11 Gibberellins and Plant Reproduction
Abstract
The involvement of the phytohormone gibberellin (GA) in land plant reproductive processes is ancient, but is best understood in flowering plants (angiosperms). GA acts in angiosperms to promote the transition from vegetative to reproductive development, and subsequent development of both male (stamen) and female (pistil) floral organs. Coordinating growth between these organs to promote successful fertilisation is a key regulatory function of GA. Its functions in female development are not well understood, but in stamens a number of downstream regulatory pathways have been determined. GA signalling directly regulates processes both in pollen development and in the surrounding anther tissues, particularly the tapetum. Female fertility also requires GA, which, while incompletely understood, regulates both pistil and ovule development. GA is a crucial trigger of fruit development upon fertilisation, and subsequently contributes to both the development of viable seeds and of fruiting structures.
11.1 Introduction
The involvement of gibberellin (GA) with land plant reproduction has ancient origins: the first evidence of functional GA signalling (as defined by GA-dependent interaction between the receptor GIBBERELLIN INSENSITIVE DWARF 1 (GID1) and the DELLA class of transcription factor) observed to date is during sporogenesis of the lycophyte Selaginella moellendorffii (Aya et al., 2011). However, this has not been seen in the bryophyte Physcomitrella patens (Hirano et al., 2007; Yasamura et al., 2007; Aya et al., 2011). The functions of GA in plant reproduction have been most extensively studied in angiosperms (flowering plants), where reproductive development has become more complex and the gamete-producing haploid generation (microgametophytes and megagametophytes) has been incorporated within the organs of a specialised reproductive structure (the flower). Similarly, from the apparent initial regulation of a single process in lycophytes (Yasamura et al., 2007), the role of GA has elaborated to regulate multiple aspects of angiosperm reproductive development, including the transition to flowering, establishment of the floral meristem, growth of floral organs and the development of both male and female reproductive cells (microspores and megagametophytes). The functions of GA in the reproduction of the monilophyte family, the sister group to seed plants (Pryer et al., 2001) that includes ferns, have not yet been studied in detail, but chemical evidence has suggested that GA derivatives act as sex-determining antheridiogens in fern gametophytes (Yamane et al., 1979; Warne and Hickok, 1989). A recent study in Lygodium japonicum confirmed the antheridiogen as a modified GA precursor (GA9Me), which is taken up by immature gametophytes and processed into bioactive GA to induce male organ formation (Tanaka et al., 2014). Furthermore, it is reported that application of bioactive GA to Ceratopteris richardii sporophytes accelerates the transition to the reproductive stage (Y. Yasamura and N. Harberd, personal communication).
The pathways through which GA regulates angiosperm reproductive development have been studied extensively in the two genetic models Arabidopsis thaliana (Arabidopsis) and rice (Oryza sativa), representing the diverging dicot and monocot branches of the family. In the case of pollen development, a high degree of functional conservation has been detected between these two species (reviewed in Plackett et al., 2011), suggesting conservation within angiosperms. In contrast, GA regulation of fruit development has been most extensively investigated in Arabidopsis and tomato (Solanum lycopersicum), representing two divergent angiosperm fruit types (dry and fleshy, respectively). As such, this chapter will focus on evidence from Arabidopsis, rice and tomato to illustrate the principal functions of GA during reproductive development, although information for other species is included where appropriate.
11.2 The Floral Transition
11.2.1 Gibberellin Promotes Flowering Through Multiple Interacting Pathways
The switch to reproductive development requires the reprogramming of the vegetative shoot apical meristem (SAM) to an inflorescence meristem (IM), which produces floral meristems (FMs) at its periphery instead of leaf primordia. This transition is regulated by a number of interacting environmental and endogenous inputs, including photoperiod, vernalisation, age and GA (reviewed in Amasino, 2010; Srikanth and Schmid, 2011). In many species, including Arabidopsis, the floral transition is accelerated by the action of GA, but in some species, for example pea (Pisum sativum), increasing GA content by chemical application or genetic manipulation delays flowering (Pharis and King, 1985; Reinecke et al., 2013).
Flowering in Arabidopsis is promoted under permissive long day (LD) photoperiods (Searle and Coupland, 2004) through the action of CONSTANS (CO) up-regulating expression of FLOWERING LOCUS T (FT), which alone is sufficient to induce flowering (Suárez-López et al., 2001). A number of Arabidopsis accessions and other species require a prolonged period of cold (vernalisation) to become competent to flower, antagonising the activity of the repressor FLOWERING LOCUS C (FLC) through epigenetic regulation; these signals are then integrated through SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) (Lee and Lee, 2010). The floral transition is also repressed through the action of microRNA156 (miR156), which negatively regulates a family of SQUAMOSA PROMOTER BINDING-LIKE (SPL) transcription factors; levels of miR156 reduce steadily with increasing plant age, with a consequent increase in SPL expression (Huijser and Schmid, 2011). FT and SOC1 (amongst others) orchestrate activation of important downstream transcription factors, including APETALA1 (AP1), FRUITFUL (FUL) and LEAFY (LFY) to reprogram SAM identity to become an IM. LFY is also key to specifying subsequent FM identity; in the absence of LFY the transition to reproductive development still occurs, but FMs are replaced by secondary IMs (Weigel et al., 1992). GA signalling promotes flowering in conjunction with these different pathways through regulating the expression of FT, SOC1, the SPL family and LFY (Figure 11.1).
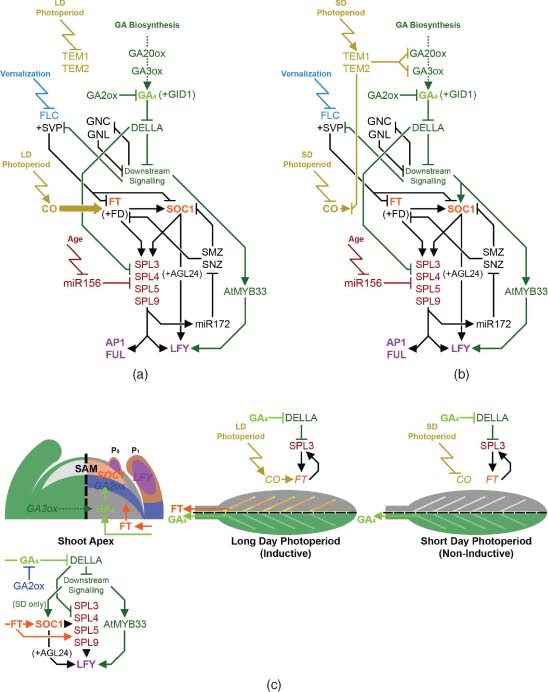
Photoperiod has an important interaction with GA signalling. Under LD photoperiods, flowering is delayed in the GA-deficient mutant ga1-3, but not entirely blocked (Reeves and Coupland, 2001), whereas under short day (SD) photoperiods ga1-3 does not flower unless treated with exogenous GA (Wilson et al., 1992). This reflects the action of GA on two separate mechanisms, focussed on the regulation of LFY and FT, respectively. LFY expression is present in ga1-3 under LD photoperiods, albeit at a reduced level, but is absent from ga1-3 when grown under SD (Blázquez et al., 1998). LFY is up-regulated under SD by GA at least in part through a SOC1-dependent pathway: SOC1 is up-regulated by GA (Borner et al., 2000; Moon et al., 2003), can rescue ga1-3 flowering under SD (Moon et al., 2003) and directly up-regulates LFY in conjunction with AGAMOUS-LIKE 24 (AGL24) (Lee et al., 2008). However, genetic evidence suggests that SOC1 also promotes flowering through additional, AGL24-independent pathways (Yu et al., 2002), and GA can activate LFY expression independently of SOC1 through the GAMYB orthologue AtMYB33 (Achard et al., 2004). In wheat (Triticum spp.), where there is a strong vernalisation requirement, it has been shown that GA signalling requires the additional presence of the vernalisation-activated AP1 homologue VERNALIZATION1 (VRN1) to up-regulate orthologues of SOC1 and LFY (Pearce et al., 2013). In Arabidopsis, the floral repressor SHORT VEGETATIVE PHASE (SVP), which acts in conjunction with the vernalisation-down-regulated repressor FLC to inhibit expression of SOC1 and FT (Li et al., 2008; Jang et al., 2009), is itself negatively regulated by GA signalling (Li et al., 2008).
