6 Molecular Components that Drive Phosphorus-Remobilisation during Leaf Senescence
Abstract
Senescence is a complex process of controlled degradation and nutrient recycling that is modulated by developmental and environmental cues. Beginning in the middle to late vegetative stage of growth, the remobilisation of phosphorus (P) from senescing leaves serves as the major source of P for sink tissues, such as young leaves, reproductive structures and storage organs. Although it is clear that plants are generally efficient at recycling P from senescing leaves, little is known regarding the molecular components involved in the process. Optimising P remobilisation during senescence will likely be a valuable contribution to future improvements in P-use efficiency of crop species, which is urgently needed to minimise the use of unsustainable P fertilizers.
6.1 Introduction
Once developing seedlings deplete their seed storage reserves, they depend on the acquisition of mineral nutrients from the soil. As plants mature, and older leaves experience increasing respiratory costs and lower light conditions through self-shading, the growth of both shoots and roots slows. The decreases in root expansion eventually lead to a sharp decline in nutrient acquisition from soil. During the late vegetative and reproductive growth of monocarpic plants (e.g. annual crops), senescing leaves serve as a major source of nutrients for sink tissues, including young leaves, storage organs, and developing seeds (Veneklaas et al., 2012), and consequently senescence is inexorably linked to nutrient-use efficiency and crop productivity. Senescence is a complex process of controlled degradation and nutrient recycling that is modulated by developmental and environmental cues.
Since, due to their prevalent low availability, nitrogen (N) and phosphorus (P) are frequently limiting for plant growth, the remobilisation of these macronutrients during senescence is particularly important to sustain growth and development. Concentrations of N are decreased by more than 80% in senescing leaves of the model plant Arabidopsis thaliana (Himelblau & Amasino, 2001) via the breakdown of cellular protein and export of amino acids (Buchanan-Wollaston et al., 2003). Concentrations of P decrease to a similar extent (78%) (Himelblau & Amasino, 2001) but, in contrast to N, the mechanisms of recycling and remobilisation of P are less well understood. In this chapter, the known molecular components involved in recycling P during senescence are reviewed, and the possible involvement of additional factors – particularly those responsive to P status – are explored. The optimisation of P remobilisation from senescing leaves through breeding or biotechnological strategies will likely make an important contribution to improving the P-use efficiency (PUE) of crops, which is necessary to decrease the global dependence on unsustainable P fertilizers.
6.2 Transcriptomes of Senescence and Phosphate-Deficiency
A number of studies have investigated the large-scale or genome-wide transcript changes that accompany leaf senescence in diverse species, including Arabidopsis (Balazadeh et al., 2008; Breeze et al., 2011; Buchanan-Wollaston et al., 2003; Gepstein et al., 2003; Guo et al., 2004; van der Graaff et al., 2006), aspen (Populus tremula) (Andersson et al., 2004), barley (Hordeum vulgare) (Parrott et al., 2007), wheat (Triticum aestivum) (Gregersen & Holm, 2007), rice (Oryza sativa) (Liu et al., 2008), and Medicago truncatula (De Michele et al., 2009). These studies have greatly contributed to the present knowledge of the molecular mechanisms involved in the initiation and modulation of senescence. Importantly, the information obtained indicates a general conservation in senescence processes among species (e.g., dicotyledons, monocotyledons, annuals, and perennials), including the attenuation of photosynthesis, the degradation of carbohydrates, fatty acids, proteins, and nucleic acids, and nutrient remobilisation. This clear overlap in senescence-associated loci among species demonstrates that knowledge gained from model systems can be generally applied across species.
It is clear that the expression of many components involved in the recycling of N is affected by senescence. Nitrogen remobilisation during senescence requires the coordinated action of proteases, enzymes involved in glutamate and glutamine biosynthesis, and amino acid transport proteins. This is reflected in the altered expression of the corresponding genes during senescence (Breeze et al., 2011; Buchanan-Wollaston et al., 2003). Perhaps not surprisingly, the transcript profiling analysis of N-starved plants has revealed that similar gene expression changes occur during N deficiency (Peng et al., 2007a; Scheible et al., 2004), as plants recycle N and tolerate the depletion of this important macronutrient. In contrast to N, much less is known regarding the mechanisms that control the remobilisation of P during senescence, despite observations that similar proportions of P are recycled during senescence relative to N (Himelblau & Amasino, 2001). As with N, gene expression changes in response to P-starvation may be a general proxy for identifying genes involved in P remobilisation during senescence. To determine the extent of transcriptome overlap between inorganic P (Pi) deficiency and senescence, transcript profiling studies on Pi-starved shoots (Bustos et al., 2010) and senescing leaves (Breeze et al., 2011) were compared. The leaf senescence study, which generated a temporal transcript profile, identified over 6000 genes that were differentially expressed during leaf senescence. The majority of these genes could be divided into two major groups – downregulated or upregulated by senescence – while approximately 300 genes showed a more complex pattern, such as an increase in transcript abundance during early senescence followed by a decrease during late senescence, or vice versa. The Pi-deficient shoot microarray study identified 1873 and 1795 genes as being upregulated and downregulated, respectively (Bustos et al., 2010). A comparison of the two studies revealed a considerable overlap in differentially expressed genes during Pi-starvation and senescence. As shown in Figure 6.1, 717 of the 1795 genes (40%) that were downregulated by Pi deficiency were also downregulated during leaf senescence, whereas 711 of the 1873 genes (38%) upregulated by Pi deficiency were also upregulated during leaf senescence. To gain insight into the functions of the overlapping gene sets, the gene ontology (GO) enrichment tool BiNGO and the Cytoscape software environment were used to identify significantly enriched (p-value <0.001) GO Slim Plants annotations (Maere et al., 2005; Shannon et al., 2003). As shown in Figure 6.2, the shared sets of upregulated and downregulated genes were enriched for genes linked to a number of distinct annotations. For example, the downregulated group was enriched in genes linked to chloroplast and ribosome structure, photosynthesis, metabolism of nucleic acids and amino acids, and development, whereas the upregulated group was enriched in genes linked to catabolic and transport processes, transcriptional regulation, and responses to stress and hormones.
