heart of the CCC technique is the biphasic liquid system since one phase is the mobile phase and the other phase is the stationary phase. However, biphasic liquid system equilibrium makes that any change in one phase also changes the other phase. It means that in CCC, the liquid system selection corresponds to the column selection in liquid chromatography and, simultaneously, to the mobile-phase selection. Gradient elution is very difficult with limited uses in CCC since any modification of the mobile-phase composition may impact the stationary-phase composition and hence its density and retained volume [2-4].
CCC is mainly a preparative technique that has an underestimated potential in large-scale purification of high-value compounds. Examples of applications will be described. The use of a support-free liquid stationary phase is unique in the chromatographic world, dedicated terms were needed in CCC, and they will be detailed at the end of this chapter.
2 The CCC Technique
2.1 A Support-Free Liquid Stationary Phase
In all modern commercially available CCC columns, centrifugal forces are used to retain the liquid stationary phase without any solid support. This is a drawback of the technique. Centrifugal fields imply rotors, motors, gears, belts, cooling units, electronic speed control, temperature control, and rotating seals as a nonexhaustive list of technical parts making the CCC “column” actually an instrument with a significant size.
2.2 A Preparative Technique
The major advantage of working with a liquid stationary phase is that all solutes injected into the CCC column can access the bulk of the stationary-phase volume. They are not confined to the surface of a solid stationary phase. Peak distortions due to column overload in LC occur at much higher concentrations injected into CCC column [5]. A CCC column is able to process one order of magnitude higher masses of material than an LC column of comparable volume [6].
If loading capacity is clearly in favor of CCC, the efficiency of a solid-phase LC column is far superior to that of a liquid stationary-phase CCC column. The solute exchange between two liquid phases with volume diffusion in CCC columns is slower than the exchange between the liquid mobile phase and a very thin layer of the solid-phase surface in LC columns. CCC columns have maximum efficiencies barely reaching 1000 plates and commonly being in the 150–400 plate range [4], while preparative LC column can have efficiency one order of magnitude higher. However, the CCC column maintains its efficiency, hence its separation power, when processing high amounts of material when peak distortion and column overload ruin the separation obtained on a preparative LC column with small amounts injected.
As in any chromatographic technique, the actual column size is linked to the amount of manageable sample. However, CCC is definitively a preparative technique since it is easier to obtain a stable liquid stationary phase in bigger instruments than in smaller ones [5]. The minimal CCC column volume is around 20–30 ml already able to handle milligrams of sample to purify per run. While working with liter volume CCC columns, it is possible to handle masses as high as kilograms of sample to purify [5, 6]. Figure 1 shows that there are two column designs allowing to obtain a workable support-free liquid stationary phase: the hydrostatic (Figure 1a) and the hydrodynamic (Figure 1b) designs.
2.3 Simple Partitioning Mechanism
2.3.1 Simple Retention Equation
2.3.2 Efficiency and Resolution
adapted from Ref. [5]
).| Code | Solute | Formula | m.w. | pKa | log K | K |
|---|---|---|---|---|---|---|
| 1 | New coccine red | C20H11N2O10S3Na3 | 604.5 | 1.0 | −5b | ∼0 |
| 2 | Caffeine | C8H10N4O2 | 194.2 | 14.0 | −0.6 | 0.25 |
| 3 | Ferulic acid | C10H10O4 | 194.1 | 4.4 | −0.07 | 0.85 |
| 4 | Umbelliferone | C9H6O3 | 162.2 | 10.3 | 0.04 | 1.10 |
| 5 | Vanillin | C8H8O3 | 152.2 | 10.2 | 0.15 | 1.40 |
| 6 | Quercetin | C15H10O7 | 302.2 | 10.3 | 0.27 | 1.90 |
| 7 | Coumarin | C9H6O2 | 146.2 | — | 0.60 | 4.0 |
| 8 | Narigenin | C15H12O5 | 272.3 | 10.1 | 0.65 | 4.5 |
| 9 | Salicylic acid | C7H6O3 | 138.1 | 2.9 | 0.78 | 6.0 |
| 10 | Estradiol | C18H24O2 | 272.4 | 10.5 | 1.04 | 11.0 |
| 11 | Carvone | C10H14O | 150.2 | — | 1.38 | 24.0 |
| 12 | β-carotene | C40H56 | 537.0 | — | 5b | large |
- a Source: Adapted from the Friesen GUESS solute list [15].
- b Compounds 1 and 12 are dyes selected to have an almost nil K for compound 1 (red dye) and very large K for compound 12 (orange dye) in the biphasic liquid system hexane/ethyl acetate/methanol/water 4/6/4/6 v/v.
| v/v | Initial % v/v | |||||||
|---|---|---|---|---|---|---|---|---|
| Letter | Heptane | Ethyl acetate | Methanol | Water | Heptane or methanol | Ethyl acetate or water | Up./low. phase ratio | Reichardt polarity low./up. |
| A | 0 | 1 | 0 | 1 | 0.0 | 50.0 | 0.88 | 100/50 |
| B | 1 | 19 | 1 | 19 | 2.5 | 47.5 | 0.92 | 90/51 |
| C | 1 | 9 | 1 | 9 | 5.0 | 45.0 | 0.965 | 88/52 |
| D | 1 | 6 | 1 | 6 | 7.1 | 42.9 | 0.96 | 85/53 |
| F | 1 | 5 | 1 | 5 | 8.3 | 41.7 | 0.95 | 84/53 |
| G | 1 | 4 | 1 | 4 | 10.0 | 40.0 | 0.95 | 83/53 |
| H | 1 | 3 | 1 | 3 | 12.5 | 37.5 | 0.945 | 82/53 |
| J | 2 | 5 | 2 | 5 | 14.3 | 35.7 | 0.91 | 80/54 |
| K | 1 | 2 | 1 | 2 | 16.7 | 33.3 | 0.88 | 79/55 |
| L | 2 | 3 | 2 | 3 | 20.0 | 30.0 | 0.84 | 78/55 |
| M | 5 | 6 | 5 | 6 | 22.7 | 27.3 | 0.80 | 77/54 |
| N | 1 | 1 | 1 | 1 | 25.0 | 25.0 | 0.70 | 76/53 |
| P | 6 | 5 | 6 | 5 | 27.3 | 22.7 | 0.69 | 77/54 |
| Q | 3 | 2 | 3 | 2 | 30.0 | 20.0 | 0.68 | 77/52 |
| R | 2 | 1 | 2 | 1 | 33.3 | 16.7 | 0.68 | 77/51 |
| S | 5 | 2 | 5 | 2 | 35.7 | 14.3 | 0.70 | 77/51 |
| T | 3 | 1 | 3 | 1 | 37.5 | 12.5 | 0.735 | 77/51 |
| U | 4 | 1 | 4 | 1 | 40.0 | 10.0 | 0.76 | 76/50 |
| V | 5 | 1 | 5 | 1 | 41.7 | 8.3 | 0.78 | 76/40 |
| W | 6 | 1 | 6 | 1 | 42.9 | 7.1 | 0.775 | 76/28 |
| X | 9 | 1 | 9 | 1 | 45.0 | 5.0 | 0.77 | 75/26 |
| Y | 19 | 1 | 19 | 1 | 47.5 | 2.5 | 0.71 | 74/25 |
| Z | 1 | 0 | 1 | 0 | 50.0 | 0.0 | 0.45 | 73/23 |
- a The upper over lower-phase volume ratios and Reichardt polarity index measured with the dye are given for the freshly prepared liquid systems. Heptane can be replaced by hexane, isooctane and/or petroleum ether with minimum polarity changes [18].
