Dimeric P-loop ATPase CFD1 and Related Proteins
Sven A Freibert
Institut für Zytobiologie und Zytopathologie, Philipps-Universität Marburg, 35032 Marburg, Germany
LOEWE Zentrum für Synthetische Mikrobiologie SynMikro, 35043 Marburg, Germany
Search for more papers by this authorJae-Hun Jeoung
Institut für Biologie, Strukturbiologie/Biochemie, Humboldt-Universität Berlin, 10115 Berlin, Germany
Search for more papers by this authorOliver Stehling
Institut für Zytobiologie und Zytopathologie, Philipps-Universität Marburg, 35032 Marburg, Germany
Search for more papers by this authorHolger Dobbek
Institut für Biologie, Strukturbiologie/Biochemie, Humboldt-Universität Berlin, 10115 Berlin, Germany
Search for more papers by this authorRoland Lill
Institut für Zytobiologie und Zytopathologie, Philipps-Universität Marburg, 35032 Marburg, Germany
LOEWE Zentrum für Synthetische Mikrobiologie SynMikro, 35043 Marburg, Germany
Search for more papers by this authorSven A Freibert
Institut für Zytobiologie und Zytopathologie, Philipps-Universität Marburg, 35032 Marburg, Germany
LOEWE Zentrum für Synthetische Mikrobiologie SynMikro, 35043 Marburg, Germany
Search for more papers by this authorJae-Hun Jeoung
Institut für Biologie, Strukturbiologie/Biochemie, Humboldt-Universität Berlin, 10115 Berlin, Germany
Search for more papers by this authorOliver Stehling
Institut für Zytobiologie und Zytopathologie, Philipps-Universität Marburg, 35032 Marburg, Germany
Search for more papers by this authorHolger Dobbek
Institut für Biologie, Strukturbiologie/Biochemie, Humboldt-Universität Berlin, 10115 Berlin, Germany
Search for more papers by this authorRoland Lill
Institut für Zytobiologie und Zytopathologie, Philipps-Universität Marburg, 35032 Marburg, Germany
LOEWE Zentrum für Synthetische Mikrobiologie SynMikro, 35043 Marburg, Germany
Search for more papers by this authorAbstract
The assembly of iron–sulfur (Fe/S) proteins in living cells requires complex machineries. The initial synthesis of a Fe/S cluster occurs on specialized scaffold proteins rather than on target apoproteins. The scaffold needs to support both facile assembly and ready transfer of clusters to Fe/S trafficking proteins that assist cluster delivery to final recipients. In the cytosolic iron–sulfur protein assembly (CIA) machinery of eukaryotes, the hetero-oligomeric CFD1-NBP35 complex is performing a scaffold function. Both proteins belong to the metal binding P-loop NTPase family whose members typically are involved in metal center assembly. In vivo, Fe/S cluster assembly on CFD1-NBP35 depends on functional nucleotide binding sites in both proteins, a sulfur compound exported from mitochondria, and the CIA electron transfer chain TAH18-DRE2. In vitro, CFD1-NBP35 bind a bridging [4Fe–4S] cluster at two conserved cysteine residues of each protein. The crystal structure of homodimeric CFD1, as a model of the heterodimer, locates these clustercoordinating cysteine residues on exposed loops. The bridging cluster coordination appears to be ideal for both facile cluster assembly and rapid transfer to downstream CIA trafficking proteins. The crucial cellular function of CFD1-NBP35 is indicated by their essential in vivo requirement for assembly of most cytosolic and nuclear Fe/S proteins.