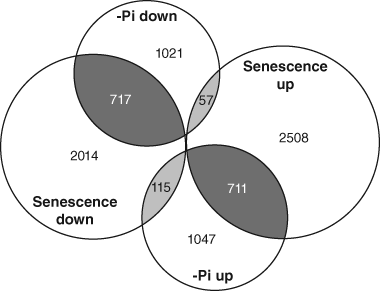
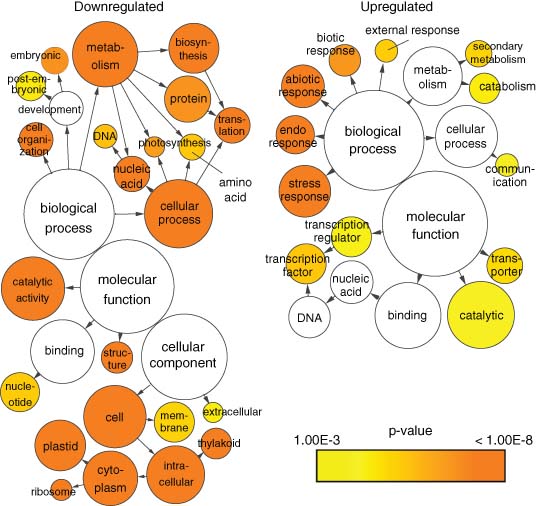
The sizeable overlap in differentially expressed genes during Pi deficiency and leaf senescence highlights the importance of recycling P during senescence, and is consistent with the presence of a complex network that has evolved to tightly control P availability in planta. Future examinations of shared gene clusters will surely aid in the identification of novel molecular components involved in P remobilisation during leaf senescence, and will help to unravel the complexity of low-Pi and senescence signalling pathways. The most predominant components implicated in P remobilisation during senescence will be described in the following sections, while potential regulators of P recycling during senescence will be discussed.
6.3 Major Biochemical Components that Mediate P-Remobilisation during Leaf Senescence
Phosphorus in plant tissues is present as either Pi or as organic P esters. The major pools of organic P are nucleic acids, phospholipids, low-molecular-mass P-ester metabolites, and phosphorylated proteins (Veneklaas et al., 2012). The proportion of Pi to the total P content is more variable than the organic forms, but tends to correlate with Pi availability (Veneklaas et al., 2012). The level of metabolically active Pi in the cytoplasm is tightly controlled, and excess Pi is stored in the vacuole. During short-term Pi deprivation, vacuolar Pi is mobilised to buffer cytoplasmic Pi, and this delays the onset of cellular Pi deficiency (Mimura et al., 1990). However, a sustained Pi deprivation will eventually exhaust the vacuolar Pi pool and lead to a pronounced reduction in cytoplasmic Pi pools. This appears to coincide with the induction of numerous intracellular and secreted hydrolases that liberate and scavenge Pi from organic P pools. These activities are also important for the normal redistribution of Pi that occurs in accordance with developmental programs, including senescence.
6.3.1 Nucleases
Approximately half of the organic P content of a plant is present in nucleic acids, and about 80% of this P pool exists as ribosomal RNA (rRNA) (Veneklaas et al., 2012). In developing leaves, the rRNA levels increase along with protein synthesis capacity, whereas in mature leaves net protein synthesis wanes and the rRNA levels fall (Hensel et al., 1993; Suzuki et al., 2010). During leaf senescence, an enhanced degradation of rRNA and other nucleic acids occurs, accompanied by the upregulation of many nuclease genes. Together with the subsequent hydrolysis of oligonucleotides and mononucleotides by phosphatases and phosphodiesterases (Plaxton & Tran, 2011), the nucleases play an important role in P recycling during leaf senescence or Pi deficiency.
Members of the T2 family of ribonucleases (RNases) are present in virtually all eukaryotes, and appear to play a variety of important functions. The T2 family can be further divided into three subclasses: class III consists of S-RNases involved in gametophytic self-incompatibility (Hua et al., 2008), whereas classes I and II comprise S-like RNases that are responsive to diverse developmental and environmental signals (MacIntosh et al., 2010). Arabidopsis AtRNS2 was the first S-like RNase gene to be identified in plants, and was shown to be abundantly expressed in most tissues (Taylor et al., 1993). It is now clear that this pattern of expression is characteristic of all class II RNases characterised to date (Liang et al., 2002; MacIntosh et al., 2010; Taylor et al., 1993). Consistent with their abundant expression and clear conservation among species, class II RNase genes encode enzymes that play a fundamental role in ribosomal RNA decay throughout plant development (Hillwig et al., 2011; MacIntosh et al., 2010). Although class II RNases, such as Arabidopsis AtRNS2 and Antirrhinum AhSL28, play a crucial housekeeping role in rRNA decay, their expression at the gene and/or protein level increases in response to Pi deficiency and leaf senescence (Bariola et al., 1999; Liang et al., 2002; Taylor et al., 1993). This suggests that the activity of class II RNases is important during normal development, but is enhanced during nutritional stress (e.g. senescence and Pi deficiency).
In contrast to the class II T2 RNases, a great diversification exists for class I RNases, which are represented in larger numbers. Members of this class generally are not abundantly expressed but are induced by diverse stimuli, including nutrient deficiency, senescence, biotic stress, wounding, and osmotic stress. Because many class I RNases are induced by Pi deficiency and senescence they are expected to play an important role in Pi recycling and scavenging under these conditions (MacIntosh et al., 2010). Although most class I RNases are secreted to cell-wall compartments (i.e. apoplast), both apoplastic and intracellular RNases have been identified. RNase LX from tomato (Solanum lycopersicum) is a well-characterised, intracellular class I RNase, originally identified as a Pi starvation-inducible (PSI) RNase from tomato suspension cells (Kock et al., 1995). Subsequently RNase LX was shown to be highly induced at the mRNA and protein levels in senescing leaves and in response to ethylene (Lehmann et al., 2001; Lers et al., 1998; Lers et al., 2006). Knockout of RNase LX delays leaf senescence and abscission, confirming an important role of this RNase in cell death processes (Lehmann et al., 2001; Lers et al., 2006). The expression of RNase LX in response to Pi deficiency and senescence increases during relatively advanced stages of these conditions (Kock et al., 1995; Lers et al., 1998). This, along with RNase LX having an acidic pH optimum but being localised to the endoplasmic reticulum (ER) (i.e. of neutral pH), suggests that this enzyme functions during cell degradation when the ER membranes become compromised, exposing soluble cell contents to degradation (Fukuda, 1997; Lehmann et al., 2001).