Figure 2 and Equation 8 point out the critical point of the CCC technique: the CCC column must be able to retain as much liquid stationary phase as possible in order to have some resolution power in separating sample constituents. They also demonstrate that CCC must not be considered as an alternative to preparative LC. Both techniques are complementary using different processes: liquid–liquid extraction for CCC and solid–phase extraction for prep-LC. The challenging problem to have a stable liquid stationary phase received two different solutions that gave two commercially available lines of CCC instruments: the hydrostatic CCC columns and the hydrodynamic ones.
2.4 The Hydrostatic CCC Column Design
Hydrostatic CCC columns are also called centrifugal partition chromatographs (CPCs) coming from the patented trade name of the Japanese Sanki company that was the sole commercial builder of hydrostatic columns from 1982 to its closure in 1996 [8].
2.4.1 A Geometrical Pattern
The hydrostatic column design is a pattern of interconnected chambers in which the liquid stationary phase is retained. An inlet and outlet at both sides of each chamber allow for liquid mobile-phase entrance and exit. The interconnected chambers are placed in a rotor so that the constant centrifugal field will tightly maintain the liquid stationary phase, while the mobile phase is percolated through it. Figure 3a shows the initial design used by the closed Japanese company. The chamber design was greatly improved following the work of the Foucault French team [8]. It was demonstrated that a double-cell design was suppressing Coriolis displacement of the liquid phase thus enhancing mixing and breaking films of phases that tended to form in the initial simple chamber design. Figure 3a shows a disk used in the Kromaton FCPC-C hydrostatic column. The disk gathers 64 double cells with a total volume of 2.8 ml formed by ∼31 µl cells and their interconnecting ducts. The total cell volume of the Figure 3a disk is about 31 µl × 64 = 2 ml and the duct volume 0.8 ml. Thirteen such disks are packed together in a 36.4 ml rotor in the small FCPC-C hydrostatic Kromaton columns. This rotor contains 64 × 13 = 832 double cells with a volume of 26 ml. The internal connecting duct volume is about 11 ml making about 29% of the nominal column volume of 37 ml [9].
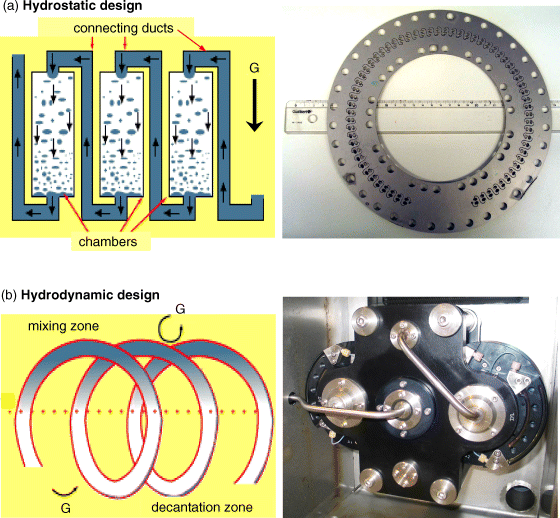
2.4.2 Hydrostatic Characteristics
The geometrical cell design of CPC columns has consequences for the chromatographic process. Of the duct volume, 0.8 ml per disk or 11 ml per column (29%) is actually a “dead” volume for chromatographic exchanges since it contains only mobile phase precluding partitioning with the other phase. Also, since only stationary phase can be retained in the cells, the maximum volume of stationary phase that can be retained by a hydrostatic CCC column is the cell volume. In the case of the FCPC-C 37 ml column previously described, the total cell volume is 26 ml, hence the maximum stationary phase possibly retained also will be 26 ml. This is only 70% of the 37 ml instrument volume. For all hydrostatic columns, the Sf maximum value is necessarily much lower than 100% with a maximum 80% value [8].
- ΔP the hydrostatic mobile phase driving pressure;
- Δρ the density difference between the mobile and stationary liquid phases;
- ω the rotor rotation speed;
- Sf the liquid stationary-phase retention ratio;
- µM the mobile-phase viscosity;
- F the mobile-phase flow rate; and
- C and D are constants linked to the column design [8].
- There is good liquid stationary-phase retention in cells.
- But there is a maximum value corresponding to the cell volume (65–80% of column volume).
- The design generates a hydrostatic pressure depending on density difference between phases and, quadratically, on rotor rotation speed.
- There is a constant centrifugal field.
- They have a simple centrifuge single-axis mechanical design.
- However, they require rotating seals at both sides of the column.
- They have a limited efficiency, and several cells are needed to generate a single theoretical plate.
2.5 The Hydrodynamic CCC Column Design
The complex hydrodynamic CCC design evolved in time through numerous variants that will not be discussed here since they did not produce reliable CCC columns. These variants have been well described in several books or reviews [2, 3, 10]. The most useful design was named coil planet centrifuge type J because it is made of drums containing a coiled open tube mounted on a rotor and rotating around their own axis and simultaneously around the rotor axis [10]. The combined rotations produce a coil motion called planetary motion, similar to Earth rotation around its axis and around the Sun simultaneously. The coil planet centrifuge denomination is not recommended because its acronym is the same as the CPC acronym for hydrostatic CCC column, a source of confusion. Commercial CCC instruments containing coils of open tubes will be called hydrodynamic CCC “column” in this chapter.
