9 Development and the Evolution of Plant Form
Abstract
Development in land plants is the process whereby a single cell becomes a large multicellular organism. Developmental networks specify every organ, tissue, or cell type from egg to juvenile to a reproducing adult. Therefore, it would follow that changes in developmental networks result in changes in form, and understanding these developmental networks and their changes would provide great insight into the evolution of plant form. Only recently has the field of evolutionary developmental biology (evo-devo) been recognized as an important evolutionary mechanism; however, this field is still in its nascent stages in plants. Much of the plant evo-devo studies have focused on the flower and to some extent on the leaf. However, to understand the evolution of plant form, these ideas and studies need to be extended across the land plants. We review what we know about the contribution of development to the evolution of plant form and discuss avenues of future research.
9.1 Introduction
The evolution of plant form has been studied for centuries using morphology, development, and comparative embryology. These studies have included both paleobotanical evidence as well as focusing on extant lineages, and these have been important for generating hypotheses on the evolution of plant morphological features. Phylogenetic analyses using molecular characters have refined some of these hypotheses, and more recently developmental genetics has provided some compelling data to further evaluate the evolution of particular plant morphological features, revealing some general concepts on the role of development in evolution (e.g., Ambrose et al. 2000; Nickrent et al. 2000; Carroll 2008).
Research in developmental genetics is focused on unraveling the gene regulatory networks that are necessary to build a particular morphological feature (reviewed in Carroll 2008). The field of evolutionary developmental biology (also known as evo-devo) is concerned with the molecular genetics of these developmental pathways and how changes in the gene regulatory networks underlie changes in form (Wagner et al. 2000; Carroll 2008). The study of these gene regulatory networks has revealed some general concepts about the logic of these developmental pathways and shown how developmental features play a role in the evolution of organism. Unlike population genetics, evolutionary developmental biology can provide an explanation for the evolution of body plans and the origin of morphological novelties (Wagner et al. 2000). In addition, evo-devo studies have been important in contributing molecular data, and together with the morphological and paleontological data have been the basis for discussions about the homology of structures.
9.1.1 A Brief Historical Overview of Evolutionary Developmental Biology
The role of development in the evolution of form arose with the explosion of developmental genetic studies in the fruit fly, Drosophila melanogaster (Drosophila) (reviewed in Carroll 2008). In the 1980s, geneticists began characterizing homeotic mutations in Drosophila. Homeosis, the transformation of one organ type into the identity of another organ, was defined by William Bateson in the late 1800s. Geneticists began to identify the molecular basis of these mutations and found that mutations in similar types of genes also resulted in homeotic mutations. These genes were referred to as Homeobox (Hox) genes, and ultimately eight linked Hox genes were identified in Drosophila that affected the specification of segments in the embryo. In addition, the order of the Hox genes on the chromosome paralleled the order of the function of these Hox genes in the development of the embryo from “head” to “tail.”
The real beginning of evo-devo studies occurred when Hox clusters were identified throughout the animal kingdom and similar patterning functions could be ascribed to these Hox clusters in morphologically diverse animals. For example, Pax6, is the mouse Hox homolog of eyeless from Drosophila and both are important for eye field selector function in both animals. Eyes evolved independently at least three times in animals and the eyes of mice are morphologically distinct from eyes in Drosophila, with a lens and retina as opposed to the ommatidia common to insects. Amazingly, ommatidia form when the mouse Pax6 gene is introduced into Drosophila (Halder et al. 1995). Although all eyes are not homologous, developmental genetic studies demonstrated that there was a common genetic component that was deployed independently in the evolution of similar structures, a phenomenon termed deep homology (Shubin et al. 1997; Shubin et al. 2009).
The beginnings of plant evolutionary developmental biology also began when homeotic mutants in flowering plants were characterized (Bowman et al. 1989; Coen & Meyerowitz 1991). Homeotic mutants such as apetala3 in Arabidopsis thaliana (Arabidopsis) have flowers whose petals are replaced by sepals and stamens replaced by carpels. Geneticists began to analyze the molecular basis for these homeotic mutations and found that mutations in similar types of genes, namely, MADS-box genes, resulted in many of these floral homeotic mutations (Sommer et al. 1990; Yanofsky et al. 1990; Jack et al. 1992). Orthologs of MADS-box genes were identified across the flowering plants and mutations in these resulted in similar homeotic mutations in morphologically diverse flowers (see Section 2.1 for more detail). These early studies spawned the isolation of hundreds of floral MADS-box genes across the flowering plants and the beginning of modern plant evo-devo.
9.1.2 General Concepts in Evolutionary Developmental Biology
Developmental pathways that underlie form are composed of gene regulatory networks (e.g., Davidson & Erwin 2006; Peter & Davidson 2011), and evo-devo studies have shown that many components of gene regulatory networks encode transcription factors or signaling molecules. Once the genes and gene regulatory network underlying a particular morphology have been elucidated, a comparative approach can be used to try and understand how changes in the gene regulatory network can account for phenotypic changes. Evolution of gene regulatory networks can occur by duplication and diversification of genes, and by mutations in the coding sequence or mutations in the cis-regulatory elements of these loci. These evo-devo studies have allowed us to understand how development can act as a mechanism in the evolution of form.
Evo-devo studies have highlighted the role of large gene families (e.g., MADS-box genes) in the evolution of developmental processes, particularly large transcription factor families. The duplication and diversification of gene families provides the raw material for evolutionary processes, and gene duplication is an important source of genetic variation (Bowman et al. 2007; Conant & Wolfe 2008; Romanel et al. 2009). Following duplication, the gene and its copy can be functionally redundant and constraints on one of the copies can be relaxed. One copy can then accumulate mutations and take on a part of the original gene function (subfunctionalization), take on an entirely new function (neofunctionalization), or accumulate mutations until it is nonfunctional (becoming a pseudogene) (Ohno 1970).
The evolution of developmental networks can occur by changes in the coding sequence, and/or the cis-regulatory sequences of a gene in the network. Changes in the coding sequences of genes will affect the interaction of proteins in the network and the expression of downstream genes. Although duplication and diversification of genes and mutations in developmental genes can result in changes in the gene regulatory network, it has been argued that mutations in cis-regulatory elements are the main mechanism underlying the evolution of form (Carroll 2008).
Research in animal and plant evolutionary developmental biology has revealed some general concepts that arise from evo-devo, including modularity, co-option, developmental constraint, redundancy, evolvability, canalization, and developmental plasticity (Arthur 2002, 2004; Jenner & Wills 2007). All of these general concepts provide a framework to describe how developmental pathways can evolve and provide insight into how plant form has evolved.