In contrast to RNase LX, many class I RNases are secreted to the apoplast. It has been hypothesised that, during Pi deprivation or leaf senescence, these extracellular RNases (along with cell-wall-targeted acid phosphatases) participate in recycling P from cytoplasmic P esters that leak across the plasma membrane (Robinson et al., 2012a; Shane et al., 2014). This recycling function would be beneficial not only during Pi limitation, but also during senescence when the breakdown of macromolecules can result in high levels of P esters and nucleic acids leaking into the apoplast (Lim et al., 2007). Arabidopsis AtRNS1 and tomato RNase LE are two extracellular class I RNases (Bariola et al., 1999; Borderies et al., 2003; Kock et al., 1995) induced by senescence and Pi deprivation (Bariola et al., 1994; Bariola et al., 1999; Kock et al., 1995; Lers et al., 1998). AtRNS1 appears to be primarily responsible for the RNase activity upregulated by senescence in Arabidopsis cell-wall fractions (Shane et al., 2014). In addition to senescence and Pi deprivation, AtRNS1 and RNase LE are also induced by wounding and abscisic acid (ABA) (Kock et al., 2004; LeBrasseur et al., 2002; Lers et al., 1998; Verslues & Zhu, 2007). The responsiveness of these class I RNases and others to stressors such as wounding and osmotic stress has led to proposed roles for these enzymes in defence, membrane permeability and nutrient recycling during processes of cell differentiation. AtRNS1 expression was observed, albeit at a relatively low level, in the vascular tissues of seven-day-old seedlings (Hillwig et al., 2008), whereas RNase LE was localised to the phloem cells following wounding (Kock et al., 2004). It is of interest to characterise the tissue specificity of these RNases during Pi deficiency and senescence in more detail. It is tempting to speculate that phloem localisation is consistent with a role for these enzymes in significant P remobilisation during Pi deficiency and senescence. The involvement of T2 RNases in P recycling appears ubiquitous in plants. The efficient P-remobilisation from senescing leaves of Hakea prostrata (harsh hakea), an ‘extremophile’ plant native to the nutrient-impoverished soils of south-western Australia, was recently correlated with enhanced activities of intracellular and cell-wall RNases, as well as purple acid phosphatases (PAPs) (Shane et al., 2014). In addition, several RNase genes, OsRNS3, OsRNS4, OsRNS5, OsRNS7, and OsRNS8, were induced by Pi deficiency in rice (MacIntosh et al., 2010; Zheng et al., 2009), whereas transcript arrays for senescing leaves identified upregulated RNases in Medicago truncatula (De Michele et al., 2009) and wheat (Gregersen & Holm, 2007).
In addition to T2 RNases, other nucleases appear to play an important role in nucleic acid degradation during senescence. The Arabidopsis BIFUNCTIONAL NUCLEASE 1 (BFN1) gene encodes a type 1 nuclease (Perez-Amador et al., 2000). AtBFN1 expression is enhanced during leaf and stem senescence, floral abscission, programmed cell death, and Pi deficiency (Bustos et al., 2010; Farage-Barhom et al., 2011; Perez-Amador et al., 2000; Wagstaff et al., 2009). The AtBFN1 protein was shown to colocalise with fragmented nuclei in membrane-coated vesicles during senescence, implicating AtBFN1 in nucleic acid breakdown and P recycling during senescence (Farage-Barhom et al., 2011). Recently, AtBFN1 was shown to be a direct target of the well-characterised, senescence-associated NAC transcription factor, AtORE1 (Matallana-Ramirez et al., 2013), further supporting a role for AtBFN1 in Pi remobilisation during leaf senescence.
6.3.2 Phosphatases
The enhanced activity of phosphatases is widely recognised as being linked to Pi remobilisation during Pi deficiency (Plaxton & Tran, 2011) and, more recently, to programmed senescence (Robinson et al., 2012a). One of the most well-studied groups of phosphatases linked to Pi metabolism and senescence is the PAP family (Chapter The Role of Intracellular and Secreted Purple Acid Phosphatases in Plant Phosphorus Scavenging and Recycling); these enzymes hydrolyse a broad range of Pi monoesters with an acidic pH optimum. Unlike the situation in mammals, plant PAPs comprise relatively large gene families; for example, the Arabidopsis genome encodes 29 PAPs (Li et al., 2002). Considering that their gene expression is responsive to a variety of developmental and environmental factors, the PAPs are expected to play a diversity of functions during development (Chapter The Role of Intracellular and Secreted Purple Acid Phosphatases in Plant Phosphorus Scavenging and Recycling). Those PAPs that have been linked to Pi remobilisation during leaf senescence will be described below.