2.5.1 Coiled Open Tubes
Commercial hydrodynamic CCC columns all contain one or several drums or bobbins of coiled Teflon® tubing placed in a rotor creating a planetary motion of the coils (Figure 3b). The spectrum hydrodynamic CCC column of Figure 1b) and Figure 3b contains two coils mounted on a rotor and equilibrating one another. It is a CCC column able to work with a volume of 68 ml (operating with one coil only) or 133 ml (using the two coils serially connected). Each coil was made winding 32.5 m of 1/8″ perfluoroalkoxy polymer tubing of 2 mm2 bore area and 1.6 mm internal diameter (volume ∼65 ml). The 32.5 m polymer tubing is wound on each coil in five layers of 15 turns on the 60 mm bobbin axis. The first internal layer has the 60 mm bobbin radius and the external layer 74.4 mm. The maximum rotation speed of the rotor is 1800 rpm. This maximum rotation generated a variable centrifugal field whose maximum value is 500 g, the earth gravitational field (g = 9.81 m/s2). A special path for the connecting tubing is used so that the winding torsion generated by the coil rotation is canceled by the counterrotation of an intermediate connecting tube that allows for direct connection without rotating seals [2, 3, 10].
2.5.2 Hydrodynamic Characteristics
The stationary-phase retention is obtained by a succession of mixing and decantation zones created by the coil planetary motions (Figure 3b). When there is addition of the centrifugal field generated by the rotor rotation to that generated by the coil rotation, both with the same direction when the tubing is passing on the rotor outside, the maximum resulting field induces decantation of the two liquid phases. As the rotation goes on, the same tubing will pass at the rotor inside where the rotor and coil centrifugal fields will have opposite direction generating a mixing zone [2-4].
- A significant amount of stationary phase can be retained by the hydrodynamic column only if the density difference between the two liquid phases is high enough (higher than 0.1 g/ml) [12].
- A hydrodynamic CCC column made of a wider bore tubing will retain the liquid stationary phase better than one made of a small bore tubing [13]. If the two CCC columns have the same volume, the chromatographic efficiency (plate number) obtained with a small bore coil is higher than that obtained with a larger bore tubing since the tubing lengths are different [13].
- As with hydrostatic CCC columns, a higher rotor rotation speed ω induces a higher centrifugal field and better stationary-phase retention.
- Reduced mobile phase viscosity is desirable.
2.6 Hydrostatic or Hydrodynamic?
When considering the cost of equipment, the end user in CCC wants to know which column should be bought. The hydrodynamic column gives sharper peaks (better efficiency and more plates) and works at a low pressure without rotary seals compared to a hydrostatic column of the same volume. However, a hydrodynamic column can be unable to retain a biphasic liquid system whose phase density difference would be too low [12]. The hydrostatic column will retain any biphasic liquid system having some resolution power and allow to work quickly with high mobile-phase flow rates. However, it needs two rotary seals and works with significant background pressure.
The good point of CCC is that a separation obtained with a liquid system and a particular CCC column can be transposed directly on any other CCC column. It could be recommended to have a small-volume hydrostatic CCC column that would allow testing quickly a variety of solvent systems for a particular separation problem [9, 13]. Once the right liquid system has been found, the separation could be scaled up on a large-volume hydrodynamic CCC system or a larger volume hydrostatic CCC “column.”
3 Biphasic Liquid Systems
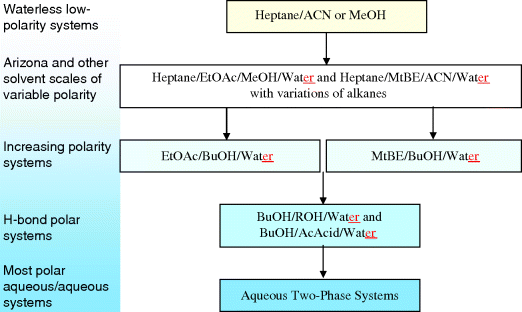
In CCC, it must be realized that the biphasic liquid system selection is the selection of the stationary phase and the mobile phase at the same time. Any change in one phase induces changes in the other phase. The selection of the right biphasic liquid system is the most difficult part in a CCC purification [14]. Figure 4 is a simplified view of possible biphasic liquid systems for CCC sorted from the least polar waterless systems to the most polar aqueous two phase systems (ATPS). This figure shows deliberately selected UV transparent solvents that allow for easy detection. For this reason, it does not show any ketone when acetone, methyl ethyl ketone, and methyl isobutyl ketone are very useful solvents in CCC.
3.1 The Heptane/Ethyl Acetate/Methanol/Water Bipasic System
3.1.1 HEMWat and Arizona Systems
Figure 4 shows the particular heptane/ethyl acetate/methanol/water biphasic system with intermediate but easily adjustable polarity. This system was called the HEMWat system [15]. It was first proposed by Oka et al. in 1991 [16]. Margraff standardized the use of this system and defined a range of 24 proportions coded by alphabetic letters (no. I and no. O) from A to Z [17]. All these proportions have exactly the same heptane/ethyl acetate ratio as the methanol/water ratio. The most polar first system A is 0/1/0/1 and the least polar system Z is the 1/0/1/0 v/v heptane/ethyl acetate/methanol/water system. The middle system N has the composition 1/1/1/1 v/v. Margraff demonstrated that if the components that should be separated are located in the ethyl acetate less polar upper phase of the two-solvent ethyl acetate–water system A and in the methanolic more polar lower phase of the two-solvent methanol–heptane system Z, then there is necessarily a four-solvent biphasic system between composition A and Z in which the components will be equally distributed [16, 17]. The AZ range of biphasic liquid system became very popular as the “Arizona” liquid system from the AZ zip code of the US Arizona state [18]. If all “Arizona” compositions belong to the HEMWat system, there is no objection to work with HEMWat compositions not respecting the equality between the two H/E and M/Wat ratios [15, 17].