3D Structure
Adapted from reference 1 and UCSF Chimera (E. F. Pettersen, T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng, T. E. Ferrin, Journal of Computational Chemistry 2004, 25, 1605–1612).2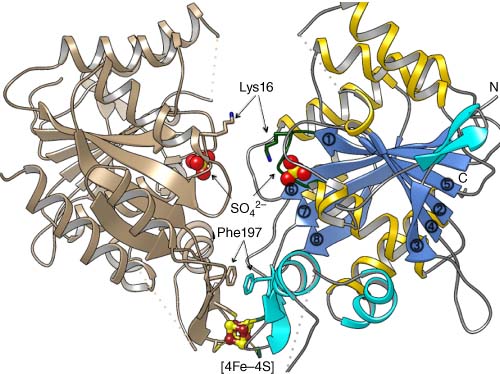
References
- 1O Stehling, JH Jeoung, SA Freibert, VD Paul, S Banfer, B Niggemeyer, R Rosser, H Dobbek and R Lill, Proc Natl Acad Sci U S A, 115, E9085–E9094 (2018). DOI: 10.1073/pnas.1807762115.
- 2EF Pettersen, TD Goddard, CC Huang, GS Couch, DM Greenblatt, EC Meng and TE Ferrin, J Comput Chem, 25, 1605–1612 (2004).
- 3A Roy, N Solodovnikova, T Nicholson, W Antholine and WE Walden, EMBO J, 22, 4826–4835 (2003). DOI: 10.1093/emboj/cdg455.
- 4A Hausmann, DJ Aguilar Netz, J Balk, AJ Pierik, U Mühlenhoff and R Lill, Proc Natl Acad Sci U S A, 102, 3266–3271 (2005).
- 5DJ Netz, AJ Pierik, M Stümpfig, U Mühlenhoff and R Lill, Nat Chem Biol, 3, 278–286 (2007). DOI: 10.1038/nchembio872.
- 6O Stehling, DJ Netz, B Niggemeyer, R Rosser, RS Eisenstein, H Puccio, AJ Pierik and R Lill, Mol Cell Biol, 28, 5517–5528 (2008). DOI: 10.1128/MCB.00545-08.
- 7EJ Camire, JD Grossman, GJ Thole, NM Fleischman and DL Perlstein, J Biol Chem, 290, 23793–23802 (2015). DOI: 10.1074/jbc.M115.667022.
- 8DJ Netz, AJ Pierik, M Stümpfig, E Bill, AK Sharma, LJ Pallesen, WE Walden and R Lill, J Biol Chem, 287, 12365–12378 (2012). DOI: 10.1074/jbc.M111.328914.
- 9LJ Pallesen, N Solodovnikova, AK Sharma and WE Walden, J Biol Chem, 288, 23358–23367 (2013). DOI: 10.1074/jbc.M113.486878.
- 10VD Paul and R Lill, Biochim Biophys Acta, 2015, 1528–1539 (1853). DOI: 10.1016/j.bbamcr.2014.12.018.
- 11JD Grossman, KA Gay, EJ Camire, WE Walden and DL Perlstein, Biochemistry, 58, 2017–2027 (2019). DOI: 10.1021/acs.biochem.8b00737.
- 12K Bych, DJ Netz, G Vigani, E Bill, R Lill, AJ Pierik and J Balk, J Biol Chem, 283, 35797–35804 (2008). DOI: 10.1074/jbc.M807303200.
- 13A Karnkowska, V Vacek, Z Zubacova, SC Treitli, R Petrzelkova, L Eme, L Novak, V Zarsky, LD Barlow, EK Herman, P Soukal, M Hroudova, P Dolezal, CW Stairs, AJ Roger, M Elias, JB Dacks, C Vlcek and V Hampl, Curr Biol, 26, 1274–1284 (2016). DOI: 10.1016/j.cub.2016.03.053.
- 14H Kohbushi, Y Nakai, S Kikuchi, T Yabe, H Hori and M Nakai, Biochem Biophys Res Commun, 378, 810–815 (2009). DOI: 10.1016/j.bbrc.2008.11.138.
- 15EL Bastow, K Bych, JC Crack, NE Le Brun and J Balk, Plant J, 89, 590–600 (2017). DOI: 10.1111/tpj.13409.
- 16J Balk and TA Schaedler, Annu Rev Plant Biol, 65, 125–153 (2014). DOI: 10.1146/annurev-arplant-050213-035759.
- 17J Godman and J Balk, Genetics, 179, 59–68 (2008). DOI: 10.1534/genetics.107.086033.