The best-characterised PAP to play a role in P recycling during leaf senescence, as well as Pi scavenging during P deprivation, is Arabidopsis AtPAP26. Kinetic studies with purified native vacuolar and secreted AtPAP26 isoforms have revealed that this PAP effectively hydrolyses Pi from a wide range of substrates with a high catalytic efficiency over a broad pH range (Veljanovski et al., 2006; Tran et al., 2010). During P deprivation, AtPAP26 was the predominant dual-targeted (vacuolar and secreted) PAP upregulated, and atpap26 knock-out mutants failed to induce most of their intracellular and secreted APase activity while exhibiting an impaired growth during P deficiency (Hurley et al., 2010; Robinson et al., 2012b; Tran et al., 2010). The loss of AtPAP26 also resulted in a delayed-senescence phenotype, and atpap26 mutants remobilised only 15% of total P from their leaves following senescence, as compared to 70% P remobilisation in the wild-type control (Robinson et al., 2012a). Seeds from atpap26 plants contained only 16% of the total P of wild-type seeds, showing clear defects in Pi remobilisation from senescing leaves and subsequent seed filling (Robinson et al., 2012a). Further characterisation revealed that senescence promoted an enhanced AtPAP26 protein abundance in both the vacuole and apoplast (Shane et al., 2014). Dramatic decreases of intracellular and apoplastic APase activities in the atpap26 mutant and correspondingly low Pi remobilisation demonstrates that AtPAP26 is the predominant Arabidopsis PAP involved in P recycling during senescence (Robinson et al., 2012a; Shane et al., 2014). A recent examination of APase activities in senescing leaves of highly P-efficient harsh hakea revealed senescence-induced intracellular and apoplastic APase activities, most likely due to functional orthologues of AtPAP26 (Shane et al., 2014). Another Arabidopsis PAP implicated in both tolerance to P deficiency and P recycling during leaf senescence is AtPAP17 (del Pozo et al., 1999; Robinson et al., 2012a; Shane et al., 2014). AtPAP17 is responsive to Pi deficiency and senescence at the RNA and protein levels (del Pozo et al., 1999; Robinson et al., 2012b), whereas AtPAP26 transcript abundance is insensitive to Pi status and is only moderately induced by senescence (Gepstein et al., 2003; Robinson et al., 2012a). AtPAP17 expression is also induced by a variety of stimuli including ABA, salinity, and hydrogen peroxide (del Pozo et al., 1999). AtPAP17 and AtPAP26 each exhibit alkaline peroxidase activity (del Pozo et al., 1999; Veljanovski et al., 2006), which might contribute to the metabolism of reactive oxygen species (ROS) following the loss of vacuolar integrity that accompanies senescence, although this point requires further investigation. Collectively, the characterisation of distinct Arabidopsis PAPs, as well as the observation that at least seven AtPAPs are induced by both Pi deficiency and senescence (Breeze et al., 2011; Bustos et al., 2010), suggest that the PAP family plays an integral role in P recycling during leaf senescence and Pi starvation.
One group of phosphatases distinct from PAPs has been shown to be tightly controlled by Pi deprivation in plants at the mRNA level. These phosphatases are members of the haloacid dehalogenase (HAD) superfamily, which is a group of diverse enzymes present in presumably all eukaryotes (Burroughs et al., 2006). Tomato LePS2;1 was the first plant HAD member to be characterised (Baldwin et al., 2001). Although LePS2;1 was originally characterised as an acid phosphatase with a low activity against the standard substrate p-nitrophenyl phosphate (p-NPP) (Baldwin et al., 2001), it was later shown to have a higher activity for a synthetic Ser/Thr phosphopeptide. Similarly, PvHAD1 from common bean (Phaseolus vulgaris) exhibited activity against a phosphopeptide substrate, but not p-NPP (Liu et al., 2010). The expression of both LePS2;1 and PvHAD1 is highly responsive to Pi deprivation and subsequent resupply of Pi, yet is not sensitive to starvation of other nutrients (Baldwin et al., 2001; Liu et al., 2010). Taken together, these observations led to the hypothesis that LePS2;1 and PvHAD1, as well as other Pi-responsive HAD homologues, including PvPS2;1 and PvPS2;2 from common bean (Liang et al., 2012), rice OsACP1 (Hur et al., 2007), and potato StPPP1 (Petters et al., 2002), play a role in Pi signalling through the modulation of protein phosphorylation events (Baldwin et al., 2008; Liang et al., 2012). However, since extensive screening of substrates for these HADs has not been carried out, they may play additional roles in Pi metabolism.
As with the HAD genes in tomato and common bean, some Arabidopsis HAD genes are highly responsive to Pi deficiency, as shown by transcript profiling studies. Indeed, under low-Pi conditions several HAD genes had the most abundant transcript levels of all Arabidopsis genes (Morcuende et al., 2007; Muller et al., 2007). Functionally characterised Arabidopsis HADs include AtPPsPase1 (At1g73010), a novel pyrophosphatase (May et al., 2011); AtPECP1 (At1g17710), a phosphoethanolamine/phosphocholine phosphatase (May et al., 2012); and AtSgpp (At2g38740), a phosphosugar phosphatase (Caparros-Martin et al., 2013). Both AtPPsPase1 and AtPECP1 are type 1B HAD members, which include enzymes that hydrolyse a diverse set of P monoesters (Burroughs et al., 2006). It appears that AtPPsPase1 and AtPECP1 are induced in the early stages of Pi deprivation, and can delay intracellular Pi deficiency by quickly liberating Pi from pyrophosphate and phospholipids, respectively. AtSgpp, a C1-type HAD, acts on a variety of phosphosugar substrates including glucose-6-phosphate (Glc-6-P) (Caparros-Martin et al., 2013). Experiments utilizing 31P-NMR analysis revealed that cytosolic Glc-6-P levels fall quickly in response to Pi starvation, which is accompanied by an increase in cytosolic Pi (Pratt et al., 2009). Therefore, as with AtPPsPase1 and AtPECP1, AtSgpp could provide a quick reserve of Pi via the hydrolysis of cytosolic phosphosugars, including Glc-6-P. Intriguingly, many Arabidopsis HAD loci are also upregulated during senescence, as revealed by the transcript profiling of senescing leaves (Breeze et al., 2011; van der Graaff et al., 2006), although a role for plant HADs in senescence has not yet been investigated. Nonetheless, future experimentation in this regard holds much promise for revealing key Pi-remobilisation mechanisms that may occur during senescence and other cell-death processes.
6.3.3 Lipid-Remodelling Enzymes
Extensive remodelling of membrane lipids occurs during leaf senescence. The breakdown of lipids is initiated by lipolytic reactions that release fatty acids, which are targeted to peroxisomes for modification via the β-oxidation pathway (Troncoso-Ponce et al., 2013). On completion of the senescence process, the total fatty acid concentration of Arabidopsis, Brachypodium (Brachypodium distachyon), and switchgrass (Panicum virgatum) leaves were decreased by at least 80% (Yang & Ohlrogge, 2009). As lipid metabolism is dynamic throughout development, the decrease in fatty acid concentration represents the sum of lipid synthesis and catabolism. Therefore, the net decrease during senescence appears to be the result of declining synthesis, as well as an increase in the rate of degradation.