3.1.2 Adapting Solvents
It was recently demonstrated that replacing hexane with heptane, a less toxic alkane, does have minor effect on the Arizona system [18]. Table 2 lists the AZ compositions in volume and percentage compositions. The Reichardt polarities of the upper organic and the lower aqueous phase are also indicated. In this normalized scale, obtained by measuring the bathochromic shift of the absorbance maximum of the Reichardt dye (a pyridinium-N-phenoxyde betaine dye), 100 is assigned to water and 0 to tetramethylsilane [19]. Table 2 shows that the polarity of the compositions changes monotonously and the polarity of the two phases decreases together as the amount of water (lower phase) and ethyl acetate (upper phase) both decrease similarly. However, the solute partitioning between the two phases can change dramatically when the liquid composition changes from one letter to the next one [16-18]. Figure 4 shows that ethyl acetate can be replaced by methyl-t-butyl ether and/or methanol by acetonitrile to obtain systems with different solving properties.
3.2 Aqueous Two-Phase Solvent and Other Systems
The most polar ATPSs are made by mixing water with a salt and a hydrophilic polymer or two hydrophilic polymers. As the ATPS name says, the two liquid phases of an ATPS are aqueous solutions. One phase of the ATPS is somewhat less polar than the other. The water/potassium phosphate/polyethylene glycol (PEG) ATPS has been particularly studied [20]. Its PEG-rich upper aqueous phase is less polar than the phosphate-rich lower aqueous phase. Water/polyethylene glycol/dextran systems or water/polyethylene glycol/ionic liquid ATPSs were also proposed to separate biological materials [21]. The interfacial tension between the two aqueous phases is always low and is responsible for high settling times of the two phases. Associating this low interfacial tension with significant viscosity, it explains the very poor stationary-phase retention of ATPSs by hydrodynamic columns (low Sf even at reduced mobile-phase flow rates). Hydrostatic columns are recommended as they are practically required to work with ATPSs [20, 21].
Given the number of possible solvents, there are countless of other biphasic liquid systems that could possibly be used in CCC. Ketones and toluene are very good intermediate polarity solvents with dipolar (π interactions) character that have a cutoff wavelength precluding the use of the convenient UV detector. They can be excellent solvent for purification associated with other kinds of detector such as the evaporative light-scattering detector (ELSD), the Corona charged aerosol detector (CAD), or the universal mass spectrometer (MS).
The very hydrophobic waterless systems can include fluorinated solvents, ionic liquids, and dipolar aprotic solvents such as N-methyl pyrrolidone, N,N-dimethyl formamide or acetamide, dimethylsulfoxide, or even furfural [22]. It must be noted that increasing environmental concerns and changing regulations are inducing changes in solvent usage in CCC. The common use of chloroform in the twentieth century was due to the excellent solving properties of this chlorinated solvent associated with its high density producing apolar organic phases denser (lower phase) than the polar aqueous phase. Chlorinated solvents are banned by the REACH European regulation of chemicals. Greener less polluting solvents are sought today [12].
4 Use of CCC: Applications
4.1 Literature Search
A rapid electronic search on the SciFinder® database turned 2142 scientific publications dealing with CCC in the course of the past 14 years, 2000–2013. From these publications, 538 (25%) were patents. It is interesting to note the unusual huge number of patents registered for chemical purification using CCC. Of these patents, 463 or 86% were registered in China. A single Chinese chemist from Nanjing was able to register 148 different patents in 3 years, 2011–2013. All 148 patents were “methods” for preparing different pure chemicals extracted from natural products mostly coming from traditional Chinese medicine. CCC is used for purification of plant extracts with various biphasic systems commonly based on the alkane/ethyl acetate/methanol/water system. Of publications of the search, 188 (9%) were review articles on a variety of CCC topics and the remaining ∼1400 (66%) items were articles in a variety of scientific journals.
As already observed, more than 60% of the published articles present purification of natural compounds coming from plants or other natural products. CCC is established as a useful tool in phytochemistry and extraction and purification of natural compounds. The second theme of the published articles is method development, the subject of about 20% of the articles showing that the CCC technique is still maturing and evolving. Industrial development, new or original solvent systems, inorganic and artificial organic purifications, and chiral separations are the subject of the remaining articles on CCC.
4.2 An Example of Small-Scale Purification
4.2.1 Sample Presentation and Preparation
The full purification of a natural product recently published is described step by step to illustrate the classical CCC procedure for rapid screening and purification. Long pepper or Piper longum L. (Piperaceae) is a variety of pepper used in Asian cuisine as a spice and also as a medicinal plant with antipyretic, hypotensive, central nervous system stimulant, and, potentially, antitumor activity [23].
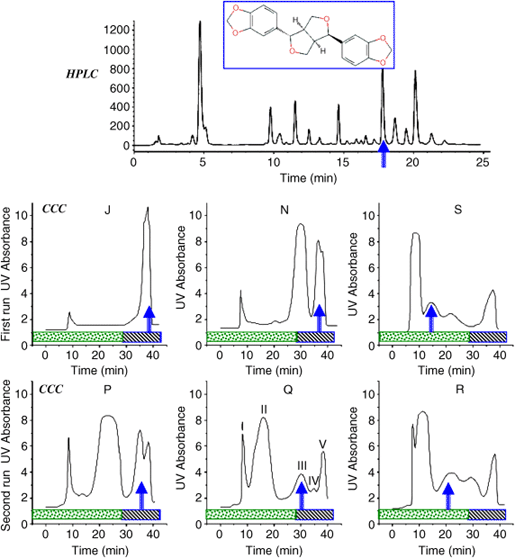
One kilogram of powdered dry log pepper fruits was extracted with 4 l of 95% ethanol for 2 h at 85 °C under reflux. The filtrated ethanolic solution gave about 80 g (8%) of intermediate polarity extract that was fractionated by CCC. Figure 5 (top) shows the HPLC chromatogram of the extract containing asarinine or (−)-episesamin, C20H18O6, m.w. 354.4, log Po/w 2.7 whose purification will be fully described as an example of the use of the CCC technique.
Adapted from Ref. [24].