For example, modularity is a property of a developmental system so that components of that system can be separated and modified independent of the rest of the system (Bolker 2000; Schlosser & Wagner 2004). One illustrative case is that of insects, which are composed of serial segments that can be multiplied or modified to have legs or wings, independent of other segments and the rest of the organism. It has been argued that modularity is an important property of developmental systems because it reduces the constraints and allows the developmental system to explore additional morphospace independent of the entire organism (Bolker 2000; Schlosser & Wagner 2004). Modularity is not only seen in body plan construction but also in the organization of protein domains and the cis-regulatory elements of a developmental gene (Riechmann et al. 2000; Carroll 2008; Charoensawan et al. 2010). Developmental networks are also modular in organization, and it is this modularity that gives the network robustness and evolvability. Another concept that has been revealed in evo-devo studies is co-option. Co-option is the recruitment of a gene (or pathway) to a new use, or the recruitment of a gene independently in evolution to a similar function (Arthur 2002).
Evolutionary developmental biology studies usually begin with the characterization of a gene involved in development and subsequently a more detailed analysis of that gene in a developmental network. Once the function of the gene or gene regulatory network has been elucidated, a comparative approach is used to try and understand which changes in the gene or gene regulatory network can account for phenotypic changes.
9.2 Plant Evolutionary Developmental Biology
There are two areas of plant research that best illustrate how evolutionary developmental studies contribute to our knowledge of the evolution of plant form. The first is the well-studied molecular genetics of flower development, which illustrates the concepts of modularity and canalization of flower development, provides tools to address questions of organ homology, the importance of duplication, and diversification of large gene families, and provides an explanation for the origin of an important evolutionary innovation—the flower. The second area of research is the molecular genetics of leaf development, which illustrates the evolution of developmental pathways, co-option of regulatory modules, data to address questions of homology, the influence of developmental constraint, and is beginning to provide an explanation for the origin of another important plant innovation—the leaf. We will also briefly discuss the development of some plant structures that have received somewhat less attention in plant molecular genetics including gametophytes and spores. Finally, the development of functional model systems across the land plants is crucial to the future of plant evo-devo.
9.2.1 The Evolution and Development of the Flower
9.2.1.1 How to Build a Flower
Most flowers in angiosperms follow a common basic plan, which consists of a standard arrangement of four concentric whorls. The two outer whorls or perianth are composed of sterile organs, most commonly sepals and petals, and the two inner whorls comprise the male and female reproductive organs.
Two decades ago, a genetic model for specifying floral organ identity was proposed based on mutant analyses in the two eudicot model species Arabidopsis thaliana and Antirrhinum majus. In both Arabidopsis and Antirrhinum, mutants had been described that showed transformations of one class of floral organs into another (Bowman et al. 1989; Schwarz-Sommer et al. 1990). These transformations were classified as a, b, or c types and generally affected two adjacent whorls. Class a mutants showed transformations of the two outer whorls, in which sepals took carpel identity and petals had stamen identity. Class b mutants developed sepals instead of petals and carpels instead of stamens. Finally, class c mutants showed transformations of stamens into petals and carpels into sepals and, in addition, they lost floral determinacy forming new flowers from the center of the floral meristem that repeated this pattern of homeotic transformations—lost determinacy.
Haughn and Sommerville made the first attempt to explain the phenotypes of these floral homeotic mutants with some simple logical rules that involved three classes of genes (A, B, C) which in different combinations of on/off states could specify the four types of floral organs (Haughn & Somerville 1988). A few years later, when the first homeotic genes started to be molecularly identified and Schwartz-Sommer et al. had already started to rework these “abc” rules (Schwarz-Sommer et al. 1990), Coen and Meyerowitz published the seminal “War of the whorls” paper, where the ABC model of floral development was clearly delineated (Coen & Meyerowitz 1991).
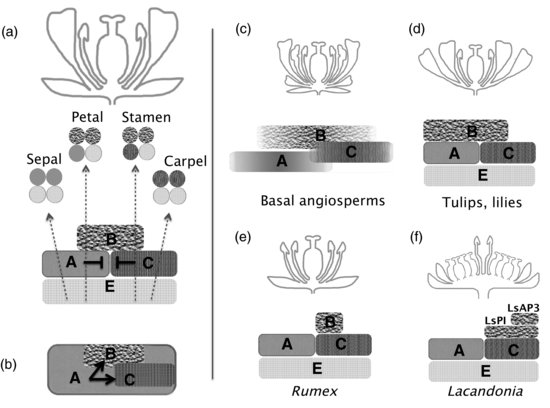
The ABC model was drawn from the three classes of homeotic mutants explained earlier and proposed that three gene functions, A, B, and C, defined by these mutant classes, would be responsible for the specification of floral organ identity. A, B, and C functions should be expressed in two adjacent whorls, overlapping with each other. Thus, A-function alone would specify sepals in the outer whorl, A + B would define petals in whorl 2, B + C stamens in whorl 3, and C alone carpels in the center of the flower. Mutual repression of A and C functions also had to be included in the model to explain a and c phenotypes, as well as an additional role of C-function in preventing indeterminate floral meristem growth (Figure 9.1a). In addition to the elegance of this simple yet powerful model, an added value was that it was inferred from the phenotypes of equivalent mutants in two relatively distant species of quite different floral morphology, and therefore strongly indicated that it could represent a general mechanism for flower development in angiosperms.
Concurrent with the proposal of the model, the identification of genetic functions to which ABC roles could be assigned strongly supported the model and provided a wealth of molecular data (Bradley et al. 1993; Goto & Meyerowitz 1994; Jack et al. 1992; Jofuku et al. 1994; Mandel et al. 1992; Sommer et al. 1990; Tröbner et al. 1992). All the identified ABC genes, except the Arabidopsis class A APETALA2 gene, were closely related in sequence, and belonged to the MADS-box family of transcription factors. Moreover, MADS-box proteins were shown to interact in protein complexes, providing a molecular clue to the mechanisms that explained the combinatorial nature of the model (Davies et al. 1996b; Egea-Cortines et al. 1999). In addition, genes with equivalent functions in different species showed interspecific homology, reinforcing the idea of the general applicability of the model throughout the angiosperms.