LFY, AP1 and FUL are each up-regulated by SPL protein activity, and have been shown to be direct transcriptional targets of SPL3 (Yamaguchi et al., 2009). SPL3, -4 and -5 have in turn been identified as downstream transcriptional targets of both SOC1 and FT (Schmid et al., 2003, Jung et al., 2012), which promote their expression independently of miR156 repression (Jung et al., 2012). However, experimental data regarding the hierarchical position of the SPL genes is complex, reporting them as both downstream targets (Jung et al., 2012) and upstream regulators of FT and SOC1 (Yu et al., 2012). The most parsimonious model consistent with this is a feed-forward loop where SPL activity inhibits the AP2-like transcription factors SCHLAFMUTZE (SMZ) and SCHNARCGZAPFEN (SNZ), which are negative regulators of both FT and SOC1 (Figure 11.1a and b; Mathieu et al., 2009). Negative regulation of SMZ and SNZ expression by SPL acts via the microRNA miR172 (Yant et al., 2010). Importantly, DELLA proteins inhibit SPL activity through direct protein binding, as demonstrated through their inhibition of the up-regulation of SOC1 expression by SPL9 (Yu et al., 2012). Similar alteration of miRNA172 expression suggests that this regulation utilises the feed-forward circuit described above. GA induction of SPL3, -4 and -5 has been shown to be dependent on SOC1 (Jung et al., 2012), although whether induction of SOC1 by GA is likewise dependent on SPL activity is currently unclear. Thus, in the absence of FT or SOC1 expression this gene network is maintained in equilibrium unless destabilised by FT expression under LD (Figure 11.1a) or GA-SOC1 signalling under SD (Figure 11.1b), whereupon it switches states in an apparently self-reinforcing manner.
The absolute reliance of flowering on a GA signal is over-ridden under LD photoperiods by the up-regulation of FT, which can itself activate SOC1 expression (Yoo et al., 2005). GA promotes FT expression under LD: the delayed flowering of ga1-3 is caused by a reduction in FT expression, which is restored by GA treatment (Hisamatsu and King, 2008), and over-expression of GA2ox7 reduces FT levels under LD (Porri et al., 2012). Up-regulation of FT by GA signalling is photoperiod-dependent, with induction of FT by GA treatment strongly reduced under SD (Hisamatsu and King, 2008). GA-dependent promotion of FT expression under LD again potentially acts through the SPL feed-forward module described above, as demonstrated with SPL3 (Figure 11.1c; Galvão et al., 2012). However, GA treatment of a non-flowering ft mutant under LD still induced flowering (Hisamatsu and King, 2008), indicating an FT-independent effect of GA under LD photoperiods, very possibly via the parallel SOC1-LFY pathway described above.
Additional regulators of flowering also influence, or are influenced by, GA signalling, although to date their roles remain less clearly defined. In addition to a repressive effect on FT expression via CO (Castillejo and Pelaz, 2008), TEMPRANILLO 1 (TEM1) and TEM2 down-regulate the GA biosynthetic genes GA 20-OXIDASE 2 (GA20ox2), GA 3-OXIDASE 1 (GA3ox1) and -2 (Osnato et al., 2012). The tem1 tem2 loss-of-function mutant displays an associated early flowering phenotype under LD (which is delayed in the absence of GA3ox1) and increased SOC1 and LFY expression under both LD and SD (Osnato et al., 2012). The same study found that expression of TEM1 and TEM2 are also photoperiod-dependent, with their expression promoted under SD (Figure 11.1b). Two other known repressors of flowering, the GATA-type transcription factors GATA, NITRATE-INDUCIBLE, CARBON METABOLISM INVOLVED (GNC) and GNC-LIKE (GNL), are negatively regulated by GA and are themselves repressors of downstream GA signalling (Richter et al., 2010).
11.2.2 Sites of Gibberellin Biosynthesis and Action During the Floral Transition
The transition to flowering necessarily occurs at the SAM, but the initiation of this process occurs in other tissues. It has been demonstrated that FT is expressed in the phloem tissue of Arabidopsis leaves (Figure 11.1c; Takada and Goto, 2003) in response to vascular CO expression (An et al., 2004) and that FT protein is subsequently transported to the SAM to initiate floral transition in both Arabidopsis (Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007) and rice (Tamaki et al., 2007). LFY expression is restricted to early and emerging Arabidopsis leaf primordia prior to floral transition under both LD and SD conditions (Figure 11.1c; Blázquez et al., 1997), while SOC1 is expressed in both the SAM and developing leaf primordia (Samach et al., 2000). In accordance with these two distinct expression patterns, targeted expression studies in Arabidopsis using GA2ox and GA-resistant DELLA proteins found that GA regulation of flowering occurs in both leaves and the SAM (Galvão et al., 2012; Porri et al., 2012, Yu et al., 2012). The relative importance of these two sites on flowering time is affected by photoperiod, corresponding to the contributions of the parallel FT and SOC1-LFY pathways under LD and SD photoperiods. Under LD conditions, blocking GA signalling in either leaves or the SAM delays flowering (Yu et al., 2012); in the leaves, GA signalling increases expression of FT and SPL genes (Figure 11.1c; Galvão et al., 2012; Porri et al., 2012), while in the SAM, SPL genes, but not SOC1, are up-regulated by GA signalling (Porri et al., 2012). Under SD conditions blocking GA signalling in leaf phloem did not affect flowering time, while a block imposed in the SAM further delayed or prevented flowering. As mentioned previously, under SD conditions up-regulation of SOC1 is dependent on GA (Moon et al., 2003), but SOC1 expression can also be up-regulated by FT (Yoo et al., 2005). Therefore GA signalling in the leaves indirectly promotes floral transition under LD through a mobile FT signal, while GA signalling in the SAM promotes flowering under both photoperiods, functioning either through SOC1 (SD) or through the downstream SPL genes (LD).
Importantly, the site of GA action does not necessarily reflect that of GA biosynthesis. Under SD photoperiods, where GA signalling is an essential requirement for flowering, levels of bioactive GA4 increase dramatically at the shoot apex (including early leaf primordia) prior to flowering, coinciding with a sharp increase in LFY expression in the same tissues (Eriksson et al., 2006). However, no increase in GA biosynthesis (as determined by gene expression) was detected, suggesting that the necessary GA signal is synthesised elsewhere. Radiolabelling studies have demonstrated movement of bioactive GA4 from rosette leaves to the SAM (Eriksson et al., 2006). Furthermore, GA-inactivating enzymes are expressed beneath the shoot apex of Arabidopsis, rice and its relative, Lolium temulentum, during vegetative development (Figure 11.1c; Sakamoto et al., 2001; Jasinski et al., 2005; Zhu et al., 2006; King et al., 2008). In the case of Arabidopsis, loss of these GA2ox enzymes progressively accelerates the transition to flowering under SD conditions (Rieu et al., 2008a). Therefore GA inactivation appears to have a role in regulating the timing of floral transition, by preventing bioactive GA synthesised in remote tissues from accessing the SAM.