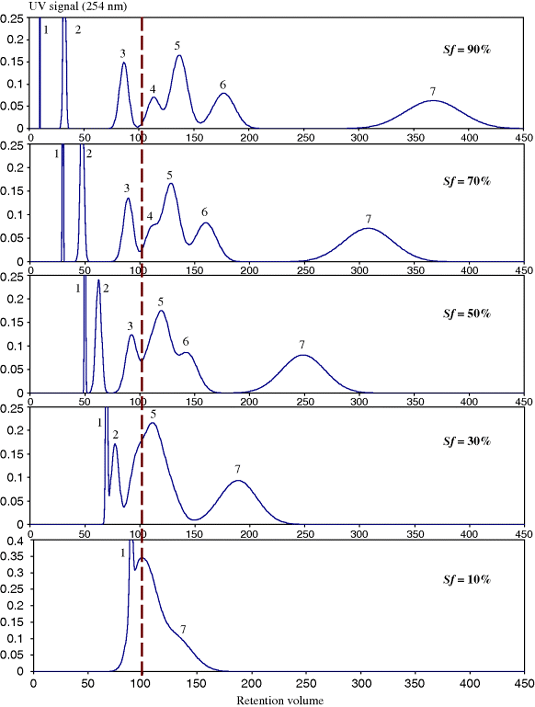
)4.2.2 Rapid Protocol for Liquid System Determination
The heptane/ethyl acetate/methanol/water system was selected working with AZ composition (Table 2). Figure 5 shows the CCC chromatograms obtained with different composition as indicated by their letter. The asarinine position is pointed out by a blue arrow. The elution–extrusion procedure was used. The first step is a classical elution with the lower aqueous phase in the head-to-tail or descending flowing direction. It is marked by a dotted gray bar on Figure 5 chromatograms [24]. After one column volume of mobile phase (40 ml or 20 min at 2 ml/min), the aqueous lower phase is replaced by the organic upper phase not changing the flowing direction. This is the extrusion step since flowing the upper phase in the head-to-tail or descending direction pushes the whole CCC column content out maintaining the respective positions of all separated compounds in the CCC column [25]. If a column volume of organic phase is used in the extrusion step (40 ml or 20 min), it was demonstrated that everything injected into the CCC column is eluted. The extrusion steps are marked by a dashed gray bar in Figure 5.
We begin trying different AZ compositions well separated to sweep a large polarity range. The rather polar Composition J (heptane/ethyl acetate/methanol/water 2/5/2/5 v/v) does not elute asarinine that stays in the organic stationary phase being eluted during the extrusion step. A similar result is obtained with middle Composition N (1/1/1/1 v/v) that does show a better partitioning of the extract. Composition S (5/2/5/2 v/v) elutes asarinine during the elution step. However, it does not show as a well-isolated peak. These three essays tell that the right AZ composition is between N and S. So, the three P, Q, and R compositions are tried (Figure 5, bottom). Composition Q (3/2/3/2 v/v) shows a fraction III (Figure 5) that contains essentially asarinine. A scaling up can be done with this liquid system.
4.2.3 Geometrical Scaling Up
Composition Q (heptane/ethyl acetate/methanol/water 3/2/3/2 v/v) was used with a larger hydrodynamic CCC column of 140 ml in which it was easy to inject 100 mg of extract [24]. This procedure produced the Figure 5 chromatogram with five fractions and fraction III being essentially ∼20 mg of the desired asarinine compound identified by HPLC, MS, and NMR [24]. Since exactly the same liquid system can be used on larger CCC columns, the scaling up is straightforward being almost geometrical: an x time larger column can separate an x time larger amount of sample in the same time if the flow rate is increased x times [6]. It was even found that the phase mixing being better in larger CCC column, it is possible to inject more than the amount predicted by simple geometrical scaling up [26].
4.3 Large-Scale CCC Purification
The purification of an industrial pest control agent with the trade name spinetoram (Dow Chemical) was done by LC on C18 silica stationary phases. A full comparison of this purification using LC and CCC was done and fully documented [27].
4.3.1 The Sample
spinetoram is a polyketide macrolide prepared by the chemical modification of a spynosyn mixture obtained by fermentation of the actinomycete Saccharopolyspora spinosa. Spinetoram has a large spectrum of activity and fast mode of action against pests attacking crops, food-producing plants, and fruit trees such as thrips, leafminer flies, whiteflies, lepidopteran insects, diamondback moth, leafroller with academic names such as Spodoptera exigua, Plutella xylostella, Frankliniella sp., Cydia pomonella, or Trichoplusia ni. As derivatives of biologically produced compounds, spinetorams pose less environmental risk than chlorinated pesticides or other totally synthetic ones [27].
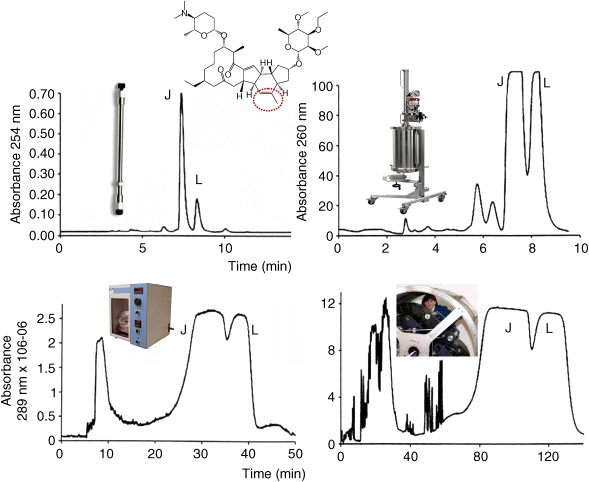
Spinetoram is a mixture of two compounds: spinetoram-J (C42H69NO10, m.w. 748, log P 4.1, ∼75%) and spinetoram-L (C43H69NO10, m.w. 760, log P 4.5, ∼25%) differing by the presence of a double bond and a methyl group at the C-6 position for the L form (dotted circle in Figure 6a, left) when the J form has two methylene groups. The biological activity of pure J or L forms is far superior to that of the mixture justifying the separation.
4.3.2 Preparative Liquid Chromatography
The common way to separate the J and L spinetoram forms is using reversed phase liquid chromatography. Figure 6a (, left) shows the analytical chromatogram easily obtained with isocratic elution with a 1 ml/min flow rate of acetonitrile/pH 9 buffer with 10 mM ammonium hydroxide 95:5% v/v on a 250 mm × 4.6 mm C18 Gemini™ 5 µm column from Phenomenex (Torrance, CA, USA). A 0.25 mg of spinetoram produced two peaks with a baseline resolution in about 10 min with 0.2 mg and 50 µg of pure spinetoram-J and -L, respectively. To produce 1 g of pure spinetoram-J, it would be theoretically necessary to repeat 5000 times this analytical separation needing 800 h and 50 l of mobile phase. This is completely unrealistic. A different system dedicated to preparative purification is used.
