Assembly of a Functional Triiron(III) Cluster in L-Ferritin
Abstract
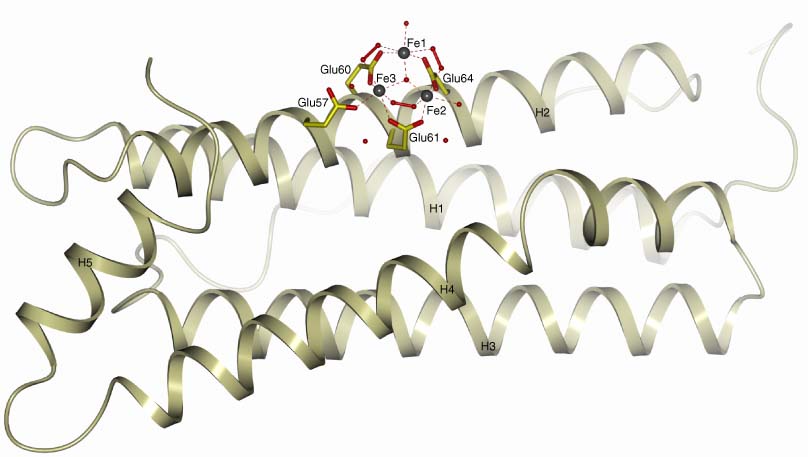
The L-subunits of mammalian ferritins are generally accepted to facilitate iron biomineral formation by providing nucleation sites. The formation of a μ3-oxo trinuclear iron cluster on the inner cage of homopolymeric recombinant human L ferritin and natural horse spleen ferritin has been observed upon diffusion of ferrous ions through metal-free ferritin crystals. Three glutamate side chains (Glu60, Glu61, and Glu64) act as bridging ligands between iron pairs, thus driving the cluster assembly. In the fully formed cluster, observed after 60′ diffusion in the human L ferritin, the iron ions are also bridged by peroxide anions, which could originate from ferrous iron oxidation by dioxygen. Substitution of Glu60, Glu61, and Glu64 by alanine residues significantly reduces the iron biomineralization rates, thus suggesting that the observed cluster represents the biomineral nucleation site.
3D Structure