Still, the original ABC model did not provide answers for some essential questions. For example, ectopic expression of ABC genes was shown to transform one type of floral organ into another, as predicted by the model, but was not sufficient to transform leaves into floral organs (Mizukami & Ma 1992; Davies et al. 1996a; Krizek & Meyerowitz 1996). These results were in conflict with an old but attractive theory of the German philosopher and writer Goethe, who proposed that all lateral organs shared a leaf-like developmental plan (Goethe 1790). This necessitated an extension of the basic model with an additional E-function, specific to the floral context, which was proposed and eventually assigned to a subclade of MADS-box genes, the SEPALLATA (SEP) subfamily, expressed in all floral whorls (Figure 9.1a).
Sepallata mutants showed flowers where only leaf-like organs developed in a whorled but indeterminate pattern, strongly supporting the idea of a common ground plan for leaves and floral organs (Pelaz et al. 2000; Ditta et al. 2004). E-function (SEP) proteins were subsequently shown to act as molecular “glue” in high order MADS-box complexes (Immink et al. 2009a). Thus, the ABC model was reformulated into what we know today as the ABCE or quartet model: whorls 1, 2, 3, and 4 contain, respectively, tetramers of A-A-E-E, A-B-B-E, B-B-C-E, or C-C-E-E MADS-box proteins, which bind to DNA of target genes to direct floral organ specific development (Figure 9.1a). Several excellent reviews have addressed the molecular details and the historical development of the quartet model (Causier et al. 2010; Immink et al. 2009b; Krizek & Fletcher 2005; Theissen & Melzer 2007).
The regulation of temporal and spatial expression of ABCE functions was shown to be crucial for establishing floral organ identity. The genetic pathways from floral meristem specification to the establishment of ABCE functions have been studied and it was shown how they were based on feed-forward loops and a final feedback loop that reinforced and conferred robustness to the floral program. In Arabidopsis, LEAFY (LFY), a plant-specific transcription factor with a major role in floral meristem specification, is also a key player in the upregulation of ABCE genes (Weigel et al. 1992). LFY is uniformly expressed in the young floral meristem, but achieves temporal and spatial regulation of floral organ identity genes through the action of different cofactors (Parcy et al. 1998). LFY appears to be sufficient to activate APETALA1 (AP1), a MADS-box gene with a dual role in floral meristem specification and A-function.
Once activated by LFY, AP1 upregulates SEP3. SEP3 then forms a protein complex with AP1, switching its activity from floral specification to A-function. Subsequently, SEP3 is also able to act as a LFY cofactor to upregulate the other SEP genes, and the B- and C-function genes, although SEP3 is not as important for LFY activity as the other LFY cofactors (reviewed in Liu et al. 2009). LFY directly interacts with UNUSUAL FLORAL ORGANS (UFO), an F-box protein, to activate the B-class gene APETALA3 (AP3) in the UFO domain of expression, which coincides with the B-class expression domain (Lee et al. 1997; Chae et al. 2008). Activation of the C-class gene AGAMOUS (AG) in the center of the floral meristem requires the concerted action of LFY and WUSCHEL (WUS), a homeodomain protein with a key role in stem cell maintenance of all aerial meristems. Finally, in a negative feedback loop, AG terminates floral meristem growth by repressing WUS expression in the center of the floral meristem (Lenhard et al. 2001; Lohmann et al. 2001).
9.2.1.2 The Evolution of Flower Developmental Pathways
Conceptually, the quartet model could be considered a paradigmatic example of an evolutionary framework. It was based on a modular structure, with combinatorial activities that resulted in different outcomes, and these activities were largely controlled by regulation of expression patterns. Moreover, since it was proposed from independent studies in two relatively distant species, conservation across species was strongly supported. These features of the model provided powerful mechanisms to explain evolutionary innovation, immediately suggesting predictions that could explain morphological novelties. Accordingly, from the early years when the model was initially proposed and MADS-box genes were identified as the main players involved, intensive research comprising an ever growing diversity of species has shown how the model shows both conservation and divergence and has provided a body of evidence to study the evolution of organ identity. Still, we remain limited by the comparatively small number of comprehensively studied species, especially in evolutionary nodes where innovations appeared and functional studies are challenging.
The elusive A-function. From molecular phylogenies and comparative development studies, it is clear now that the best-supported part of the model refers to the specification of stamens and carpels by B and C functions. The A-function, however, remains controversial. Arabidopsis is the only species so far where clear loss-of-function A-class mutants have been described. Arabidopsis ap1 mutants show a partial loss of floral meristem identity and also some loss of A-function, with sepals transformed into bract-like organs and petals that do not form (Bowman et al. 1993). In the other A-class mutant, ap2, sepals are transformed into carpels and petals into stamens (Bowman et al. 1989). The other few mutants in AP1-like genes characterized in different species (for example, squamosa in Antirrhinum, proliferating inflorescence meristems in legumes or macrocalyx in tomato; Huijser et al. 1992; Taylor et al. 2002; Vrebalov et al. 2002) do not show these dual defects, being mainly affected in the floral meristem specification function. Likewise, mutants in AP2 orthologs like lip1 and lip2 show only mild defects in sepal and petal development but not misregulation of C-class genes.
For these reasons, many authors have favored a variant of the ABCE model, an earlier version known as the BC model (Litt 2007; Schwarz-Sommer et al. 1990), or its more recent reformulation, the (A)BC model (Causier et al. 2010). This alternative to the quartet model proposes that the (A) function would comprise both AP1-like and SEP genes and would not have a major role in perianth organ specification. (A)-class main roles then would be to specify the floral meristem, establishing a floral context where the default identity for floral organs would be sepals, and subsequently to regulate the expression pattern of B- and C-class genes. Once established the floral program, only B and C functions would be required for the specification of floral organ identity—carpels (C), stamens (B + C), and petals (B), with sepals being defined by the absence of B and C activities (Figure 9.1b).
The debate on quartet versus (A)BC model is hampered by the scarcity of mutants in different species, which makes it difficult to distinguish the general from the particular. But even after revisiting the original A-function, postulated as required for sepal and petal identity and to restrict C-function expression to the inner whorls, the quartet model in which A-function would require AP1-like genes still deserves some credit. The evolution of a dual perianth composed of sepals and petals appears to be fixed only in core eudicots, coincidental in evolutionary time with a whole genome duplication that originated the euAP1 clade (Litt & Irish 2003; see Section “Duplication and diversification of large gene families”). Moreover, while the in vivo formation of MADS tetramers has not been definitively proven yet, indirect evidence obtained in vitro or in yeast strongly supports the quartet ensemble, being one of these AP1/SEP/AP3/PI (Honma & Goto 2001; Ferrario et al. 2003). Also, the simultaneous ectopic expression of AP1 and the B-class genes PI and AP3 has been shown to convert cauline leaves into petals, without further development of floral meristems (Pelaz et al. 2001), and, for instance, the ap1-like mutant in Medicago truncatula (mtpim) shows conversions of sepals into leaf-like organs, casting some shadows on the idea that sepals could be the “default” floral organ state (Benlloch et al. 2006).