A Prochrom® LC110 column of 25 cm × 11 cm containing 1.8 kg of XTerra C8 10 µm silica stationary phase (Waters, Milford, MA, USA) could be loaded with 2.5 g of crude spinetoram contained in 10 ml of a 250 g/l solution. To obtain the chromatogram in Figure 6a (right), it was necessary to adapt the mobile-phase composition that was acetonitrile/pH 9 buffer (10 mM NH3) 60:40% v/v because of the different silica stationary phase. The flow rate was 0.8 l/min allowing to obtain 2 g of 97% pure spinetoram-J in 10 min using 4 l of mobile phase. This gives a theoretical time of 5 min with 2 l of mobile phase per gram of pure spinetoram-J, orders of magnitude better than the repetition of the analytical purification.
4.3.3 Countercurrent Chromatography
Using a protocol similar to the one described for the P. longum natural product, the best AZ system for the spinetoram separation is the W system heptane/ethyl acetate/methanol/water 6/1/6/1 v/v (Table 2) used in the reversed phase mode (aqueous mobile phase in the head-to-tail or descending flowing direction). A loading study was done showing that the analytical 22 ml minidynamic extraction (Slough, UK) was able to separate up to 30 mg of spinetoram as shown by Figure 6b (left) in 45 min with a 1 ml/min flow rate. In this study, 22 mg and 7.5 mg of pure spinetoram-J and -L were, respectively, obtained. Repeating the operation to obtain 1 g of pure spinetoram-J, it would be theoretically necessary to do 45 CCC separations needing more than 30 h and 2 l of mobile phase. If this is not very realistic, it is definitively much better than the theoretical 800 h and 50 l of the RPLC method (Figure 6a, left).
The Brunel University CCC center has a pilot-scale 18 l hydrodynamic CCC machine (inset photo Figure 6b, right). Injecting 111 g of crude spinetoram into this CCC column and using exactly the same Arizona W system made it possible to recover 78 g of 97% pure spinetoram-J and 29 g of 98% pure spinetoram-L in 2.5 h, using 55 l of mobile phase. This gives a theoretical time of 2 min using 0.7 l of mobile phase per gram of pure spinetoral-J. Table 3 compares the results in terms of time, solvent, and productivity for the two prep-LC and CCC methods. The kilo of Spinetoram-J was contained in 85 l of CCC mobile phase compared to 290 l of prep-LC mobile phase, a 340% higher volume of phase that must be removed to recover the solid product. In conclusion, on this particular purification, CCC uses three times less solvent and energy to produce the same mass of pure compound with the same yield and purity than prep-LC with silica stationary phase [27].
| Parameter | Prep-LC | CCC |
|---|---|---|
| Experiment duration | 8 min | 2 h 20 min |
| Loading per run | 2.5 g | 111 g |
| Number of runs for 1 kg of J | 460 | 14 |
| Time to purify 1 kg of J | 61 h | 32 h |
| Phase volume needed | 2640 l | 700 l |
| Volume of fractions with spinetoram-J | 290 l | 85 l |
| Purity J/l | 97%/98% | 97%/98% |
| Yield J/l | 63%/15% | 63%/15% |
- a Source: Data from Ref. [27].
5 A Glossary of CCC Terminology
5.1 Terms Related to CCC Instrumentation
Archimedean Screw
In rotating bobbins or spools with coiled tubes, the thread of the tube produces a force that pushes the contained liquid toward one end of the tube or the other. This force is called an Archimedean screw. Its intensity and direction depend on the rotational speed and direction and on the way the coil was made. When two immiscible liquid phases are contained in the coiled tube, the denser liquid is subjected to a stronger force than the lighter liquid phase pushing it to gather at one end of the coil called head. The denser liquid phase gathers at the tail end that is also the low-pressure point of the coil (possible air suction).
β Ratio
It was demonstrated [2-4] that the β ratio must be higher than 0.5 for the liquid contained in the coiled tube to follow a looped cardioid motion. This motion produces an inversion of the centrifugal field direction, hence the mingling decantation of the two liquid phases. If β is lower than 0.5, the loop does not form and without inversion of the centrifugal field direction, the liquid stationary phase is not retained. All hydrodynamic CCC columns contain multilayer coils so the higher, lower, and average β values are often indicated corresponding, respectively, to the external, internal, and average tubing layer radius in the bobbin.
Centrifugal Partition Chromatograph (CPC)
See Section 2.4.
Channel Equivalent to a Theoretical Plate
The number of channels in a CPC hydrostatic machine that are needed to produce one theoretical plate. This is s measure of the efficiency of the liquid system and chromatographic efficiency.
Column Volume
One of the principal instrumental parameters in CCC is the total volume of the column, which is important when understanding analyte elution and when calculating countercurrent chromatograms. Total column volume (VC) also determines the load capacity of a particular machine. It is recommended to use the experimental column volume obtained by measuring the retention volume of a compound injected into the column containing a single liquid phase. This experimental VC volume will include extracolumn called “dead” volumes such as injection valve, detector cell, and connecting tubing; but all these extracolumn volumes are filled by mobile phase during the run and can be integrated into the VM volume for calculations.
Coil Planet Centrifuge
A hydrodynamic CCC machine containing one or several spools or bobbins of coiled tube mounted on a rotor, first introduced by Ito [1]. A two-axis arrangement produces the rotation of each spool about itself with a simultaneous rotation around the parallel central axis (planetary motion). Various forms of coil planet centrifuge are possible.
Cross-Axis Chromatograph
A kind of hydrodynamic CCC machine containing two spools of coiled tube mounted on a rotor in such a way that the axis of rotation of the spools is at right angle to the central axis of rotation of the rotor. This type of motion is sometimes described as an X-type coil planet centrifuge. It was never commercialized as it is mechanically very fragile.
Droplet CCC
The first hydrostatic CCC column working with gravity only. See Section 2.4.
Dual Mode
A particular way to use a CCC column to elute strongly retained solutes. After elution of a given volume of mobile phase, the phase role is interchanged. The mobile phase becomes the stationary phase and vice versa. Since the density difference is reversed, the flowing direction of the phases is also reversed.