The effects of GA on flowering and shoot elongation are separable, despite both occurring in shoot tissues. In many plant species stem internode elongation (‘bolting’) is triggered by the floral transition; however, this can begin either before (e.g. sugar beet (Beta vulgaris)) or after (e.g. Arabidopsis) the floral transition has occurred (Mutasa-Göttgens and Hedden, 2009). In Arabidopsis, GA3ox expression is present beneath the shoot meristem both before and after the floral transition (Mitchum et al., 2006; Hu et al., 2008), suggesting that additional factors inhibit cell elongation in response to GA in a stage-specific manner. The separation of stem elongation and floral induction is currently poorly understood, with possible explanations including differential DELLA interacting partners between the SAM and internode tissues, or differential sensitivity to different forms of bioactive GA. In Arabidopsis, both stem elongation and flowering time are more sensitive to GA4 than to GA1 when applied under SD (Xu et al., 1997), consistent with LFY induction being more responsive to GA4 (Eriksson et al., 2006). In the case of Lolium, certain species of GA including GA1 and GA4 are inactivated by GA2ox enzymes surrounding the SAM, whilst GA5 is resistant to GA2ox activity due to unsaturation on C-2 (King et al., 2008). Correspondingly, GA5 is present within the vegetative SAM and accumulates in response to a LD stimulus, whereas GA1 and GA4 are excluded (King et al., 2001). Treatment with GA5 was shown to have a disproportionate effect on floral induction relative to stem elongation, compared to treatment with GA1 or GA4 (King et al., 2008). Thus selective inactivation of GA may discriminate between stem growth and floral induction in some species. In rice, the transition to reproductive development apparently alters the composition of bioactive GA produced, with GA1 or GA4 predominating in vegetative and reproductive tissues, respectively (Kurogochi et al., 1979; Kobayashi et al., 1984, 1988). The rice cytochrome P450 mono-oxygenase ELONGATED UPPERMOST INTERNODE (EUI) has been found to selectively deactivate GA4 by 16α-17 epoxidation in elongating internodes during the reproductive phase (Zhu et al., 2006), further supporting the existence of distinct functions and regulation of 13-hydroxylated and non-13-hydroxylated GAs during reproductive development in at least some plant species.
11.2.3 Gibberellin and Flowering in Perennial Species
Although most of our understanding of the floral transition is derived from annual species, some information about the regulation of flowering in perennial species is now available. The perennial growth habit is primarily achieved through the maintenance of a sub-population of vegetative meristems despite the presence of floral inductive signals. Molecular studies of perennialism suggest that this is achieved via an elaboration of the core floral transition module identified in annual species, with particular emphasis on enhanced roles of known repressors of floral transition such as FLC (in Arabis Alpina; Wang et al., 2009) and TERMINAL FLOWER1 (TFL1) (in Rosaceae species; reviewed in Kurokura et al., 2013), an antagonist of FT action (Hanano and Goto, 2011).
In an interesting parallel, in many angiosperm perennial species GA acts as an inhibitor of flowering (Pharis and King, 1985; Wilkie et al., 2008; Bangerth, 2009). GA is thought to negatively regulate seasonal flowering in Rose through promoting expression of the TFL1 homologue RoKSN (Randoux et al., 2012). In Citrus spp., where GA treatment also inhibits flowering, it has been found that GA treatment leads to reduced FT expression in leaves (Muñoz-Fambuena et al., 2012) and in apical buds (Goldberg-Moeller et al., 2013) under otherwise flowering-inductive conditions, in an apparent reversal of its effect in Arabidopsis. Treatment of Citrus trees with the GA biosynthesis inhibitor paclobutrazol (PAC) (Rademacher, 2000) under inductive conditions increases the conversion of apical meristems to flowers (Delgado et al., 1986), an effect that is reversible by simultaneous GA treatment (Martínez-Fuentes et al., 2013). Interestingly, increasing numbers of developing fruit negatively affect the flower-promoting effects of PAC treatment (Martínez-Fuentes et al., 2013), suggesting that GA functions as part of a feedback mechanism at the whole-plant level, either as a mobile signal or as part of a localised downstream signalling response to another mobile signal.
11.3 Floral Development
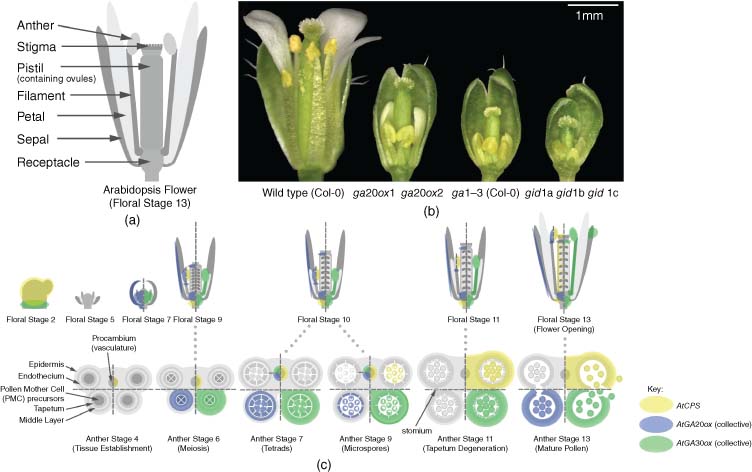
After the transition to flowering, the IM sequentially produces FMs in place of leaf primordia. In contrast to the indeterminate nature of the IM, development of individual flowers is both highly determinate and uniform, with a fixed pattern and chronology. In the case of Arabidopsis, floral development has been staged in detail (Smyth et al., 1990). Extensive mutant analysis of Arabidopsis has demonstrated that GA has regulatory functions at multiple points throughout floral development, and is a very important determinant of successful reproduction. GA-deficient and GA-insensitive mutants in numerous monocot and dicot species are infertile, with severely underdeveloped floral organs (Koornneef and Van der Veen, 1980; Pharis and King, 1985; Cecconi et al., 2002; Griffiths et al., 2006; Aya et al., 2009).
11.3.1 Floral Patterning and Early Development
The earliest stages of Arabidopsis floral development are driven by the action of LFY, which is strongly expressed in the developing FMs from their initiation, although not in the IM itself (Weigel et al., 1992). The first lateral meristems produced by the IM are not in fact flowers, but develop as inflorescence branches (Ratcliffe et al., 1999). Expression of floral meristem identity genes is promoted by LFY, and recent research shows that, beyond the floral transition, GA signalling in fact delays the start of FM specification, increasing the number of inflorescence branches produced, while conversely, accumulation of DELLA protein promotes FM identity (Yamaguchi et al., 2014). In Arabidopsis, LFY has been shown to promote depletion of bioactive GA from inflorescence tissues through up-regulation of the EUI homologue EUI-LIKE P450 A1 (ELA1) (Zhang et al., 2011; Nomura et al., 2013), leading to an accumulation of DELLA protein (Yamaguchi et al., 2014). It has now been shown that DELLA proteins actively promote expression of the floral identity gene AP1 (see Section 2.1), binding to its promoter in association with SPL9 independently of LFY (Yamaguchi et al., 2014).
Beyond FM specification, floral organs are established and emerge sequentially in concentric whorls (Figure 11.2a), with identity within each whorl determined through a complex of interacting MADS box transcription factors following the now well-established ABCE model (reviewed in Airoldi, 2010; Irish, 2010). LFY drives early expression of a number of these genes, including the B-class (petal and stamen) and C-class (stamen and carpal) factors APETALA3 (AP3) and AGAMOUS (AG) (Lohmann et al., 2001; Lamb et al., 2002). LFY expression in the early FM is not regulated by GA signalling (Yu et al., 2004), in contrast to its vegetative-phase expression, and continues until floral stage 5 (emergence of stamen and petal primordia).