Comparative transcriptomic studies of leaf senescence have provided important clues as to how lipid metabolic pathways are regulated during senescence (Troncoso-Ponce et al., 2013). For example, a comparison of three robust microarray studies of natural leaf senescence in Arabidopsis revealed that the transcript levels for virtually all of the genes encoding core fatty acid biosynthetic enzymes decreased during senescence, whereas many genes encoding lipases were upregulated. Similarly, genes encoding components involved in β-oxidation were upregulated. These observations are consistent with the need to disassemble and degrade lipids during the senescence process. In contrast, the transcript abundance for several biosynthetic genes for galactolipids and sulfolipids is increased during senescence. Notably, many of these senescence-induced genes are also induced by Pi deficiency (Chapter Membrane Remodelling in Phosphorus‐Deficient Plants) (Bustos et al., 2010; Misson et al., 2005; Morcuende et al., 2007), including those involved in phospholipid hydrolysis (phospholipase A, phospholipase D, several members of the glycerophosphoryl diester phosphodiesterase (GDPD) family, and AtPECP1), as well as genes involved in galactolipid and sulfolipid biosynthesis, such as AtMGD2, AtMGD3, AtDGD2, AtSQD1, and AtSQD2 (Bustos et al., 2010). The coordinated upregulation of phospholipase genes and galactolipid and sulfolipid biosynthetic genes under Pi-deficient conditions collectively results in the liberation of Pi from phospholipids and replacement with lipids containing other head groups (Chapter Membrane Remodelling in Phosphorus‐Deficient Plants). It is unclear whether a similar P-recycling program is initiated during leaf senescence, or if upregulation of the galactolipid and sulfolipid biosynthetic genes reflects a necessary part of membrane turnover. The leaves of garden pea that were beginning to show signs of senescence had higher levels of galactolipid (monogalactosyldiacylglycerol; MGDG) synthesis as compared to younger leaves (Hellgren & Sandelius, 2001). Unlike extraplastidic membranes, the chloroplastic membranes generally contain low levels of phospholipids but high levels of MGDG and digalactosyldiacylglycerol (DGDG) galactolipids (Dörmann & Benning, 2002). It is possible that this increased synthesis of galactolipids such as MGDG during senescence reflects a need to repair or stabilise thylakoid membranes throughout the catabolic program. The lipid concentrations of leaves from several Proteaceae species growing on severely P-impoverished soils exhibit very low phospholipid concentrations, but increased galactolipid and sulfolipid concentrations in mature leaves (Lambers et al., 2012). The same phenomenon was observed in Arabidopsis leaves, though to a much lesser extent. These results indicate that P redistribution is a process already in place as the leaves mature, and that this recycling mechanism is linked with P remobilisation during senescence.
6.3.4 Pi Transporters
The concerted action of RNases, phosphatases and lipid-remodelling enzymes liberates Pi from a variety of organic compounds during leaf senescence. This recycled Pi must be loaded into the phloem of senescing leaves and unloaded at sink tissues by Pi transporters. As discussed in detail in Chapter Phosphate Transporters, many members of the Pht1 family of Pi transporters play important roles in Pi acquisition from soil and the subsequent root-to-shoot translocation of Pi (Nussaume et al., 2011). The expression of several Pht1 transporters has also been detected in the phloem of leaves from Pi-sufficient plants, implicating these transporters in Pi remobilisation, including barley HvPht1;6 (Rae et al., 2003), rice OsPht1;1 (Sun et al., 2012), OsPht1;2 (Ai et al., 2009) and OsPht1;8 (Jia et al., 2011), and Arabidopsis AtPht1;5 (Nagarajan et al., 2011). The overexpression of either OsPht1;1, OsPht1;8, or APht1;5 conferred an enhanced remobilisation of shoot Pi from mature to younger organs (i.e. mature leaves to younger leaves and/or reproductive tissues). In addition, loss of function and RNAi-suppressed lines of OsPht1;8 restricted translocation of Pi from the panicle axis to the grain (Jia et al., 2011). Similarly, atpht1;5 mutants accumulated more P in shoots and less in roots as compared to wild-type (Nagarajan et al., 2011), indicating an impaired remobilisation of Pi. A number of other Pht1 genes are expressed in shoot tissues during Pi deficiency, and these may play a role in Pi remobilisation during low-Pi conditions. These include AtPht1;4 (Nagarajan et al., 2011), rice OsPht1;6 (Ai et al., 2009), OsPht1;9 and OsPht1;10 (Wang et al., 2014), tomato LePht1;1 and LePht1;7 (Chen et al., 2014), potato StPht1;1 (Leggewie et al., 1997), and soybean GmPht1;1 (Song et al., 2014). Whether these transporters function during leaf senescence is unclear. The expression of petunia PhPht1;1, is induced in senescing corollas, and appears to be directly regulated by ethylene (Chapin & Jones, 2009). AtPht1;5-overexpression lines exhibit altered root morphology, which could be rescued by inhibiting ethylene biosynthesis or signalling (Nagarajan et al., 2011). These observations may indicate a conserved mechanism of ethylene-induced expression of Pht1 transporters during senescence.
Other transporters that play a role in Pi remobilisation are members of the SPX superfamily (see below) and the Pht2 Pi transporter family. AtPht2;1 is a low-affinity Pi transporter localised to the chloroplast envelope, where it is necessary for Pi import to sustain optimal photosynthetic activity (Versaw & Harrison, 2002). The mutation of AtPht2;1 led not only to reduced growth (which likely was due to Pi limitation of photosynthesis) but also to a restricted Pi remobilisation from mature to young leaves in response to Pi deficiency (Versaw & Harrison, 2002). The wheat orthologue of AtPht2;1, TaPht2;1, likely has a similar function in the delivery of chloroplastic Pi and overall Pi distribution (Guo et al., 2013). A greater expression of TaPht2;1 was recently observed in the stems of a P-efficient wheat cultivar as compared to a P-inefficient cultivar, which may contribute to its higher PUE (Aziz et al., 2014).
6.4 Regulatory and Signalling Components of Senescing Leaves
6.4.1 Transcription Factors
Senescence is a highly regulated process that is accompanied by the altered transcription of thousands of genes. Not surprisingly, transcriptional regulators play a major role in modulating the onset and elaboration of senescence processes. The largest groups of transcription factors implicated in controlling senescence are from the NAC, WRKY, AP2/EREBP, MYB, and zinc finger families (Balazadeh et al., 2008; Buchanan-Wollaston et al., 2003; Gregersen & Holm, 2007; Guo et al., 2004). A comparison of transcriptomic data from several global expression studies on Pi-deprived plants revealed an enrichment of the same five groups of transcription factors (Nilsson et al., 2010). In the following sections, attention will be focused on the characterised senescence-related transcription factors most likely to contribute to Pi-remobilisation processes during leaf senescence.