- 18C Grosche, A Diehl, SA Rensing and UG Maier, Genome Biol Evol, 10, 2061–2071 (2018). DOI: 10.1093/gbe/evy156.
- 19AA Vashisht, CC Yu, T Sharma, K Ro and JA Wohlschlegel, J Biol Chem, 290, 14218–14225 (2015). DOI: 10.1074/jbc.M115.650762.
- 20M Uhlen, L Fagerberg, BM Hallstrom, C Lindskog, P Oksvold, A Mardinoglu, A Sivertsson, C Kampf, E Sjostedt, A Asplund, I Olsson, K Edlund, E Lundberg, S Navani, CA Szigyarto, J Odeberg, D Djureinovic, JO Takanen, S Hober, T Alm, PH Edqvist, H Berling, H Tegel, J Mulder, J Rockberg, P Nilsson, JM Schwenk, M Hamsten, K von Feilitzen, M Forsberg, L Persson, F Johansson, M Zwahlen, G von Heijne, J Nielsen and F Ponten, Science, 347, 1260419 (2015). DOI: 10.1126/science.1260419.
- 21K Bych, S Kerscher, DJ Netz, AJ Pierik, K Zwicker, MA Huynen, R Lill, U Brandt and J Balk, EMBO J, 27, 1736–1746 (2008).
- 22AD Sheftel, O Stehling, AJ Pierik, DJ Netz, S Kerscher, HP Elsässer, I Wittig, J Balk, U Brandt and R Lill, Mol Cell Biol, 29, 6059–6073 (2009). DOI: 10.1128/MCB.00817-09.
- 23SE Calvo, EJ Tucker, AG Compton, DM Kirby, G Crawford, NP Burtt, M Rivas, C Guiducci, DL Bruno, OA Goldberger, MC Redman, E Wiltshire, CJ Wilson, D Altshuler, SB Gabriel, MJ Daly, DR Thorburn and VK Mootha, Nat Genet, 42, 851–858 (2010). DOI: 10.1038/ng.659.
- 24AE Maclean, VE Kimonis and J Balk, Hum Mol Genet, 27, 3697–3709 (2018). DOI: 10.1093/hmg/ddy247.
- 25MM Wydro, P Sharma, JM Foster, K Bych, EH Meyer and J Balk, Plant Cell, 25, 4014–4027 (2013). DOI: 10.1105/tpc.113.117283.
- 26L Lezhneva, K Amann and J Meurer, Plant J, 37, 174–185 (2004).
- 27S Schwenkert, DJ Netz, J Frazzon, AJ Pierik, E Bill, J Gross, R Lill and J Meurer, Biochem J, 425, 207–214 (2010). DOI: 10.1042/BJ20091290.
- 28JM Boyd, AJ Pierik, DJ Netz, R Lill and DM Downs, Biochemistry, 47, 8195–8202 (2008).
- 29JM Boyd, WP Teoh and DM Downs, J Bacteriol, 194, 576–583 (2012). DOI: 10.1128/JB.05988-11.
- 30JM Boyd, RM Drevland, DM Downs and DE Graham, J Bacteriol, 191, 1490–1497 (2009). DOI: 10.1128/JB.01469-08.
- 31C Zhao, Z Lyu, F Long, T Akinyemi, K Manakongtreecheep, D Soll, WB Whitman, DJ Vinyard and Y Liu, FEBS Lett, 594, 924–932 (2019). DOI: 10.1002/1873-3468.13673.
- 32R Lill, R Dutkiewicz, SA Freibert, T Heidenreich, J Mascarenhas, DJ Netz, VD Paul, AJ Pierik, N Richter, M Stümpfig, V Srinivasan, O Stehling and U Mühlenhoff, Eur J Cell Biol, 94, 280–91 (2015). DOI: 10.1016/j.ejcb.2015.05.002.
- 33R Lill, Nature, 460, 831–838 (2009).
- 34R Lill and SA Freibert, Annu Rev Biochem (2020) in press.