(Adapted from Plackett et al. (2011). Reproduced with permission of Elsevier.)
(See insert for colour representation of this figure.)Bioactive GA is present in early floral tissues, as evidenced by the expression of GA 3-OXIDASE1 (GA3ox1), first reported in central FM tissues at stage 3 (sepal initiation), which later becomes confined to the receptacle in early floral stages (Hu et al., 2008). As with LFY, GA3ox is not expressed in IM tissues. Early GA3ox expression is reported to continue through to floral stage 7 (stamen filament differentiation) and beyond (Mitchum et al., 2006; Hu et al., 2008). After LFY expression declines continued expression of AP3 and AG becomes dependent on GA (Yu et al., 2004), AG apparently establishing a positive feedback loop with GA3ox1 (amongst others) to maintain its own expression (Gómez-Mena et al., 2005). AG expression is required throughout floral development to maintain floral organ development, most evidently that of stamens (Ito, 2011; Ito et al., 2011).
Despite these early functions, the floral plan remains essentially unchanged in Arabidopsis GA mutants, although some GA-dependent instability in floral organ number has been detected during the early phase of flowering, potentially due to the initial size of the developing FM (Plackett, 2012). In other species GA has a greater influence on early floral organ development. The GA-deficient tomato mutant stamenless-2 exhibits partial conversion from stamen to carpel identity (Sawhney, 1992), while GA treatment or constitutive GA signalling in developing tomato flowers increases the number of floral organs that develop (Sawhney, 1983; Carrera et al., 2012). In the monoecious species maize (Zea mays) loss of the ent-copalyl diphosphate synthase (CPS) orthologue ANTHER EAR1 (AN1) induces stamen development in otherwise female-only florets (Bensen et al., 1995).
11.3.2 Gibberellin and Fertility
The importance of GA action on flowering plant fertility is clearly illustrated by mutants that are completely deficient in or are insensitive to GA, where both male and female reproductive organs are sterile (Koornneef and Van der Veen, 1980; Jacobsen and Olszewski, 1991; Griffiths et al., 2006; Aya et al., 2009). In the case of these severe phenotypes, floral organ development undergoes premature arrest (see below), while less severely deficient mutants display fertility defects caused by impaired floral organ growth (Figure 11.2b). However, GA signalling also appears to be restricted during floral development: exogenous GA treatment in Arabidopsis measurably reduces fertility (Jacobsen and Olszewski, 1993; Plackett et al., 2014), and constitutive GA signalling in DELLA loss-of-function mutants causes sterility in monocot and dicot species (Lanahan and Ho, 1988; Ikeda et al., 2001; Plackett et al., 2014). In Arabidopsis this observation is complicated by ecotype-specific factors. In the Landsberg erecta (Ler) ecotype, some fertility is maintained despite the loss of all five DELLA paralogues (Fuentes et al., 2012), whereas in the Columbia (Col-0) ecotype loss of just two, GA INSENSITIVE (GAI) and REPRESSOR OF ga1-3 (RGA), causes a pollenless, male-sterile phenotype, although female fertility is retained (Plackett et al., 2014). In m-onocots, the reported sterility phenotype of the equivalent rice slender (slr1) mutant remains undescribed in any detail (Ikeda et al., 2001), but the barley slender (sln1) mutant is reported as pollenless (Lanahan and Ho, 1988).
The later stages of GA biosynthesis are governed by the GA20ox and GA3ox multi-gene families (see Chapter Gibberellin Biosynthesis in Higher Plants), and mutants lacking individual (or multiple) paralogues from these families display semi-dwarf phenotypes associated with reduced GA biosynthesis, including reduced size of all floral organs (Hu et al., 2008; Rieu et al., 2008b; Plackett et al., 2012). The Arabidopsis semi-dwarf mutant ga20ox1 ga20ox2 displays a detectable reduction in fertility, both in the number of seeds set per silique and a failure of early flowers to set siliques (Rieu et al., 2008b). This latter phenotype is associated with reduced growth of stamens relative to the pistil at flower opening (Figure 11.2b), creating a mechanical block to pollination at anthesis. The timing of pollination is an important factor, as ovules remain receptive to fertilisation only for a limited time once mature (Vivian-Smith and Koltunow, 1999). Spontaneous recovery of fertility in later flowers without GA treatment occurs in both these mutants and in ga1-3 (Plackett et al., 2012), which has a correspondingly severe initial floral phenotype. Phenotypic recovery includes increased floral organ growth and, in the case of ga1-3, overcoming early arrest of pollen development. The common nature of this recovery suggests that it is downstream, or independent of GA regulation. This hypothesis is supported by detection of similar GA levels between early (infertile) and late (fertile) flowers (Hu et al., 2008).
Sites of Gibberellin Biosynthesis and Signal Transduction During Floral Development
The sites of GA biosynthesis in Arabidopsis floral tissues have mostly been deduced indirectly from GA3ox expression patterns, using GUS reporter constructs (Figure 11.2c). Prolonged GA3ox expression is observed in the receptacle, but is also expressed in the stamen filament and anther from approximately floral stage 7 (Mitchum et al., 2006; Hu et al., 2008). Some limited expression has also been observed in the vasculature of sepals. This pattern suggests that the stamens and receptacle act as a source of bioactive GA for other floral organs. In the case of petals, this is supported by a strong correlation in size with stamens in the flowers of mutants such as ga20ox1 ga20ox2 (Figure 11.2b; Rieu et al., 2008b). No GA3ox expression was detected in pistils prior to pollination and the start of silique development. Expression of CPS, the first step in GA biosynthesis, is reported in the receptacle, stamen vasculature and anther tissues of developing flowers (Figure 11.2b; Silverstone at al., 1997), but is first detected in the developing pistil only at floral stage 12, immediately prior to flower opening, where it occurs in the pistil vasculature and ovule funiculi. GA20ox expression was detected in the receptacle, stamen filament, anther tissues, pistil apex and sepal vasculature during floral development (Figure 11.2b; Plackett et al., 2012).
DELLA expression patterns during floral development have not been comprehensively mapped in either Arabidopsis or rice, but expression data during the development of some critical anther tissues is now available for these species (Honys and Twell, 2004; Hirano et al., 2008; Tang et al. 2010). Mutant analysis in Arabidopsis indicates that RGA, GAI, RGA-LIKE1 (RGL1) and -2 are all involved in regulating floral development to some degree (Lee et al., 2002; Cheng et al., 2004; Tyler et al., 2004; Plackett et al., 2014). An RGL2::GUS line indicates expression in the receptacle, sepals, petal vasculature, stamen filaments and pistil tissues, with strongest expression at the top of the style (Lee et al., 2002). Interestingly, RGL2 is apparently excluded from anther tissues, whereas in situ hybridisation identified RGL1 expression specifically in the anthers of stamen primordia (Wen and Chang, 2002), suggesting functional specialisation. RGL1 was also detected in developing ovules. A comparison between mutant phenotypes in Col-0 and Ler suggests that the functions (and potentially the expression patterns) of specific DELLA paralogues during floral development can vary between ecotypes (Plackett et al., 2014).