The NAC transcription factor family is a large, plant-specific family that plays a role in a number of developmental and stress-responsive processes. The family was originally defined by the NO APICAL MERISTEM and CUP-SHAPED COTYLEDON transcription factors (Aida et al., 1997; Souer et al., 1996). Transcript levels for NAC loci from a variety of plant species, including Arabidopsis (Breeze et al., 2011; Guo et al., 2004; Lin & Wu, 2004; van der Graaff et al., 2006), rice (Liu et al., 2008), Medicago truncatula (De Michele et al., 2009), wheat (Gregersen & Holm, 2007), barley (Parrott et al., 2007), and wallflower (Erysimum linifolium) (Price et al., 2008), are upregulated during senescence. Many NAC genes are also highly responsive to Pi deficiency, as well as ABA and osmotic (e.g. drought, salt, and mannitol) stimuli. Therefore, the NAC transcription factor family likely plays a key role in the coordination of programmed senescence and environmental cues, including Pi and water status.
One of the best-characterised senescence-related NAC transcription factors is AtORE1/ANAC092 from Arabidopsis. The disruption or overexpression of AtORE1 confers delayed or early senescence, respectively (Balazadeh et al., 2010; Oh et al., 1997). AtORE1 directly promotes the expression of AtBFN1, AtSWEET15, and AtSINA1 (Matallana-Ramirez et al., 2013). This reveals an overlap between senescence and Pi deficiency, as AtBFN1 is likely an important nuclease involved in P recycling during senescence (as described above). The expression of AtSINA1, AtSWEET15, and AtORE1 itself is also induced by Pi deficiency (Bustos et al., 2010; Marchive et al., 2009). Putative targets of AtORE1 were identified using an oestradiol-inducible AtORE1-overexpression line (Balazadeh et al., 2010). Of the 170 possible AtORE1 targets, more than one-third (n = 58) were induced in Pi-deficient leaves (Bustos et al., 2010). Similar to AtORE1, knock-out or overexpression of the NAC transcription factor gene, AtNAP, conferred a delayed or early senescence, respectively (Guo & Gan, 2006). AtNAP targets SAG113, which encodes a protein phosphatase 2C that regulates ABA-mediated stomatal movement and water loss during senescence (Zhang & Gan, 2012; Zhang et al., 2012), and is also highly induced in response to Pi deprivation (Bustos et al., 2010). Considering the extensive overlap among ABA, Pi deficiency, and senescence transcriptomes (Figure 6.2) (Breeze et al., 2011; Woo et al., 2012), AtNAP could be an important point of crosstalk among the associated signalling pathways.
A recent transcript profiling study identified three Arabidopsis NAC loci that did not respond to a Pi starvation treatment, but had elevated transcript abundance during a subsequent readdition of Pi (Woo et al., 2012). One of these, ANAC080, is strongly upregulated during senescence (Breeze et al., 2011). A similar pattern of Pi-responsive expression (i.e. no response to Pi starvation, but induced expression by subsequent Pi readdition) was observed for five NAC genes in rice. One of these genes, Os01g66120, was identified as being upregulated during senescence in flag leaves (Liu et al., 2008). It is tempting to speculate that the expression of ANAC080 and Os01g66120 is sensitive to a dramatic increase in intracellular Pi which would accompany senescence.
As with NACs, many WRKY transcription factor genes are upregulated by senescence in many plant species, and several WRKYs in Arabidopsis promote senescence, including AtWRKY6 (Robatzek & Somssich, 2001; Robatzek & Somssich, 2002), AtWRKY53 (Miao et al., 2004), and AtWRKY75 (Li et al., 2012). Notably, AtWRKY6 and AtWRKY75 also regulate responses to Pi deficiency (Chen et al., 2009; Devaiah et al., 2007). In addition to promoting senescence through its regulation of AtFRK1/AtSIRK, and likely by targeting a number of other loci, including two NAC transcription factors (Robatzek & Somssich, 2002), AtWRKY6 represses the expression of AtPHO1, which is required for normal Pi distribution (Chen et al., 2009). It appears that AtWRKY6 is targeted for proteolysis via its polyubiquitination by a specific E3 ligase isozyme (At1g74410) that is induced by Pi deficiency, which then relieves AtWRKY6 repression of AtPHO1 (Chen et al., 2009). Interestingly, the expression of this E3 ligase is induced during the onset of senescence, but is downregulated during late senescence (Breeze et al., 2011). AtWRKY6 also appears to target several additional genes, including many that are induced by Pi starvation, some positively and some negatively (Chen et al., 2009; Robatzek & Somssich, 2002). Similar to AtWRKY6, AtWRKY75 also affects the expression of several PSI genes. RNAi knockdown of AtWRKY75 resulted in an attenuated low-Pi induction of Pht1 Pi transporters and HAD family phosphatases (Devaiah et al., 2007; Nagarajan et al., 2011). AtWRKY75 was implicated in promoting senescence when atwrky75 null mutants were observed to have a delayed senescence phenotype (Li et al., 2012). The loss of another Arabidopsis WRKY, AtWRKY53, also resulted in delayed senescence, whereas overexpression conferred an accelerated senescence (Miao et al., 2004). Several loci are predicted targets of AtWRKY53 such as AtSQD1 and other WRKYs, including AtWRKY6. Antagonistic and synergistic interactions among WRKYs have been reported previously (Rushton et al., 2010), which makes the interpretation of their overall impacts on cellular processes very challenging. Nevertheless, it appears likely that AtWRKY6, AtWRKY53, and AtWRKY75 have overlapping roles in responses to Pi deprivation and Pi remobilisation from senescing leaves.