- 35U Mühlenhoff, S Molik, JR Godoy, MA Uzarska, N Richter, A Seubert, Y Zhang, J Stubbe, F Pierrel, E Herrero, CH Lillig and R Lill, Cell Metab, 12, 373–385 (2010).
- 36L Banci, F Camponeschi, S Ciofi-Baffoni and R Muzzioli, J Am Chem Soc, 137, 16133–16143 (2015). DOI: 10.1021/jacs.5b10592.
- 37AG Frey, DJ Palenchar, JD Wildemann and CC Philpott, J Biol Chem, 291, 22344–22356 (2016). DOI: 10.1074/jbc.M116.744946.
- 38O Stehling, AA Vashisht, J Mascarenhas, ZO Jonsson, T Sharma, DJ Netz, AJ Pierik, JA Wohlschlegel and R Lill, Science, 337, 195–199 (2012). DOI: 10.1126/science.1219723.
- 39K Gari, AM Leon Ortiz, V Borel, H Flynn, JM Skehel and SJ Boulton, Science, 337, 243–245 (2012). DOI: 10.1126/science.1219664.
- 40O Stehling, J Mascarenhas, AA Vashisht, AD Sheftel, B Niggemeyer, R Rösser, AJ Pierik, JA Wohlschlegel and R Lill, Cell Metab, 18, 187–198 (2013). DOI: 10.1016/j.cmet.2013.06.015.
- 41VD Paul, U Mühlenhoff, M Stümpfig, J Seebacher, KG Kugler, C Renicke, C Taxis, AC Gavin, AJ Pierik and R Lill, eLife, 4, e08231 (2015). DOI: 10.7554/eLife.08231.
- 42H Nakashima, MJ Grahovac, R Mazzarella, H Fujiwara, JR Kitchen, TA Threat and MS Ko, Genomics, 60, 152–160 (1999). DOI: 10.1006/geno.1999.5898.
- 43A Christodoulou, CW Lederer, T Surrey, I Vernos and N Santama, J Cell Sci, 119, 2035–2047 (2006).
- 44S Misra, MA Crosby, CJ Mungall, BB Matthews, KS Campbell, P Hradecky, Y Huang, JS Kaminker, GH Millburn, SE Prochnik, CD Smith, JL Tupy, EJ Whitfied, L Bayraktaroglu, BP Berman, BR Bettencourt, SE Celniker, AD de Grey, RA Drysdale, NL Harris, J Richter, S Russo, AJ Schroeder, SQ Shu, M Stapleton, C Yamada, M Ashburner, WM Gelbart, GM Rubin and SE Lewis, Genome Biol, 3, research0083.1 (2002). DOI: 10.1186/gb-2002-3-12-research0083.
10.1186/gb‐2002‐3‐12‐research0083 Google Scholar
- 45NM El-Sayed, PJ Myler, G Blandin, M Berriman, J Crabtree, G Aggarwal, E Caler, H Renauld, EA Worthey, C Hertz-Fowler, E Ghedin, C Peacock, DC Bartholomeu, BJ Haas, AN Tran, JR Wortman, UC Alsmark, S Angiuoli, A Anupama, J Badger, F Bringaud, E Cadag, JM Carlton, GC Cerqueira, T Creasy, AL Delcher, A Djikeng, TM Embley, C Hauser, AC Ivens, SK Kummerfeld, JB Pereira-Leal, D Nilsson, J Peterson, SL Salzberg, J Shallom, JC Silva, J Sundaram, S Westenberger, O White, SE Melville, JE Donelson, B Andersson, KD Stuart and N Hall, Science, 309, 404–409 (2005). DOI: 10.1126/science.1112181.