Downstream of GA signalling, GAMYB has been identified as an important transcription factor during floral development of multiple species (Murray et al., 2003; Kaneko et al., 2004; Millar and Gubler, 2005). The interpretation of GAMYB expression patterns in floral tissues is complicated by negative post-transcriptional regulation by miRNA159 (Achard et al., 2004; Tsuji et al., 2006; Alonso-Peral et al., 2010), which apparently restricts expression exclusively to anther tissues in both Arabidopsis and rice (Millar and Gubler, 2005; Aya et al., 2009).
Male Reproductive Development
The two principal functions of GA during stamen development are to promote filament elongation at flower opening through increased filament cell elongation (Cheng et al., 2004) and, in the anther, to promote successful pollen development and locule opening (anthesis). GA-deficient or insensitive plants demonstrate arrested anther/pollen development in numerous species (Nester and Zeevaart, 1988; Goto and Pharis, 1999; Izhaki et al., 2002; Aya et al., 2009). However, the precise timing of this arrest varies between the species and mutants studied. Anthers of the GA-deficient Arabidopsis mutant ga1-3 undergo development arrest at the unicellular microspore stage, prior to pollen mitosis (Cheng et al., 2004), whereas GA-deficient tomato mutants demonstrate an earlier arrest prior to the onset of meiosis (Nester and Zeevaart, 1988; Jacobsen and Olszewski, 1991). In the GA-deficient rice mutant oscps1-1 normal anther development fails after microspore release from the tetrads, but defects manifest earlier in GA-insensitive gid1-4, where meiosis begins, but does not complete (Aya et al., 2009). Inhibition of GA biosynthesis by PAC treatment of Petunia hybrida flowers caused a post-meiotic arrest of pollen development (Izhaki et al., 2002). The mechanisms through which anther developmental arrest is enacted are not yet clearly understood, but presumably indicate the existence of ‘checkpoints’ during anther development that require specific conditions to be met before development proceeds. The failure of Arabidopsis pollen development in the absence of DELLA (Plackett et al., 2014) highlights the importance of these checkpoints and the role of GA in the transition through them.
The tissues most closely associated with the failure in male fertility are pollen and the surrounding tapetum cell layer. Pollen development is highly dependent on the tapetum, both for nutrition and the deposition of pollen wall components (reviewed in Scott et al., 2004). This dependency is clearly illustrated in experiments in which the tapetum is ablated in developing tobacco anthers, resulting in pollen abortion (Koltunow et al., 1990). An important aspect of tapetum development is programmed cell death (PCD) to allow deposition of wall materials onto the pollen; impairing tapetum PCD progression in many mutants results in male sterility through pollen abortion (Kawanabe et al., 2006; Vizcay-Barrena and Wilson, 2006). Cell-type-specific transcriptome analysis in developing rice anthers indicates active GA signalling in both the pollen mother cells (PMCs)/microspores and the tapetum from meiosis until pollen maturity, with expression of GA biosynthetic genes strongest in the tapetum immediately prior to PCD and in post-meiotic pollen at the bicellular and tricellular stages (Hirano et al., 2008; Tang et al., 2010). Similarly, direct and indirect experimental approaches in Arabidopsis collectively predict active GA biosynthesis in developing pollen from meiosis until maturity (Honys and Twell, 2004; Hu et al., 2008) and in anther tissues, most strongly in the tapetum, from meiosis until tapetum degeneration (Figure 11.2c). Transmission frequencies of GA signalling mutant alleles between generations in rice and the Arabidopsis gid1 triple mutant suggest that signalling proteins synthesised in premeiotic PMCs persist during post-meiotic development and are capable of maintaining GA signalling in haploid microspores despite the absence of a functional allele (Griffiths et al., 2006; Chhun et al., 2007). Thus GA signalling directly regulates both tapetum and pollen development at meiosis and during subsequent post-meiotic development. Recently the possibility has been raised that GA signalling acts to coordinate development between these two cell types: reintroduction of functional DELLA protein into a male-sterile DELLA loss-of-function background successfully rescued pollen development if expressed after the completion of meiosis in either developing pollen or the tapetum (Plackett et al., 2014).
GA regulation of tapetum development has to date been most closely studied in rice including identification of a number of downstream targets of GA signalling (Figure 11.3), which acts almost entirely through the GAMYB transcription factor (Aya et al., 2009). In rice GA biosynthesis and signalling mutants activation of the tapetum PCD pathway is specifically blocked (Aya et al., 2009). Analysis of these mutants identified transcription factors involved in the regulation of tapetum PCD (Li et al., 2006); at least one of these downstream factors, Osc6, contains a GAMYB-binding domain within its promoter. Other direct targets were identified in pollen wall exine biosynthesis and Ubisch body formation (Aya et al., 2009), critical for successful formation of the pollen wall (Piffanelli et al., 1998; Wang et al., 2003). A very high degree of conservation has been detected in anther genetic pathways between rice and Arabidopsis (Figure 11.3; reviewed in Plackett et al., 2011), suggesting that GA regulates the same pathways in Arabidopsis as in rice. However, these species represent only one of two types of tapetum (secretory rather than amoeboid) present in angiosperms (Huysmans, 1998) and additional functions might be present in the tapeta of other species. The tomato tapetum comprises two separate single-cell layers either side of a crescent-shaped locule, distinguished as the inner and outer tapetum, which undergo endomitosis to become binucleate at maturity (Brown, 1949). In GA-deficient gib-1 anthers, development of the outer tapetum arrests with fewer cells than wild type and these cells also remain uninucleate, but subsequently undergo endoreduplication in response to GA treatment (Jacobsen and Olszewski, 1991; van den Heuvel et al., 2001). DELLA protein activity has also been shown to regulate cell cycle activity and promote endoreduplication in leaves (Claeys et al., 2012). The genetic pathways regulated by GA during microspore development are currently uncharacterised. It has recently been shown that the basic helix-loop-helix (bHLH) transcription factor INDEHISCENT (IND) modulates Arabidopsis male fertility responses to GA: loss of IND function partially rescues phenotypic defects in mild (but not severe) GA mutants (Kay et al., 2013). The precise mechanism underlying this remains undetermined, but does not appear to involve direct regulation of GA biosynthesis via AtGA3ox1 expression (Kay et al., 2013), as seen during silique development (see Section 4.1). IND expression during anther development appears to be restricted to the locule/ developing pollen, based on expression data from a GUS reporter line (Kay et al., 2013).
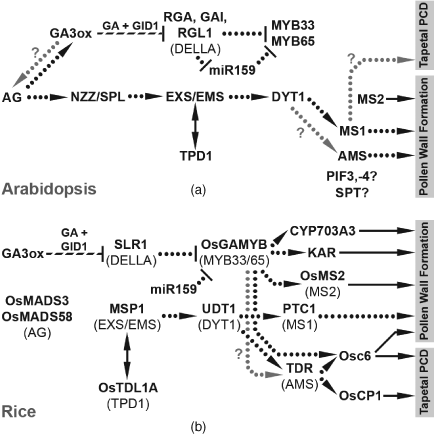
The final phase of anther development centres on locule opening and release of mature pollen (anthesis). Anthers from the first flowers of some Arabidopsis ga20ox and ga3ox mutants are indehiscent, suggestive of late-stage developmental arrest (Hu et al., 2008; Rieu et al., 2008b). After a period of down-regulated expression beyond tapetum degeneration, GA3ox reaccumulates in anther wall tissues immediately prior to anthesis (Hu et al., 2008). Jasmonate (JA) signalling apparently acts downstream of both GA and auxin during stamen maturation to promote pollen maturation, filament elongation and anther dehiscence (reviewed in Song et al., 2013). These processes are dependent on the MYB transcription factors MYB21, -24 and -57, which are downstream targets of JA signalling (Cheng et al., 2009; Song et al., 2011). During late stamen development GA signalling promotes accumulation of JA through up-regulation of the JA biosynthesis gene DEFECTIVE IN ANTHER DEHISCENCE1 (DAD1) (Cheng et al., 2009), itself a direct downstream target of AGAMOUS (Ito et al., 2011; see Section 3.1), resulting in up-regulation of the MYB genes in a JA-dependent manner (Cheng et al., 2009). However, JA treatment of GA-deficient mutants fails to restore stamen development, despite MYB up-regulation (Cheng et al., 2009), demonstrating that GA regulates additional, JA-independent pathways during stamen maturation. These additional pathways are currently uncharacterised.