The Arabidopsis MYB transcription factor AtPHR1 and its homologue AtPHR1-like1 (AtPHL1) have a global impact on modulating Pi-starvation responses (Bustos et al., 2010). AtPHR1 orthologues have been characterised in a number of other species, and are likely ubiquitous among angiosperms (Ren et al., 2012; Valdes-Lopez et al., 2008; Wang et al., 2013; Zhou et al., 2008). In response to low-Pi conditions, AtPHR1 activates the expression of numerous downstream loci via the PHR1-binding site (P1BS) cis-regulatory motif (Rubio et al., 2001). The transcript profiling of a atphr1 loss-of-function mutant revealed that AtPHR1 controls most transcriptional activation and repression responses to Pi deficiency, revealing its global impact on low-Pi signalling events (Bustos et al., 2010). To identify putative targets of AtPHR1, a transcriptomic analysis was carried out on a phr1 mutant line containing a dexamethasone-inducible AtPHR1 transgene (OxGR:PHR1 phr1) (Bustos et al., 2010). This study identified 319 potential direct targets of AtPHR1, and showed that the majority of these genes were also induced by Pi deficiency in wild-type seedlings. Considering the major contribution of AtPHR1 to Pi-related gene expression and the extensive overlap between transcriptome changes in response to Pi deficiency and senescence, it is likely that AtPHR1 activity is involved in Pi remobilisation during leaf senescence. Indeed, many putative AtPHR1 targets are highly induced during leaf senescence, including many of the genes described herein, such as AtRNS1, AtPAP12, AtPAP17, AtPPsPase1, AtPECP1, AtSgpp, AtPLDZ2, AtPLA (At1g31480), AtGDPD1, AtSQD1, AtSQD2, AtMGD2, AtMGD3, AtDGD2, AtPht1;1, AtPht1;4, AtPht1;5, AtSPX1, and AtSPX2. It is of interest to investigate the extent to which AtPHR1 activity is responsive to senescence-related signals.
6.4.2 The SPX Superfamily
Originally characterised as components of Pi sensing and transport in yeast (Schneider et al., 1994; Spain et al., 1995), SPX domain proteins have more recently been shown to participate in Pi signalling and transport in plants (Secco et al., 2012). SPX superfamily members can be divided into the SPX, SPX-EXS, SPX-MFS, and SX-RING classes based on the presence of additional protein domains (Secco et al., 2012). The majority of proteins exclusively harbouring the SPX domain appear to regulate acclimatisation to Pi starvation via a negative regulation of Pi-signalling components (Secco et al., 2012; Wu et al., 2013). Although the mechanisms are not fully understood, SPX domains appear to facilitate binding with other proteins. OsSPX4 was shown recently to bind and repress the central Pi-responsive transcriptional activator OsPHR2 (Lv et al., 2014). During Pi-deficient conditions, OsSPX4 protein is targeted for degradation, which releases OsPHR2 to activate downstream PSI genes; hence, OsSPX4 is controlled at the protein level by Pi deficiency rather than at the transcript level (Lv et al., 2014). In contrast, mRNA expression of other SPX class I members is highly responsive to low Pi (Duan et al., 2008; Wang et al., 2009a; Wang et al., 2009b). AtSPX1, AtSPX2, and AtSPX3 are also induced in senescing leaves, suggesting a role for these SPX proteins in Pi remobilisation during senescence.
AtPHO1, AtPHO1;H1, and OsPHO1;2 are SPX proteins that also contain an EXS domain, placing them in class 2 (Secco et al., 2012). These SPX-EXS proteins all play a role in Pi translocation from root to shoot (Poirier et al., 1991; Secco et al., 2010; Stefanovic et al., 2007). AtPHO1 and AtPHO1;H1 are induced in senescing leaves, which suggests that they may also play a role in Pi remobilisation in shoots. Further, AtPHO1 is necessary for stomatal responses to ABA (Zimmerli et al., 2012), supporting the hypothesis that Pi deficiency, senescence, and ABA-related pathways are interconnected.
OsSPX-MFS1 is a member of the class 3 family of SPX proteins, which also include a MFS domain. Heterologous expression in yeast showed that OsSPX-MFS1 encodes a bona fide Pi transporter (Wang et al., 2012). Mutant analysis revealed that loss of OsSPX-MFS1 restricted the translocation of Pi from mature to young leaves (Wang et al., 2012). It is of interest to examine the behaviour of OsSPX-MFS1 and its rice paralogues (Secco et al., 2012) during leaf senescence.
Finally, a member of the Arabidopsis SPX-RING class, AtNLA, was shown recently to act as an E3 ligase in the degradation process of Pht1 Pi transporters (Lin et al., 2013; Park et al., 2014). The mutation of AtNLA resulted in early senescence (Peng et al., 2007b), but this phenotype could be suppressed by mutation of AtPht1;1 or AtPHF1, an ER-resident protein required for the correct targeting of Pht1 proteins to the plasma membrane (Gonzalez et al., 2005; Kant et al., 2011). A recent network modelling of senescence transcriptomes predicted AtNLA as one of several integration points, in which AtNLA had a significant association with AtWRKY53 and an autophagy-related gene, AtATG8F (Penfold & Buchanan-Wollaston, 2014), further supporting a role for AtNLA in the senescence-dependent remobilisation of Pi. In summary, SPX-containing proteins have diverse functions linked to Pi signalling and transport that likely have overlapping roles in remobilisation during Pi deficiency and programmed senescence.
6.4.3 Ubiquitination Components and miRNAs
The characterisation of Pi-related ubiquitination components has revealed that proteasome degradation plays an important role in Pi starvation signalling pathways, particularly with regard to modulating Pi transport. As described above, the SPX-RING protein AtNLA is an E3 ligase that assists in the degradation of Pht1 proteins, with an apparent preference for AtPht1;4 (Lin et al., 2013; Park et al., 2014). Although the precise mechanism is unclear, AtNLA functions cooperatively with AtPHO2, an E2 conjugase (Aung et al., 2006; Bari et al., 2006; Delhaize & Randall, 1995) to target Pht1 proteins (Lin et al., 2013; Park et al., 2014). AtPHO2 also targets AtPHO1, a SPX-EXS protein that is essential for correct Pi translocation (Liu et al., 2012). The mutation of either AtPHO2 or AtNLA results in an enhanced Pi accumulation in the shoots (Aung et al., 2006; Kant et al., 2011). Interestingly, under high-Pi and low-N conditions, the increase in shoot Pi concentration in atpho2 or atnla mutants is exacerbated, and results in an early senescence phenotype (Kant et al., 2011). These results highlight the crosstalk between P- and N-signalling pathways, and show that both P and N status are tightly connected to the onset of leaf senescence (Chapter Interactions between Nitrogen and Phosphorus Metabolism).