Dual CCC
Dual countercurrent chromatography performs genuine countercurrent chromatography in which both upper and lower phases move through a coiled column in the opposite direction. Dual CCC has been achieved with both a multilayer coiled separation column and a spiral disk.
Gravitational (g) Force
The force or field of forces exercised to hold the stationary phase in the CCC column. This field is calculated as a function of rotational speed (rpm) and rotational radius. The relative gravitational field is calculated by g = (1.119 × 10−5) R (rpm)2, where R is the distance from the center of the main rotor to the planetary axis expressed in centimeters.
Head
In hydrodynamic CCC, the end of the coil where the liquid (or any small light object such a bubble or dense object such a bead) is pushed by the Archimedean screw action when the rotor is spinning. Therefore, the head is the high-pressure column side.
Hydrodynamic CCC
A CCC machine containing planetary spools or bobbins, on which tubing is wound. Two axes of rotation and a gear arrangement produce a periodically changing centrifugal acceleration field. The machine generates the centrifugal field needed to retain the liquid stationary phase. During a run, the machine can be considered a column containing a volume VS of liquid stationary phase and VM of mobile phase. Most hydrodynamic machines do not have any rotary seal.
Hydrostatic CCC
A CCC machine containing channels, chambers, or geometrical units interconnected according to a repetitive pattern in a centrifuge rotor and working with a constant centrifugal field (one axis of rotation). These machines are also called centrifugal partition chromatographs, a term that was once a trademark. CPC instruments use rotating seals and generally operate at higher back pressures than hydrodynamic CCC columns. The old droplet CCC (DCCC) contained only interconnected tubes and worked with the gravitational field. The DCCC is a hydrostatic column without centrifuge.
High-Performance CCC Columns (HPCCCs)
A XXIst terminology to design modern hydrodynamic CCC columns able to generate high g fields and to work with combined high flow rates and high stationary-phase retention ratios, producing fast and efficient separations [13, 26].
High-Speed Countercurrent Chromatographs (HSCCCs)
A hydrodynamic CCC system that uses a multilayer coil separation column rotating in a planetary motion introduced by Ito [2, 10]. The term high speed was used because the new hydrodynamic CCC columns were able to separate mixtures in several hours, while several days were needed with gravitational hydrostatic droplet CCC columns. Since a several hour separation is today a very low-speed separation, it is misleading and even ridiculous to use this term and the acronym HSCCC. In case of a several hour CCC separation, it is recommended to ban this term and the HSCCC acronym just using the term and acronym CCC.
Planetary Motion
Planetary motion involves two axes. The “sun” axis holds a rotor on which one or several coils are mounted. Each coil can rotate around its own axis, called the “planet” axis. The presence of these two distinct axes allows for a planetary rotation of the coil around the central or “sun” axis. Both the rotor and the coil(s) rotate in the same direction, and a simple gear arrangement produces a coil rotation exactly double the rotor rotation. Numerous experiments were done varying the relative speed and/or the direction of rotation. The synchronous rotation arrangement, referred to as “J-type,” was found both to have the simpler mechanical arrangement and to retain stationary phase and any other design. It is used exclusively in modern CCC hydrodynamic instruments.
Rotary Seal
A device that allows to connect a rotating tube to a fixed tube without leaks.
Spiral Disk CCC
A spiral channel cut into an inert plastic disk allows multilayer coil centrifuge-type separation with an enhanced retention of stationary phase. Several spiral disks piled together in one unit essentially replace the coil of a traditional CCC instrument [28].
Tail
In hydrodynamic CCC, the tail is the end of the coil opposite to the head. It is also the low-pressure column side; the pressure may even be negative inducing suction.
Toroidal Coil Centrifuge (TCC)
A hydrostatic CCC device in which the geometrical pattern is simply a turn of tubing arranged at a fixed radius in a uniform centrifugal force field.
Two-Dimensional CCC
Following the fashion “two-dimensional” or “orthogonal” trend that associates in-line two separation modes, successive CCC separations may be arranged in a “multidimensional” CCC experiment. In brief, carefully selected volumes of the effluent from the first CCC column are introduced into a second CCC column with a different biphasic liquid system to enhance the separation of target analytes [29].
5.2 Terms Related to CCC Operation
Ascending Mode
Term used in hydrostatic CCC columns or centrifugal partition chromatographs indicating that the mobile phase is entering through the bottom of the column and leaving it out of the top. The mobile phase is the lighter liquid, while the stationary phase is the denser one. This mode corresponds to the tail-to-head mode for hydrodynamic machines. There is no objection to use the two ascending and tail-to-head terms indifferently for all CCC columns.
Back-Extrusion CCC
In back-extrusion CCC, the direction of the flow through the CCC instrument is changed at some point during the chromatographic run. The overall effect is comparable to both elution-extrusion and dual-mode CCC [5].
Classical Elution
Elution of analytes in the “right” way, that is, if the mobile phase is the upper phase, it must be pumped in the CCC column in the ascending or tail-to-head direction. If the mobile phase is the denser lower phase, it must be pumped in the CCC column in the descending or head-to-tail direction.
Cocurrent CCC
A special use of the CCC technique in which the “stationary” phase is slowly pumped in the same direction but at a lower speed than the mobile phase [5].
Descending Mode
The term used in hydrostatic CCC columns indicating that the mobile phase is entering through the top of the column and leaving it out of the bottom. The mobile phase is the denser liquid, while the stationary phase is the lighter one. This mode corresponds to the head-to-tail mode for hydrodynamic columns.
Dual Mode
A particular way to use a CCC column to elute strongly retained solutes. After elution of a given volume of mobile phase, the phase role is interchanged. The mobile phase becomes the stationary phase and vice versa. Since the density difference is reversed, the flowing direction of the phases is also reversed.
Elution–Extrusion CCC (EECCC)
EECCC is a recently developed and fully parameterized CCC method that takes advantage of the liquid nature of the stationary phase by combining classical elution and extrusion in a single run. EECCC allows coverage of the whole polarity range of analytes from KD = 0 to infinity. After an initial elution stage in classical mode, extrusion of the stationary phase is achieved by switching the supply of the flowing liquid from the mobile phase to the originally stationary phase, while maintaining the centrifugal force through continued rotation. The point at which extrusion is begun (called the switch volume, VCM) can be adjusted to optimize the resolution of target analytes and minimize the runtime. When VR is equal to VCM + VC (VC being the total volume of the column), all analytes will have exited the column [24, 25].