After anthesis and the transfer of pollen to the stigma (pollination), GA also functions to promote pollen germination and pollen tube growth, as demonstrated in the rice GA biosynthesis mutants (Chhun et al., 2007) and by over-expression of a GA2ox gene in transgenic Arabidopsis pollen (Singh et al., 2002). Interestingly, GA treatment has also been shown to inhibit Arabidopsis pollen tube growth in vitro (Singh et al., 2002), suggesting that unrestricted GA signalling is detrimental. The mechanism through which GA affects pollen tube growth, which unlike most plant cell types extends through polarised tip growth (Hepler et al., 2001), appears to act through DELLA-mediated signal transduction (Swain et al., 2004). Seed counts from hand-pollination experiments suggest that loss of IND and/or the MADS-box transcription factors SHATTERPROOF1 (SHP1) and SHP2 partially restores pollen tube growth in GA-deficient 35S::GA2ox pollen (Kay et al., 2013).
Female Reproductive Development
In angiosperms the female gametophyte is typically reduced to an embryo sac of a few highly specialised cells that develops within the tissues of the sporophytically-derived ovule (reviewed in Shi and Yang, 2011). Although not explicitly investigated to date there is mounting evidence that female reproductive development and fertility are also directly influenced by GA. The Arabidopsis ga20ox1 ga20ox2 mutant produces fewer seeds than wild type even when manually fertilised with wild-type pollen without any obvious signs of seed abortion (Rieu et al., 2008b), suggesting that ovule number is reduced in this background. This is consistent with qPCR evidence for GA20ox2 expression in pistil tissues (Plackett et al., 2012) and ovule-specific microarray analysis (Yu et al., 2005; Schmidt et al., 2011). Microarray analysis of developing Arabidopsis ovules suggests that both the GA biosynthetic and signalling pathways are expressed in these tissues (Yu et al., 2005; Schmidt et al., 2011), including the megaspore mother cell (MMC), which undergoes meiosis to generate four megaspores, one of which will develop into an embryo sac (Schmidt et al., 2011). Interestingly, beyond the MMC neither direct laser-capture microdissection of key embryo-sac cells (Wuest et al., 2010) nor indirect comparative expression profiling against embryo-sac-less mutants (Yu et al., 2005; Johnston et al., 2007; Jones-Rhoades et al., 2007; Steffan et al., 2007) have yet provided convincing evidence of GA biosynthesis or signalling occurring within the female gametophyte. This is in stark contrast to the prominent role of GA in male gametophyte development (see Section 3.2) and possibly reflects the evidence that female gametogenesis predates the evolution of GA signalling, which apparently first arose to regulate sporogenesis (see Section 1). In contrast to the apparent absence of GA-dependent female gametophyte development, it has been determined that auxin is a critical regulator of embryo-sac patterning (reviewed in Sundaresan and Alandete-Saez, 2010). In situ hybridisation has identified RGL1 expression in the integuments of developing ovules, surrounding the female gametophyte (Wen and Chang, 2002), suggesting that GA signalling does occur in sporophyte-derived ovule tissues.
After ovule and female gametophyte development, female fertility in angiosperms is dependent on the pistil/gynoecium to successfully mediate fertilisation by the male gametophyte (pollen). Of particular importance are the stigma, which regulates pollen recognition and germination, and the style/transmission tract, through which pollen tubes grow to reach the ovaries. The growth of both of these tissues is responsive to GA and is enhanced in pistils of the DELLA global loss-of-function mutant (Fuentes et al., 2012), which displays a constitutive GA signalling phenotype. Female fertility is reduced in this mutant, which sets fewer seeds than wild-type controls even when manually pollinated with wild-type pollen (Fuentes et al., 2012). The subsequent siliques displayed ovule abortion, indicative of inefficient fertilisation. IND has been implicated in GA-mediated female fertility through analysis of reciprocal crosses (Kay et al., 2013) in addition to its roles in male fertility (see Section 3.2) and fruit development (see Section 4.1). In tomato, loss of DELLA repression or GA treatment also increases style growth (Carrera et al., 2012).
11.4 Seed and Fruit Development
Upon fertilisation, ovules develop into seeds containing a diploid embryo and triploid endosperm, while the surrounding pistil develops into a fruit (reviewed in Gillaspy et al., 1993; Seymour et al., 2013). In the absence of fertilisation, progression of the ovary into fruit development is repressed (Rodrigo and Garcia-Martínez, 1998; Vivian-Smith et al., 2001; Vriezen et al., 2008) and an unfertilised pistil eventually senesces. There is a wide range of fruit forms within the angiosperms, two of the best-studied being the Arabidopsis silique and the tomato berry. Amongst other signals, both GA and auxin are important regulators of fruit-set and development (reviewed by Ruan et al., 2012; Sotelo-Silveira et al., 2014). In Arabidopsis, tomato and pea continued GA signalling through either exogenous GA treatment, or loss of DELLA repression is sufficient to trigger and substantially maintain fruit development in the absence of fertilisation (parthenocarpic growth) (Ozga and Reinecke, 1999; 2003; Serrani et al., 2007a, 2007b; Dorcey et al., 2009; Carrera et al., 2012; Fuentes et al., 2012). However, GA-induced parthenocarpic Arabidopsis fruits stall prematurely even under continued GA treatment (Vivian-Smith and Koltunow, 1999). This has been shown to correlate with ovule senescence (Carbonell-Bejerano et al., 2010), and that this onset of GA-insensitivity is influenced by ethylene signalling (Carbonell-Bejerano et al., 2011). Similarly, GA-induced parthenocarpic seedless tomato fruits remain under-developed and smaller than those arising from pollination (reviewed in de Jong et al., 2009a). GA signalling therefore cannot entirely complement fruit development in the absence of developing seeds in these two species.
11.4.1 Fruit Development
Fruit development and maturation involves tissue growth through cell division and expansion and also the differentiation of new tissue types. As discussed previously, these processes are dependent on ovule fertilisation and continuing seed development. In both Arabidopsis and tomato, fertilisation up-regulates GA biosynthesis within ovule tissues, downstream of auxin biosynthesis and signalling (Serrani et al., 2008; Vriezen et al., 2008; Dorcey et al., 2009). The up-regulation of GA biosynthesis at fertilisation was shown in Arabidopsis to be ovule-specific, but active GA signalling was present in both ovules and developing siliques (Figure 11.4a; Dorcey et al., 2009), suggesting that GA might act as a mobile signal to promote silique-set in response to seed-set. In pea, RT-PCR and transcriptome analyses suggest that pollination/fertilisation triggers up-regulation of GA biosynthesis independently in both ovules/seeds and surrounding pericarp tissues (Ozga et al., 2003; 2009). During subsequent fruit development, however, GA biosynthesis in the pericarp quickly becomes dependent on the presence of developing seeds (Ozga et al., 2003). Growth of de-seeded pea pericarps can be rescued by application of either GA1 (Ozga and Reinecke, 1999) or the auxin species 4-chloroindole-3-acetic acid (4-Cl-IAA) (Reinecke et al., 1995). Regulation of pea pericarp GA biosynthesis by seeds appears to be mediated through 4-Cl-IAA, treatment with which stimulates GA biosynthesis in the pericarp through up-regulation of both PsGA20ox1 and PsGA3ox1 and down-regulation of the C19-GA catabolic gene PsGA2ox2 (Ozga et al., 2003; 2009). Interestingly, treatment with another auxin species found in pea, indole-3-acetic acid (IAA), does not significantly regulate expression of GA biosynthesis genes in the pea pericarp (Reinecke et al., 1995; Ozga et al., 2003; 2009) and in fact inhibits GA-dependent growth through stimulation of ethylene biosynthesis (Johnstone et al., 2005). In contrast, 4-Cl-IAA treatment apparently reduces the sensitivity of pericarp tissues to ethylene (Johnstone et al., 2005). These observations suggest separable signalling pathways in pea pericarps for these two naturally occurring auxin species.