An additional layer of regulation of Pi-transport activity is via microRNA (miRNA)-mediated transcript degradation. AtNLA and AtPHO2 are the targets of two PSI miRNAs, miR827 and miR399, respectively (Aung et al., 2006; Bari et al., 2006; Hsieh et al., 2009). Current evidence supports a role for miR827 and miR399 targeting AtNLA and AtPHO2 transcripts in roots which relieves the polyubiquitination-dependent repression of Pi transporters and subsequent increases in Pi acquisition and translocation (Kant et al., 2011). It is unclear whether this mechanism functions in shoots, but both AtPht1;4 and AtPHO1, which are targets of AtNLA and/or AtPHO2, show some expression in shoot tissues and have been implicated in Pi remobilisation (Nagarajan et al., 2011; Zimmerli et al., 2012). Two miRNAs playing important roles in regulating leaf senescence, miR164 and miR319, are also responsive to Pi deficiency (Gu et al., 2010; Zhu et al., 2010). Both miRNAs target transcription factors that promote leaf senescence. The NAC transcription ORE1 is targeted by miR164 (Kim et al., 2009), whereas miR319 targets members of the TCP family of transcription factors, which play roles in leaf developmental processes including senescence (Schommer et al., 2008). The levels of miR164 and miR319 decline in response to leaf aging, which relieves suppression of their targets. Studies aimed at screening for Pi-responsive miRNAs have generally found that Pi deficiency increases the transcript abundance of miR164 and miR319 in roots, but decreases their transcript levels in shoot tissues (Gu et al., 2010; Kuo & Chiou, 2011; Zhu et al., 2010). Taken together, these results indicate that miRNA expression may be an important point of interaction between senescence and Pi sensing and/or remobilisation.
6.5 Role of Hormones during Leaf Senescence
Phytohormones are key modulators of plant developmental processes, including senescence. In general, ethylene, ABA, strigolactones, jasmonic acid and salicylic acid promote senescence, whereas cytokinins, gibberellins and auxins delay senescence. Recent reviews have characterised the roles of these hormones in Pi-signalling pathways (Chiou & Lin, 2011) and senescence (Kusaba et al., 2013; Sarwat et al., 2013). Those hormones that appear to be particularly relevant to the coordination of activities that impact the remobilisation of Pi during leaf senescence are highlighted in the following subsections.
6.5.1 Ethylene and Strigolactones
Ethylene biosynthesis is induced in response to Pi deficiency (Nagarajan & Smith, 2012) and senescence (Sarwat et al., 2013), whereas mutation of the ethylene-signalling component EIN2 delays senescence (Oh et al., 1997) and suppresses the expression of PSI genes (Lei et al., 2011). These results confirm that the biosynthesis and responsiveness of ethylene increases during both leaf senescence and Pi deficiency. Similarly, strigolactone synthesis and signalling is enhanced in senescing leaves and during Pi deficiency. It is likely that the accumulation of ethylene and strigolactones which occurs throughout leaf development increases the sensitivity of older leaves to senescence-inducing signals. Considering their wide impact on modulating adaptive root and shoot responses to low-Pi conditions, ethylene and strigolactones are likely involved in monitoring and controlling the Pi status of senescing leaves.
6.5.2 Abscisic Acid
As with ethylene and strigolactones, ABA is a positive regulator of senescence (Sarwat et al., 2013). A comparison of a transcriptome analysis of Pi-deficient Arabidopsis with hormone-related transcript profiling studies revealed that Pi-deficiency had much more overlap with genes which were differentially expressed by ABA than with other hormones (Woo et al., 2012). In this comparison of senescence and Pi-deficiency transcriptomes, a shared enrichment of many genes related to ABA was also observed (Breeze et al., 2011; Bustos et al., 2010). Considering its well-characterised role in modulating responses to osmotic stressors, ABA may play an important role in integrating environmental conditions into the overall senescence program.
6.5.3 Cytokinins
In contrast to ethylene, strigolactones and ABA, cytokinins are negative regulators of both senescence and responses to Pi deficiency (Chiou & Lin, 2011; Sarwat et al., 2013). Indeed, the overexpression of isopentenyltransferase (IPT), an enzyme that catalyses the rate-limiting step in cytokinin synthesis, delayed senescence (Gan & Amasino, 1995). The ectopic expression of IPT in bentgrass suppressed leaf senescence induced by deficiencies of P or N (Zhang et al., 2010), which suggests that the effect of cytokinins (as with some other hormones) on senescence is sensitive to nutrient status. Cytokinins appear to mediate changes in sink/source relationships, and their impact on senescence appears closely linked with carbon metabolism (Zwack et al., 2013). Taking into consideration the well-known crosstalk that exists among P-, N-, and sugar-signalling pathways (Schluter et al., 2013; Wingler et al., 2006), cytokinins may be an important integrator of senescence and nutrient status.
6.6 Concluding Remarks
In light of the decreasing availability of high-purity, low-cost phosphate-rock reserves, improvements in the PUE of crop plants will be essential to sustain global agriculture. Due to the need for P recycling during plant development to maintain adequate growth – particularly during seed maturation – Pi remobilisation from senescing tissues represents a valuable target for improving PUE. Several studies have revealed differences in Pi-remobilisation components among species or cultivars that correlate with PUE, such as the expression and activities of acid phosphatases, ribonucleases, phospholipid-remodelling factors, and Pi transporters (Aziz et al., 2014; Fita et al., 2012; Lambers et al., 2012; Liang et al., 2012). Collectively, these studies have revealed genetic differences in P-recycling components and have validated the manipulation of Pi remobilisation as an effective strategy to improve PUE. Future investigations should seek to unravel the complex integration of senescence and Pi-signalling pathways to identify novel molecular targets, the manipulation of which via breeding and biotechnological effort can be used to improve the PUE of crop plants.
Acknowledgements
The studies conducted in the laboratory of Aaron Smith are funded by the National Science Foundation.