Extrusion
The process of pushing out the stationary-phase portion of the CCC column. After performing classical elution for a certain period of time, the noneluted analytes migrate inside the column. Extrusion provides access to these analytes without the need to reach the elution volume, and this can be achieved by pumping stationary phase into the column. The third stage of EECCC is called the extrusion stage [24, 25].
Gradient CCC
In gradient CCC, the composition of the mobile phase is adjusted during the CCC run to help hasten the elution of highly retained compounds from the column. The composition of the liquid stationary phase also changes [14].
Head to Tail
The term indicating that the mobile phase is entering a hydrodynamic column through its head and leaving it out of the tail. The mobile phase in this configuration is the denser liquid, while the stationary phase is the lighter one. This mode corresponds to the descending mode for hydrodynamic columns. There is no objection to use the two descending and head-to-tail terms indifferently for all CCC columns.
Retention Volume (VR)
The volume of mobile phase needed to elute a particular analyte. Retention volumes are often calculated by multiplying the retention time and the flow rate. VR depends on the liquid phase ratio inside the CCC column and on the solute liquid–liquid distribution ratio (Equation 3).
Stationary-Phase Volume Retention Ratio (Sf)
This is the major difference between the CCC techniques and all other chromatographic techniques: the liquid stationary phase volume is not a constant. It depends on the experimental conditions so that the special parameter, Sf, is needed to quantitate it. Sf is the ratio of the volume of the stationary phase retained in the CCC machine to that of the machine volume (Equation 1).
(Note: Sf is often expressed as a percentage. The simpler term stationary-phase retention ratio may also be used.)
Tail to Head
The term indicates that the mobile phase is entering a hydrodynamic CCC column through its tail and leaving it out of the head. The tail coil side is the low-pressure point of the hydrodynamic CCC column; the pressure can be negative (suction). In classical mode, the mobile phase in this configuration is the lighter liquid, while the stationary phase is the denser one. This mode corresponds to the ascending mode for hydrostatic columns. There is no objection to use the two tail-to-head and ascending terms indifferently for all CCC columns.
5.3 Terms Related to the Liquid System
Aqueous Two-Phase System
A biphasic liquid system in which both phases are aqueous solutions (e.g., a phosphate salt solution and a polyoxyethylene glycol solution).
Biphasic Liquid System
A mixture of two or more solvents producing two immiscible liquid phases.
Displacer
In pH zone-refining CCC, the displacer is contained in the mobile phase. It will ionize first the solutes and, last, the retainer contained in the stationary phase.
Distribution Constant (K)
- A good synonym for K is partition ratio. The term partition coefficient is not recommended by IUPAC [30].
- The distribution constant is proportional to the CCC retention time if the solute can exist in only a single definite form (no ionization, no complexation, no chemical reaction possible). In that case and in that case only, K = D, and the solute retention volume is directly proportional to K as related by Equation 13.
- The distribution constant of a particular solute depends only on the physicochemical properties of the liquid system used, composition and temperature, but not on the CCC column.
Distribution Ratio (D)
The ratio of the total analytical concentration of a solute in the liquid stationary phase, regardless of its chemical form, to its total analytical concentration in the mobile phase.
- The term ratio is preferable to coefficient.
- As defined above, the distribution ratio varies with experimental conditions, for example, pH and the presence of complexing agents. It should not be confused with distribution constant, K (or partition coefficient, P, a term not recommended but still used), which applies to a particular chemical species and is by definition invariable.
- The distribution ratio of a solute is directly proportional to its CCC retention time or volume.
Extractant
The active component(s) dissolved in one liquid phase only that is primarily responsible for transfer of a solute from one phase to the other.
Nonaqueous Solvent Systems
Most biphasic solvent system formulations utilize water in the hydrophilic (aqueous) phase, nonaqueous solvent systems do not. Nonaqueous solvent systems, for example, heptane/methanol, hexane/dimethyl sulfoxyde, hexane/dichloromethane/acetonitrile, or heptane/acetonitrile/acetic acid, are used as low-polarity solvent system to purify for example, fatty acids, petroleum fractions, carotenoids, or terpenoid lipophilic natural products [31].
Partition Coefficient (P or K)
The solute distribution ratio is very commonly called partition coefficient even though this was not recommended by IUPAC in its nomenclature recommendation for chromatography [30].
pH Zone Refining
A form of displacement chromatography in CCC in which ionizable solutes are sorted in bands (zones) by a pH gradient created between an acidic liquid stationary phase and a basic mobile phase, or vice versa. pH zone refining does not work with nonionizable solutes.
Retainer
In pH zone-refining CCC, the retainer is contained in the stationary phase. It will retain the injected solutes in their neutral molecular form until they are ionized by the displacer contained in the mobile phase.
Solvent Front
At the beginning of a CCC experiment, the column/coil is full of stationary phase. The solutes are injected into the mobile phase. The stationary phase is displaced by the mobile phase. The first drop of mobile phase seen at the outlet of the machine corresponds to the solvent front. The volume of displaced stationary phase is equal to the volume of mobile phase inside the machine, VM. Knowing the solvent front allows an estimate of the stationary phase volume retention ratio, Sf, to be made.
6 Conclusions
Countercurrent chromatography is a liquid chromatographic method that uses a liquid stationary phase and an immiscible liquid mobile phase. There is no countercurrent circulation of fluids in CCC, the name being somewhat a misnomer. The term centrifugal partition chromatography would be much better as a generic term for CCC. For historical reasons, CPC is reserved to hydrostatic CCC columns, not making things easy to understand to newcomers to the technique. The technique is essentially a preparative technique. The modern trend is to develop reliable CCC “columns” based on the hydrostatic or the hydrodynamic designs of different volumes. The CCC column volume is adapted to the desired productivity. Small CCC “columns” allow to quickly find the right biphasic liquid system adapted to the purification. Scaling up to a larger volume CCC instrument is relatively straightforward as it is mostly linear. It is pointed out that the CCC practice implies knowledge of the biphasic liquid system's physic-chemical behavior that is poorly taught today.