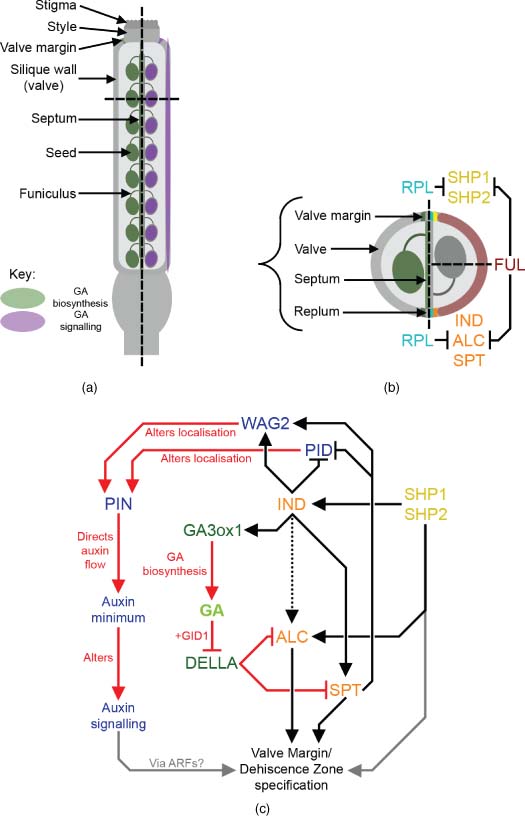
Reproduced with permission from John Wiley & Sons.
(See insert for colour representation of this figure.)Similarly, developing Arabidopsis siliques also directly synthesise GA, with GA3ox gene expression reported in the septum, valve margins and ovule funiculi (Figure 11.4a and b; Hu et al., 2008; Arnaud et al., 2010). DELLA expression patterns have not been resolved to the level of individual silique or seed tissues, but all five paralogues are expressed during silique development, with indications of differential expression patterns (Fuentes et al., 2012). GA signalling regulates both silique growth and tissue differentiation processes. Mutant analysis in Arabidopsis showed a direct effect of GA20ox genes in promoting silique elongation, independent of the number of developing seeds (Rieu et al., 2008b; Plackett et al., 2012), and a similar seed number-independent effect on pericarp growth was recently reported in transgenic pea over-expressing PsGA3ox1 (Reinecke et al., 2013). Further genetic dissection of the Arabidopsis GA3ox gene family found that GA synthesised in silique tissues and GA derived from seeds both contribute to silique elongation (Hu et al., 2008). Analysis of DELLA global (Ler) mutant siliques indicates that GA signalling promotes cell expansion in all cell layers within the silique wall (Figure 11.4a; Fuentes et al., 2012). Interestingly, GA signalling also promoted cell division in global mutant siliques, but only in specific cell layers (Fuentes et al., 2012). Analysis of a similar DELLA loss-of-function mutant in tomato, procera, suggests that GA signalling also promotes endoreduplication within tomato pericarp cells (Carrera et al., 2012). Surprisingly, Fuentes et al. (2012) detected a potentially DELLA-independent growth-promoting effect of GA signalling. The mechanism through which this putative response functions remains unclear, but requires the GID1 receptor, the 26S proteasome and SPATULA (SPT), a growth-repressing bHLH transcription factor known to interact with GA signalling in other developmental contexts (Josse et al., 2011), as well as binding with DELLA proteins in vitro (Gallego-Bartolomé et al., 2010).
GA-deficient Arabidopsis siliques fail to differentiate a separation layer within the valve margin dehiscence zone, and in consequence display defects in silique dehiscence (Arnaud et al., 2010). The dehiscence zone is specified by the actions of the bHLH transcription factors IND and SPT, which acts downstream of IND, to generate a localised auxin minimum (Liljegren et al., 2004; Sorefan et al., 2009; Girin et al., 2011). ALCATRAZ (ALC) also acts downstream of IND, partially redundantly with SPT (Groszmann et al., 2011), and is required to specify the separation layer within the dehiscence zone (Rajani and Sundaresan, 2001). Expression of IND is spatially regulated by the actions of FRUITFULL (FUL) and REPLUMLESS (RPL) (Figure 11.4b; Ferrándiz et al., 2000; Roeder et al., 2003). GA deficiency phenocopies the valve margin defects observed in alc loss-of-function mutants, and DELLA proteins bind to ALC in vitro (Arnaud et al., 2010). GA3ox1 expression in the valve margins is directly up-regulated by IND (Arnaud et al., 2010), and it is proposed that IND acts to promote SPT and ALC function through GA signalling by triggering degradation of DELLA protein (Figure 11.4c; Arnaud et al., 2010). Interestingly, SHP1 and SHP2, which also specify valve-margin identity through a separate pathway (Liljegren et al., 2000), have been found to influence GA responses in pollen tube growth (see Section 3.2), but in this context any interaction with GA signalling remains to be determined. Tomato fruits do not dehisce at maturity, and to date much greater attention has been paid to the roles of auxin and ethylene in fruit ripening (reviewed in Seymour et al., 2013). It would be very interesting to determine whether the GA-IND module is conserved between Arabidopsis and tomato fruit development.
Comparison of subsequent fruit development between Arabidopsis and tomato identifies an interesting difference. In Arabidopsis, della global mutant parthenocarpic siliques do not respond to auxin treatment (Fuentes et al., 2012), suggesting that all auxin responses are mediated through GA signalling, but tomato procera (DELLA-deficient) parthenocarpic fruits show increased growth under auxin treatment (Carrera et al., 2012). GA and auxin treatments also induce differing patterns of parthenocarpic growth in tomato (Serrani et al., 2007a), including the development of pseudoembryos in response to auxin, but not GA. As such, not all regulation of tomato fruit growth by auxin is GA-dependent. In pea, 4-Cl-IAA also has GA-independent effects on pericarp growth (van Huizen et al., 1996). Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7), a negative regulator of parthenocarpic fruit growth in tomato (de Jong et al., 2009b) appears to antagonise some of the downstream responses to both auxin and GA signalling during fruit development (de Jong et al., 2011). Expression of this gene is negatively regulated by pollination and auxin treatment, but not GA (de Jong et al., 2009b), suggesting that cross-talk between auxin and GA signalling during fruit development is likely to be highly complex.
11.4.2 Embryo and Seed Development
The transition from ovule to seed development is initiated by fertilisation of the egg cell within the female gametophyte, which develops into an embryonic sporophyte (reviewed in West and Harada, 1993). The second fertilisation event, that of the central cell, results in development of the endosperm within the seed. The embryo is dependent on nutrients mobilised from the endosperm during its early development and, in many cases, also during germination (reviewed in Lopez and Larkins, 1993; see Chapter Gibberellins and Seed Germination). The maternally-derived ovule tissues surrounding the female gametophyte develop into the seed coat (testa). Embryo and seed development have been classified into two broad stages: morphogenesis (establishing embryo polarity, the embryo axis comprising the root and shoot apical meristems (RAM and SAM) and the immature cotyledons) and maturation (accumulation of storage reserves within the embryo and/or endosperm, followed by desiccation and entry of the embryo into a dormant state) (West and Harada, 1993).