It is thus likely that AP1-like genes are part of the floral organ identity toolkit, while not so much in charge of the spatial repression of the C-function. This role in Arabidopsis would be mainly fulfilled by AP2, in turn repressed in the center of the flower by the microRNA miR172 or other cadastral regulators. In other species, AP2-like genes have not been assigned equivalent functions. Notably, however, a similar cadastral function is performed in Petunia hybrida or A. majus by a parallel module composed of NF-YA transcription factors regulated by a different microRNA, miR169 (Cartolano et al. 2007). The parallel AP2/miR172 and NF-YA/miR169 pathway constitutes an example of variation in molecular networks, which is not translated into structural differences in the flowers. It could also be a consequence of parallel redundant networks, which differ in relative importance in different species. This is suggested by the weak ap2-like phenotype of loss-of-function mutants in LIP1 and LIP2, two AP2-related genes from Antirrhinum (Keck et al. 2003), or the common role in C-function repression performed by similar transcriptional cofactors in Arabidopsis (LEUNIG) and Antirrhinum (STYLOSA) (Conner & Liu 2000; Navarro et al. 2004)
The B function works with borders: from fading to sliding. The debate affecting the specific role of putative A-function genes in floral organ specification does not touch the widely accepted universality of B and C-function roles in specifying petals, stamens, and carpels. B-class and C-class genes have been identified from a huge number of species and corresponding mutants have been characterized for several of them, which in general exhibit the expected homeotic defects predicted by the quartet model (reviewed in Irish & Sussex 1992; Krizek & Fletcher 2005; Kater et al. 2006).
Most common differences in floral architecture affect perianth organ identity. While core eudicots appear to have fixed the whorled arrangement of sepals–petals–stamens–carpels, considerably more variation occurs in basal angiosperms and monocots. Basal angiosperms generally show a perianth where no clear distinction can be made between sepals and petals. Perianth organs are often arranged in spiral phyllotaxis and show variable merosity. In some species, all perianth organs are petaloid, but in others, outer tepals are morphologically different from inner tepals, and intergrading organs of intermediate morphology are observed. Also, in some basal angiosperms like Asimina species, clearly different outer sepals and inner petals develop.
In all basal angiosperms, homologs to ABCE genes are found and, in general, expression patterns are consistent with predictions of floral organ identity by the quartet model. AG-like gene expression is found only in reproductive organs, likely reflecting this restricted expression pattern as ancestral. However, MADS-box genes related to the B-class genes AP3 or PI are expressed in broader domains, encompassing the whole of the perianth and frequently also the carpels in many basal angiosperms (Kim et al. 2005). The presence of B-class genes in the perianth is consistent with a modified version of the (A)BC model where broad B-gene expression explains the petaloid identity of these organs in most basal angiosperms. Also, in Asimina species, B-class gene expression is restricted to the petal domain, suggesting that a reduction in the ancestral broad domain of B-class expression could have originated the Asimina dual perianth (Kim et al. 2005). To explain gradual transitions in floral organ identity found in many basal angiosperms, the “fading borders” hypothesis has been proposed. This model suggests that gradients of expression of organ identity genes, and especially of B-genes, could generate domains with different relative proportions of overlapping activities that led to organs of intermediate morphology (Figure 9.1c). Precise information of expression patterns of B-genes in different basal angiosperm species is limited, but studies in Amborella or Persea support this scenario (Soltis et al. 2007a, 2007b; Theissen & Melzer 2007).
Many nongrass monocot species, such as lilies or tulips, have whorled tepaloid perianths. In general, although with a few exceptions, these species express B-class genes related to AP3 and PI in whorls 1, 2, and 3 (Kanno et al. 2003), supporting the prediction of van Tunen et al. (1993) of a “sliding border” for the B-class genes, expanding to the whorl 1 and therefore conferring petaloid identity to these organs (Figure 9.1d).
In fact, from these and other studies in basal angiosperms and monocots, the petaloid character of the perianth together with a broad and may be undefined expression pattern of B-class genes could be inferred as ancestral. Dual perianth organ identity would have evolved independently many times in different clades of angiosperms, possibly through the refinement of B-class gene expression domains. From the perspective of the quartet model, authors like Theissen and Melzer (2007) have suggested that the establishment of positive autoregulatory loops through changes in promoters or increasingly restricted protein complex formation could have contributed to define the borders of B- and C-function activities.
B and C genes are already present in gymnosperms, so they predate the origin of angiosperm flower, suggesting an ancestral role already in the specification of male and female reproductive structures. Interestingly, B-type proteins from gymnosperms tested so far have been shown to form only homodimers (Wang et al. 2010). In contrast, in basal angiosperms and monocots, homodimerization, facultative, and obligate heterodimerization of B-class proteins have been shown to occur (Su et al. 2008). Finally, in core eudicots, B-proteins form obligate heterodimers, and only these heterodimers are able to provide B-function, both maintaining its own expression and acting on other downstream targets (reviewed in Theissen & Melzer 2007; Immink et al. 2009a). This trend toward obligate heterodimerization possibly coupled to autoregulation, could have thus provided a mechanism for restricting and sharpening the borders of B-activity domain to specify defined whorls or petals and stamens.
Variations in the quartet model and the evolution of novel morphologies. Novel floral morphologies not only necessarily included in these evolutionary trends but also caused by changes in floral organ identity can be interpreted using the ABCE model. For example, the eudicot Rumex acetosa, has a two whorled perianth composed only of sepaloid organs. Accordingly, only the stamens express B-class genes, suggesting that an inward shift of their expression has originated this new morphology (Figure 9.1e; Ainsworth et al. 2005).
Similarly, shifts in B-class gene expression appear to underlie the highly unusual floral morphology of the monocot Lacandonia schismatica. In Lacandonia, small flowers form with a tepaloid perianth of sepaloid morphology and an inverted arrangement of the reproductive organs, where numerous carpels surround a central whorl of stamens. Studies on B- and C-function genes in this species have shown how the B-class gene LsPI is expressed broadly in young floral meristems to restrict later to the domain that will give rise to stamens and carpels, overlapping with the C-class gene LsAG. In contrast, the expression of the other B-class gene, LsAP3, is restricted to the central zone of the meristems where stamens will develop. Thus, simultaneous expression of LsAP3, LsPI, and LsAG is only achieved in the central whorl of stamens, while in the surrounding carpels only LsPI and LsAG are found, and all of them are absent in the tepaloid organs (Figure 9.1f; Alvarez-Buylla et al. 2010).