- 46B Loftus, I Anderson, R Davies, UC Alsmark, J Samuelson, P Amedeo, P Roncaglia, M Berriman, RP Hirt, BJ Mann, T Nozaki, B Suh, M Pop, M Duchene, J Ackers, E Tannich, M Leippe, M Hofer, I Bruchhaus, U Willhoeft, A Bhattacharya, T Chillingworth, C Churcher, Z Hance, B Harris, D Harris, K Jagels, S Moule, K Mungall, D Ormond, R Squares, S Whitehead, MA Quail, E Rabbinowitsch, H Norbertczak, C Price, Z Wang, N Guillen, C Gilchrist, SE Stroup, S Bhattacharya, A Lohia, PG Foster, T Sicheritz-Ponten, C Weber, U Singh, C Mukherjee, NM El-Sayed, WA Petri Jr CG Clark, TM Embley, B Barrell, CM Fraser and N Hall, Nature, 433, 865–868 (2005). DOI: 10.1038/nature03291.
- 47SA Freibert, AV Goldberg, C Hacker, S Molik, P Dean, TA Williams, S Nakjang, S Long, K Sendra, E Bill, E Heinz, RP Hirt, JM Lucocq, TM Embley and R Lill, Nat Commun, 8, 13932 (2017). DOI: 10.1038/ncomms13932.
- 48FW Studier, Protein Expr Purif, 41, 207–234 (2005). DOI: 10.1016/j.pep.2005.01.016.
- 49SA Freibert, BD Weiler, E Bill, AJ Pierik, U Mühlenhoff and R Lill, Methods Enzymol, 599, 197–226 (2018). DOI: 10.1016/bs.mie.2017.11.034.
- 50DJ Netz, M Stümpfig, C Doré, U Mühlenhoff, AJ Pierik and R Lill, Nat Chem Biol, 6, 758–765 (2010). DOI: 10.1038/nchembio.432.
- 51G Vitale, E Fabre and EC Hurt, Gene, 178, 97–106 (1996).
- 52DD Leipe, YI Wolf, EV Koonin and L Aravind, J Mol Biol, 317, 41–72 (2002). DOI: 10.1006/jmbi.2001.5378.
- 53JD Grossman, EJ Camire and DL Perlstein, Methods Enzymol, 599, 293–325 (2018). DOI: 10.1016/bs.mie.2017.11.005.
- 54TI Croll, Acta Crystallogr D Struct Biol, 74, 519–530 (2018). DOI: 10.1107/S2059798318002425.
- 55DT Cromer and DA Liberman, Acta Crystallogr A Found Adv, 37, 267–268 (1981).
- 56DT Cromer and D Liberman, J Chem Phys, 53, 1891–1898 (1970).
- 57S Brennan and PL Cowan, Rev Sci Instrum, 63, 850–3 (1992).
- 58TJ Dolinsky, JE Nielsen, JA McCammon and NA Baker, Nucleic Acids Res, 32, W665–W667 (2004). DOI: 10.1093/nar/gkh381 32/suppl_2/W665[pii].
- 59NA Baker, D Sept, S Joseph, MJ Holst and JA McCammon, Proc Natl Acad Sci U S A, 98, 10037–10041 (2001). DOI: 10.1073/pnas.181342398.
- 60H Schindelin, C Kisker, JL Schlessman, JB Howard and DC Rees, Nature, 387, 370–376 (1997). DOI: 10.1038/387370a0.
- 61FA Tezcan, JT Kaiser, D Mustafi, MY Walton, JB Howard and DC Rees, Science, 309, 1377–1380 (2005). DOI: 10.1126/science.1115653.
- 62JH Jeoung, T Giese, M Grunwald and H Dobbek, Biochemistry, 48, 11505–11513 (2009). DOI: 10.1021/bi901443z.
- 63JH Jeoung, T Giese, M Grunwald and H Dobbek, J Mol Biol, 396, 1165–1179 (2010). DOI: 10.1016/j.jmb.2009.12.062.
- 64YH Fong, HC Wong, MH Yuen, PH Lau, YW Chen and KB Wong, PLoS Biol, 11, e1001678 (2013). DOI: 10.1371/journal.pbio.1001678.
- 65MH Yuen, YH Fong, YS Nim, PH Lau and KB Wong, Proc Natl Acad Sci U S A, 114, E10890–E10898 (2017). DOI: 10.1073/pnas.1712658114.