The importance of GA to seed development can be inferred from transgenic and mutant studies in Arabidopsis, tomato and pea, where depletion of bioactive GA from seed tissues causes abortion at early stages of development (Swain et al., 1993; Singh et al., 2002; Olimpieri et al., 2011). In the case of pea, seed abortion is apparently linked to GA depletion specifically in the embryo and/or endosperm rather than the testa and/or surrounding fruit tissues. Fertilisation of the lhi GA-deficient mutant with wild-type pollen complements the abortion phenotype (Swain et al., 1993), while abortion of homozygous lhi seeds increases even if developing on a phenotypically wild-type heterozygous parent (Swain et al., 1995). Early abortion correlates with a peak in bioactive GA levels during this phase of pea seed development, but has not been associated with any particular stage of embryo development (Swain et al., 1993).
GA biosynthesis (as predicted by promoter GA3ox::GUS expression) has been reported in both the embryo and endosperm of developing Arabidopsis and pea seeds (Hu et al., 2008; Nadeau et al., 2011). Embryonic AtGA3ox expression occurs from the heart stage onwards, with separate tissues expressing different paralogues: AtGA3ox1 is expressed in the nascent SAM, AtGA3ox2 in the nascent RAM, and AtGA3ox3 in two zones subtending the developing cotyledons either side of the embryo axis (Mitchum et al., 2006; Hu et al., 2008). A detailed study of developing pea seeds also identified PsGA3ox expression and accumulation of bioactive GA in the embryo axis, but not the developing cotyledons (Nadeau et al., 2011). The localisation and timing of the accumulation of bioactive GA in the developing embryo appears to be tightly regulated. In pea the quantities of bioactive GA synthesised in seed tissues are limited through 2β-hydroxylation activity (Nadeau et al., 2011). Interestingly, PsGA2ox expression was detected most strongly in the endosperm and seed coat, but was also present in the embryo axis and cotyledons later in development, where reduced GA biosynthesis might promote the transition from embryo growth to maturation (Nadeau et al., 2011). In Arabidopsis AtGA3ox2 expression is actively repressed in certain tissues of the developing embryo by FUSCA3 (FUC3), through direct binding to the AtGA3ox2 promoter, and LEAFY COTYLEDON2 (LEC2), through an indirect mechanism (Curaba et al., 2004). These two transcription factors are important regulators of embryo development during both embryogenesis and seed maturation (reviewed in Harada, 2001): in the absence of these genes, AtGA3ox2 expression was detected in embryos from the heart and torpedo stages, respectively, initially with increased expression in root precursor cells, but later expanding into the epidermis and vascular tissues of the embryo (Curaba et al., 2004). The significance of restricting GA3ox expression from these tissues during embryo development is as yet unclear.
In contrast to Arabidopsis vegetative tissues, which almost exclusively synthesise GA4 (the product of the non-13-hydroxylation branch of the GA biosynthesis pathway; Talon et al., 1990) (see Chapter Gibberellin Biosynthesis in Higher Plants), in developing Arabidopsis seeds GA1 (the 13-hydroxylated equivalent) was found in equal abundance to GA4 (Curaba et al., 2004). Interestingly, loss of either LEC2 or FUS3 causes differential qualitative changes to GA biosynthesis in the embryo, resulting in an increase in GA1 or GA4, respectively (Curaba et al., 2004). A study of seed development in grain-producing legumes found similarly mixed GA profiles in three out of the four species investigated (Slater et al., 2013). In contrast, there is evidence in pea for preferential biosynthesis of GA1, with the GA4 precursor GA9 being specifically targeted for inactivation by GA2ox enzymes, resulting in the absence of GA4 (Nadeau et al., 2011). The functional significance of synthesising different bioactive GA species in seed tissues is as yet unknown.
During seed development GA is also synthesised within the endosperm (Hu et al., 2008; Nadeau et al., 2011). AtGA3ox4 is expressed throughout the early Arabidopsis endosperm shortly after fertilisation, becoming localised to the chalazal endosperm by the heart stage of embryo development (Hu et al., 2008). In barley seeds it has been shown that the GA-responsive transcription factor HvGAMYB is expressed in the developing endosperm, where it is capable of regulating the expression of endosperm-specific genes (Diaz et al., 2002). Arabidopsis and pea represent two different endosperm types: cellularised and liquid, respectively. GA-deficient lhi pea seeds that do not abort grow more slowly and are smaller at maturity than wild-type (Swain et al., 1993; 1995), which might reflect regulation of nutrient mobilisation or uptake by GA signalling (Swain et al., 1997). Conversely, loss of DELLA activity in Arabidopsis seeds causes increased seed weight at maturity and is also associated with altered seed oil content (Li et al., 2013). In pea, the testa has also been shown to synthesise bioactive GA, where it is thought to promote mobilisation/digestion of accumulated starch deposits for absorption by the developing embryo in conjunction with GA-induced alterations in seed coat morphology to facilitate nutrient transfer (Nadeau et al., 2011). An implication of the lhi reciprocal cross evidence outlined in the previous discussion of seed abortion is that GA synthesised in the testa cannot easily migrate to the embryo, suggesting that GA biosynthesis and signalling is regulated independently in these two tissues.
The final maturation stages of seed development involve a cessation of embryo growth, seed desiccation and entry of the embryo into dormancy, a process promoted by the phytohormone abscisic acid (ABA) to prevent precocious germination (see Chapter Gibberellins and Seed Germination). In GA-deficient gib1 tomato mutants, morphological indicators of the transition from embryogenesis to maturation are delayed, though probably as an indirect consequence of slower embryo development (de Castro and Hilhorst, 2006). A reduction in bioactive GA levels in seed tissues during this late phase of development has been observed in several species (White et al., 2000; Nadeau et al., 2011), whilst conversely a peak in ABA concentration late in seed development is associated with the onset of maturation/dormancy (White et al., 2000; Weber et al., 2005; de Castro and Hilhorst, 2006; Slater et al., 2013).
The maintenance of dormancy by ABA and the antagonistic action of GA during germination is well-documented (see Chapter Gibberellins and Seed Germination), and this also appears to be the case during seed maturation. ABA-deficient maize (Zea mays) kernels fail to enter dormancy and instead germinate during kernel development (Neill et al., 1986). Inhibiting GA biosynthesis in this background suppresses precocious germination (White et al., 2000), suggesting that the balance of ABA and GA levels in developing seeds regulates entry into maturation/dormancy. In maturing tomato seeds, GA deficiency has no significant effect on the timing or magnitude of peak seed ABA content (de Castro and Hilhorst, 2006), while there is evidence from mature Arabidopsis seeds that ABA synthesis is actively promoted by the presence of DELLA protein (Lee et al., 2010). In Arabidopsis, the endosperm is a major contributor of ABA to maintaining seed dormancy under favourable germination conditions (Bethke et al., 2007; Lee et al., 2010), but at present it is unknown from what tissue the signal(s) to induce seed dormancy originate.
Acknowledgements
ARGP was supported by a Rothamsted quota studentship and is currently funded by the European Research Council; additional support was from the Biotechnology and Biological Sciences Research Council of the United Kingdom, for funding to ZAW's lab and strategic support to Rothamsted Research.