An additional example of morphological innovation that can be explained by modifications in the quartet model is provided by species of the basal eudicot genus Aquilegia. Most Aquilegia species have a perianth composed of two morphologically different types of petaloid organs and an additional whorl of a novel type of organ, the staminodium, placed between stamens and carpels. Aquilegia has one PI-like gene but three AP3-like genes. Detailed studies of B-class gene function have shown how the three AP3-like genes are expressed in different spatial and temporal patterns, including petals, stamens, and staminodia, and that each organ type expresses a specific combination of B-type genes. Moreover, downregulation of AqvPI caused transformation of both stamens and staminodia into carpels, indicating that staminodia are probably derived from stamens, and suggesting that gene duplication and subsequent subfunctionalization of AP3 genes could have originated this novel organ identity program (Kramer et al. 2007).
As we have seen, variations in the ABC architecture are able to explain floral morphological diversity. However, the same ABC system has also been shown to specify very different looking organs. Grass flowers differ significantly from their eudicot relatives in the identity of the sterile perianth organs, which is composed of grass-specific organs known as glumes, lemma, palea, and lodicules. The glumes were considered as bracts, while homology of lemma, palea, and lodicules to other angiosperm floral organs has been the subject of debate. The lodicule is a small glandular-like organ, placed between the palea/lemma and the stamens. At anthesis, lodicules expand to separate the lemma and palea and thus expose the anthers to the wind. Functional studies of flowers of grasses, such as rice or maize, have shown how mutations in B-class genes lead to homeotic transformations of lodicules into palea-like organs and stamens into carpels, strongly supporting the homology of lodicules and petals (Ambrose et al. 2000; Nagasawa et al. 2003; Xiao et al. 2003; Whipple et al. 2004). The petaloid nature of lodicules represents a remarkable example of a novel type of organ derived from a preexisting identity program. The unique morphology of lodicules would then be more likely the result of a change in the set of downstream targets of B-type genes throughout the course of grass evolution (Whipple et al. 2007).
Duplication and diversification of large gene families. Gene duplication is a common phenomenon in plants which, when followed by neo- and/or subfunctionalization, can lead to large gene families and gene networks, enabling functional diversification and the subsequent evolution of morphological novelties. Given the central role of MADS-box genes in floral development and evolution, considerable efforts have been directed to identify a vast number of members of this family across the land plants and to reconstruct their phylogeny. These studies have revealed the highly complicated structure of the family where relationships of orthology and paralogy give rise to multiple cases of neo- and subfunctionalization, gene co-option, and even convergent evolution (Jaramillo & Kramer 2007). Still, general patterns on MADS phylogenies correlate diversification of the MADS family with major steps in floral evolution. These studies have shown how key MADS-box genes related to floral organ specification could have undergone simultaneous duplication, probably through polyploidization, just before or coincident with the origin of the angiosperms (Irish & Litt 2005; Zahn et al. 2005). Moreover, the patterns of duplication of ABCE genes (AP1, AP3/PI, AG, and SEP subfamilies) are related and basically coincident with the origin of major clades within angiosperms, therefore suggesting a role for these duplications in angiosperm diversification.
For instance, in gymnosperms only one copy of a B-class homolog is present, whereas at least two copies (related to AP3 and PI) are found in angiosperms (Kim et al. 2004). Then, two clades of AP3-like genes are found only in core eudicots (euAP3 and TM6) indicating a second event of duplication before the origin of this clade (Kramer et al. 1998). Likewise, angiosperms possess two lineages of C-like genes, one comprising AG-like genes related to C-function and another involved in ovule identity specification originated from a duplication predating angiosperm origin (Kramer et al. 2004). AG-like genes have also undergone more duplication events before and within the monocot and the dicot clades. These duplications have resulted in paralogs where changes mainly in expression patterns but also in protein function have given rise to functional diversification (Hasebe et al. 1998; Davies et al. 1999; Causier et al. 2005; Airoldi et al. 2010). Likewise, for E-function genes we found a similar scenario. Two lineages of SEP genes (SEP1/2/4 and SEP3) are thought to be present in the common ancestor of angiosperms while no clear homologs of SEP genes have been found in gymnosperms, suggesting that SEP genes were lost in the ancestor of extant gymnosperms (Zahn et al. 2005; Theissen & Melzer 2007). The essential role of E-class genes in the specification of all floral organs, as key components of the floral quartets, suggests that the origin and diversification of the SEP-clade might have played a key role in the origin of the angiosperm flower (Wang et al. 2010).
Notably, the clade where the putative A-function AP1-like genes belong (euAP1) appears at the base of the eudicots as the result of a duplication in the more ancient AP1/FUL lineage (Litt & Irish 2003). The origin of the euAP1 clade coincides with that of the euAP3 lineage (see Section “The elusive A-function”) and with an important morphological innovation—the fixation of the whorled structure of the eudicot flower, with a dual perianth of clearly distinct sepals and petals. Although as previously discussed, the role of AP1-like genes in the specification of perianth organs is still debateable, the temporal concurrence of euAP1/euAP3 clades, sepals, and petals suggests a role for these eudicot gene clades in this morphological innovation.
9.2.1.3 The Origin of the Flower
Although floral organ identity is well studied and the quartet model constitutes a powerful working framework, the evolutionary origin of the flower remains an open question. The fossil record is still far from comprehensive, and we have not yet identified fossils unequivocally representing stem-group angiosperms. Therefore, we lack a clear picture of how the ancestral parents of extant flowers looked like, what was the temporal sequence of the morphological novelties that appeared in the first flowers, and what were the molecular changes responsible for these innovations. In spite of these uncertainties, Goethe's theory of leaves and floral organs sharing a common ground plan is nowadays accepted and strongly supported by the sepallata mutant phenotypes (Goethe 1790; Pelaz et al. 2000; Ditta et al. 2004). Thus, flowers are viewed as “compressed” branches and floral reproductive organs (stamens and carpels) derive from leaves bearing reproductive structures (sporophylls) along the axis of these branches.
Three major evolutionary steps can be proposed to transform these ancestral reproductive branches into “modern” flowers. Since most reproductive branches in gymnosperms are unisexual, the first step could be the origin of bisexuality. In addition, reproductive branches in gymnosperms are usually indeterminate, so determinacy would have to be acquired. Finally, sterile perianth organs should have originated. Although there is no definitive data on the order or even the nature of these steps, this sequence appears to be a plausible scenario.
Several recent publications have discussed current theories on the origin of bisexuality based on paleobotanic data and the rapidly increasing amount of molecular data (Frohlich & Chase 2007; Specht & Bartlett 2009; Melzer et al. 2010). Briefly, three different hypotheses are currently debated, which basically differ with respect to the nature (male or female) of the ancestral branch of sporophylls.
The Mostly Male Theory (MMT; Frohlich & Parker 2000) proposes that flowers are derived from male strobili of a gymnosperm-like ancestor. In the male strobili of this ancestor, the male sporophylls near the apex would have first become bisexual by developing ectopic ovules, and subsequently, these bisexual sporophylls would have lost male sporangia and become functionally female. Additional evolutionary steps toward the flower would be the loss of the residual female strobili, the transformation of sporophylls in the male/female strobili by closure of the apical female sporophylls to form carpels and the basal male sporophylls into stamens (Figure 9.2).
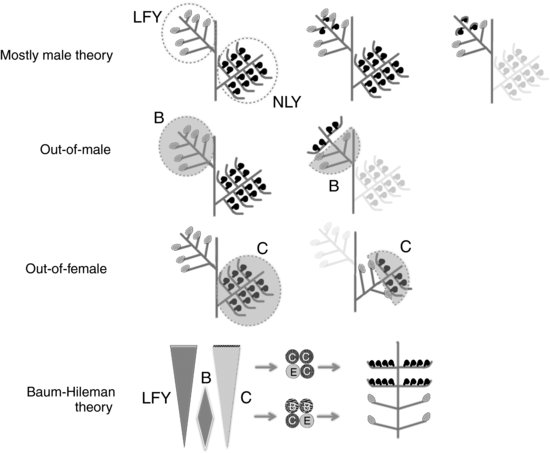
The MMT was proposed mainly on the basis of evolutionary studies on LEAFY gene function. LFY plays a central role in the specification of floral meristems in angiosperms and, subsequently, in the upregulation of floral organ identity genes. Although in angiosperm species only one LFY gene is found, in gymnosperms two paralogs are present, one LFY ortholog, and NEEDLY (NLY), for which no direct ortholog has been found in angiosperms. Initial expression studies in certain gymnosperms showed that each paralog was expressed predominantly in male (LFY) or female cones (NLY). Thus, the absence of NLY in angiosperms pointed to the female strobilus having been lost from the common ancestor to flowering plants and therefore to the male origin of the ancestral flower (Mouradov et al. 1998). Several independent observations supported this theory. For example, the Arabidopsis lfy mutant is able to produce carpels but no stamens, suggesting that LFY is only essential for male specification. In addition, it has been described that ectopic ovules form in different single mutants of several angiosperm species and even in the leaves of a gymnosperm like Ginkgo, suggesting that ectopic ovules are easily produced. However, recent conflicting data showing coexpression of LFY and NLY in both female and male cones from other gymnosperms (Carlsbecker et al. 2004; Vazquez-Lobo et al. 2007) have weakened this theory.
Two other sister hypotheses on flower origin, the out-of-male (OOM)/out-of-female (OOF) theories, both proposed by Theissen et al. (2002), are based on the observation that some extant conifers possess bisexual cones. These theories incorporate molecular data and the ABCE model of floral organ identity in an attempt to explain how bisexuality of the flower was originated by temporary or spatial shifts in patterns of expression of genes determining the male/female identity of reproductive organs, downstream of LFY/NLY (Figure 9.2). The most important difference of OOM/OOF and MMT is that the former do not involve extensive loss of female developmental programs and therefore, postulate that carpels have female origin instead of the ectopic formation of ovules on male organs proposed by MMT. The OOM hypothesis proposes that in an ancestral male strobilus, the expression of male-identity B-class MADS-box genes could have been downregulated in the distal part to leave female organs in these apical positions (Theissen et al. 2002) by an unknown signal of hormonal or other nature. Conversely, the OOF theory postulates that B-class genes could have been upregulated in the basal portion of a female cone, likewise producing a bisexual axis. Some recent studies on pollen versus seed cones in extant conifers show most spontaneous bisexual cones appear to be modified seed cones rather than modified pollen cones, thus favoring the out-of-female hypothesis (Rudall et al. 2011).
A further theory has been proposed by Baum and Hileman (2006; Baum and Hileman Theory (BHT)), which can be considered a variant of the OOM hypothesis, but with much more molecular detail. BHT incorporates increasing knowledge of molecular mechanisms of flower identity specification in extant species to formulate a highly speculative model that, in addition to the origin of bisexuality, goes further in an attempt to explain the acquisition of floral determinacy and even perianth origin. The BHT takes into account the central role of LFY in the upregulation of B- and C-function genes and the quartet model of floral organ identity. According to this hypothesis, LFY protein levels would increase with time in male reproductive branches, as has been observed in extant angiosperm inflorescences (Blázquez et al. 1997, 1998; Schmid et al. 2003). The BHT incorporates two more assumptions: that this tendency of temporal LFY accumulation would have been reinforced during evolution, and also that C-function proteins should be able to accumulate at much higher quantitative levels than B-function proteins.
Increasing levels of LFY together with this higher capacity of C-function accumulation would create a distal maxima of C-function. This apical excess of C-function would then sequester all available SEP proteins, preventing the formation of B-SEP complexes and thus resulting in the development of megasporophylls at the apex of the strobilus (Figure 9.2). The BHT also suggests that a change in WUSCHEL cis-regulatory sequences during evolution could have occasioned WUS repression by the apical excess of C-function, resulting in floral determinacy. This hypothesis also speculates further on the origin of the sterile perianth, involving a newly acquired essential role of WUS as a LFY cofactor to spatially define the activation of C-function genes. The final requirement of WUS for C-function activation, together with the central spatial pattern of WUS in the meristem, would be its exclusion from the outer/basal region of the ancestral flower. This would result in the loss of stamen identity in these regions and the development of sterile organs of petaloid appearance and therefore implying the andropetaloid origin of these organs. An attractive aspect of the BHT is that it makes a huge number of predictions that potentially could be experimentally tested. However, as already discussed, functional studies in representative species of most interesting clades, including gymnosperms and basal angiosperms, are currently out of reach, and we will have to wait before more conclusive evidence is gathered.
9.2.2 The Evolution and Development of Leaves
9.2.2.1 What is a Leaf?
Leaves are one of the most conspicuous features of land plants and arise as lateral determinate organs from an indeterminate apical meristem. The leaves of most vascular plants are all lateral organs that are vascularized and have determinate growth (Gifford & Foster 1989), with a broad lamina with adaxial–abaxial asymmetry, which is well suited for photosynthesis and respiration. Although leaves can be easily recognized by their position and structure, the leaves that are found throughout the land plants are not considered homologous (Bower 1935).
9.2.2.2 Leaf Evolution and Homology Across the Land Plants
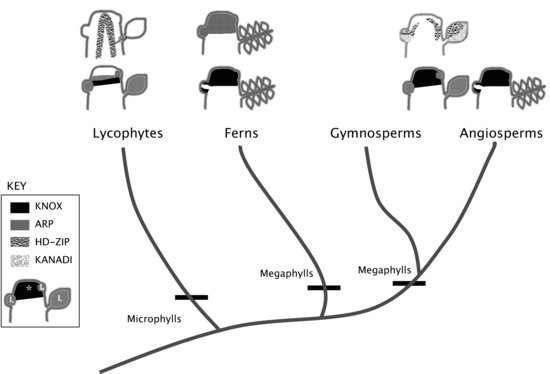
The evolution and homology of leaves has been a longstanding question among botanists. Leaves in the bryophytes are found only in the gametophyte generation and are not considered homologous to the leaves found in any of the vascular plants (Domin 1931). Vascular plant leaves are found only in the sporophyte generation and are classified as either microphylls or megaphylls, the former found in lycophytes and the latter in the euphyllophytes (Figure 9.3). Microphylls are defined by their generally small size, single unbranched vascular strand, and lack of a leaf gap. Megaphylls are generally large, have complex venation, and are associated with a leaf gap. However, there are exceptions to these definitions, some lycophyte microphylls have branched vasculature and some fossil lycophyte microphylls were large (Gifford & Foster 1989). In spermatophytes, exceptions to the megaphyll definition also exist, such as the needles found in pines. Microphylls and megaphylls are not considered homologous, and megaphylls are proposed to have arisen anywhere from two to seven times independently in the euphyllophytes. There are several hypotheses regarding the evolution of megaphylls, the most popular being the telome theory, which proposes that megaphylls evolved through a transformation of branches (Wilson 1953; Kenrick & Crane 1997).
Paleobotanical studies have been important in constructing hypotheses of leaf evolution in vascular plants. Fossils of vascular plants from the Silurian and Devonian have highly branched structures that lack laminate leaves and Paleozoic fossils indicate that laminate leaves evolved independently in the euphyllophyte clades (Gifford & Foster 1989). Although there is a wide range of vascular plant leaf morphology, these morphologies exist in all euphyllophyte clades indicating a high level of convergence in leaf development (Boyce & Knoll 2002). Analyses of fossil leaf morphology and vasculature show that spermatophytes and monilophytes underwent similar leaf developmental trajectories. Boyce and Knoll (2002) suggest that this indicates modifications of a common developmental network and that this shared developmental network constrained the early evolutionary direction of leaf development and the number of leaf morphologies that could be attained. Comparative development of angiosperm leaves provides some support for these hypotheses, however, molecular genetic support across the euphyllophytes is still lacking.
9.2.2.3 Genes and Pathways Necessary for Simple Leaf Development in Angiosperms
Molecular genetic studies in angiosperms have elucidated a large part of the regulatory network necessary for the development of a simple angiosperm leaf. The development of lateral organs involves the specification of organ position on the flanks of the meristem, establishment of boundaries between the determinate organ and indeterminate meristem, and specification of the cell types of the lateral organ. This would appear to be a logical sequence of events but there is in fact a lot of feedback between the different processes composing the leaf regulatory network. In many angiosperms, the KNOX-ARP (Class I KNOTTED1-like HOMEOBOX (KNOX) and ASYMMETRIC LEAVES1/ROUGH SHEATH2/PHANTASTICA (ARP MYB domain proteins) regulatory module is important for setting the domains between an indeterminate shoot apex and a determinate lateral organ (Figure 9.3; Vollbrecht et al. 1991; Lincoln et al. 1994; Long et al. 1996; Waites et al. 1998; Timmermans et al. 1999; Tsiantis et al. 1999; Byrne et al. 2000; Reiser et al. 2000; Semiarti et al. 2001). And in many angiosperms, the interaction between Class III homeodomain leucine zipper (HD-ZIPs), KANADI (KAN), and YABBY (YAB) proteins are important for vascular development, meristem identity, and adaxial–abaxial asymmetry in lateral organs (Baima et al. 1995; McConnell & Barton 1998; Siegfried et al. 1999; Eshed et al. 2001; McConnell et al. 2001; Emery et al. 2003; Eshed et al. 2004; Prigge et al. 2005; Byrne 2006). Simple leaf development studies have focused on the KNOX/ARP and HD-ZIP/KAN/YAB modules. We will review the developmental network involving these two genetic modules mainly from work done in the angiosperm Arabidopsis thaliana unless otherwise specified.
Lateral determinate organs are produced on the flanks of the indeterminate meristem, and determinate primordia initiate at sites of auxin maxima (Reinhardt et al. 2000; Benkova et al. 2003; Reinhardt et al. 2003). Auxin influx and efflux carriers (e.g., PIN-FORMED1 (PIN1)) direct auxin flow in the shoot (Reinhardt et al. 2000; Reinhardt et al. 2003; Bainbridge et al. 2008), and plants unable to synthesize auxin or those treated with auxin transport inhibitors have defects in lateral organ formation (Okada et al. 1991). In addition, pin1 mutants, that are unable to create auxin maxima, have defects in lateral organ formation (Okada et al. 1991; Galweiler et al. 1998).
PIN1 and KNOX homeodomain transcription factors are expressed in a complementary pattern in the shoot (Heisler et al. 2005). Generally, KNOX proteins are expressed in the vegetative and floral meristems and downregulated in leaf primordia and floral organs (Vollbrecht et al. 1991; Kerstetter et al. 1994; Long et al. 1996; Reiser et al. 2000). The Class I KNOX mutant shootmeristemless (stm) germinates with no apical meristem and although adventitious branches may form, the meristems of these branches soon terminates as well (Barton & Poethig 1993; Long et al. 1996). Class I KNOX proteins are required for the maintenance of indeterminacy and prevention of cell differentiation in the shoot apical meristem. ARP and KNOX are also expressed in complementary patterns in the shoot. The ARP protein, ASYMMETRIC LEAVES1 (AS1) is expressed in leaf primordia and developing leaves (Figure 9.3; Byrne et al. 2000, 2002). A loss of ARP function results in KNOX expression in the leaves and the resulting plant resembles a KNOX overexpression phenotype. The ARP protein AS1 forms a heterodimer with the LOB domain protein, AS2, which binds to the cis-regulatory region of Class I KNOX genes and represses KNOX transcription (Guo et al. 2008). In addition, Class I KNOX negatively regulate AS1 (Byrne et al. 2000).
The complementary expression domains and antagonism of ARP and KNOX proteins have been proposed as an important developmental module to specify determinate leaves from the flanks of the indeterminate SAM. In addition, auxin and the ARP protein, AS1, have been shown to act together to repress KNOX expression in the leaves (Hay et al. 2006). The auxin defective and as1 double mutants produce leaves with more indeterminate features than either single mutant and KNOX genes are ectopically expressed in these indeterminate regions of the double mutant.
The boundary between the indeterminate apical meristem and determinate lateral organs is finely tuned by three NAC-domain transcription factors, CUP-SHAPED COTYLEDON1–3 (CUC1, CUC2, CUC3) (Aida et al. 1997; Takada et al. 2001; Vroemen et al. 2003). CUCs are expressed in the embryonic shoot apical meristem and in the boundary region between the apical meristem and lateral determinate organs (Aida et al. 1999; Ishida et al. 2000; Takada et al. 2001; Vroemen et al. 2003; Hibara et al. 2006). Plants with mutations in any two of the three Arabidopsis CUC genes have fused lateral organs while plants overexpressing CUC1 have meristems forming on cotyledons (Aida et al. 1997; Takada et al. 2001; Vroemen et al. 2003; Hibara et al. 2006). The 35S:CUC1 plants have ectopic STM expression in the cotyledons while a loss of CUC function results in the loss of STM expression and therefore a loss of meristem formation during embryogenesis (Aida et al. 1999; Takada et al. 2001). In addition, a loss of STM results in an expansion of CUC gene expression and a plant with no apical meristem and fused cotyledons (Aida et al. 1999). Therefore, CUC genes promote KNOX expression during embryogenesis and KNOX expression represses CUC expression in the meristem. CUCs have redundant roles in SAM formation during embryogenesis and lateral organ boundary formation and maintenance throughout the plant lifecycle (Aida et al. 1997, 1999; Ishida et al. 2000; Takada et al. 2001; Vroemen et al. 2003; Hibara et al. 2006). Given the relationship between KNOX and auxin and KNOX and CUC, it is not surprising that there is an interaction between CUCs and auxin. Plants mutant for the auxin efflux protein, pin1, have expanded CUC2 expression suggesting that auxin represses CUC expression to the boundary of the initiating primordia (Vernoux et al. 2000).
After leaf initiation, the angiosperm leaf soon acquires polarity, which is important for proper cell type specification in this lateral organ. The Class III HD-ZIP, KAN (GARP-type transcription factors), and YABBY proteins are important for meristem identity and adaxial–abaxial asymmetry in lateral organs (Figure 9.3; Siegfried et al. 1999; Eshed et al. 2001; Kerstetter et al. 2001; McConnell et al. 2001; Emery et al. 2003; Prigge et al. 2005). HD-ZIPs are expressed in the SAM, vasculature, and adaxial region of lateral organs and are necessary for the initiation of the SAM and axillary meristems, specification of adaxial identity in lateral determinate organs such as leaves and floral organs, and determination of vascular tissue patterning (Baima et al. 1995; McConnell & Barton 1998; McConnell et al. 2001; Emery et al. 2003; Prigge et al. 2005; Byrne 2006). KANs are expressed in the abaxial region of lateral organs and are necessary to specify abaxial identity and vascular patterning (Eshed et al. 2001; Kerstetter et al. 2001; Emery et al. 2003; Eshed et al. 2004). YABBYs are expressed in the abaxial region of lateral organs and are necessary for abaxial identity in lateral organs (Sawa et al. 1999; Siegfried et al. 1999; Sarojam et al. 2010).
A loss of HD-ZIP function results in peg-shaped cotyledons with abaxial epidermal features while a loss of KAN or YABBY proteins results in radial lateral organs that are adaxialized (Siegfried et al. 1999; Eshed et al. 2001; Emery et al. 2003; Eshed et al. 2004; Sarojam et al. 2010). KANs are ectopically expressed in the HD-ZIP loss of function mutants and HD-ZIPs are ectopically expressed in the KAN loss of function mutants (Eshed et al. 2001). HD-ZIP proteins and KAN proteins are mutually antagonistic (Eshed et al. 2001; Kerstetter et al. 2001; Reinhart et al. 2002; Rhoades et al. 2002; Emery et al. 2003). Mutations that result in abaxialized lateral organs also have meristems that cease to function early in development (Siegfried et al. 1999; Eshed et al. 2001; Emery et al. 2003). Micro RNAs 165/166 are important for repressing HD-ZIP activity and restricting its action to the adaxial side of the leaf (McConnell et al. 2001; Rhoades et al. 2002; Emery et al. 2003). In model angiosperm species, the juxtaposition of abaxial and adaxial identity in leaves has been proposed to be necessary for laminar outgrowth (Waites & Hudson 1995; McConnell et al. 2001; Rhoades et al. 2002; Emery et al. 2003; Eshed et al. 2004).
Molecular genetic studies have been important to further elucidate the developmental network necessary to specify the leaves. Genes involved in auxin signaling along with KANs promote abaxial identity (Pekker et al. 2005). Proteins that are important for leaf determinacy also play a role in the axial patterning of the leaves. The ARP protein AS1 along with the LOB domain protein AS2 promotes adaxial identity by positively affecting HD-ZIP expression (Lin et al. 2003, 2005). In addition, AS1 and AS2 repress the activity of YABBY genes in the adaxial region (Iwakawa et al. 2007).
9.2.2.4 Genes and Pathways Necessary for Compound Leaf Development in Angiosperms
Comparative molecular genetic studies have shown that the leaf regulatory network that specifies the leaf primordia from the shoot apical meristem is similar to the regulatory network that species leaflets from the rachis of a compound or dissected leaf (Figure 9.4). Differences in KNOX and ARP expression patterns and regulation have provided a molecular genetic explanation for the morphological differences between simple versus compound leaves in some angiosperm species (Kessler & Sinha 2004; Hay and Tsiantis 2006; Kellogg 2006; Kimura et al. 2008). In simple-leaved angiosperms, KNOX proteins are expressed in the SAM, downregulated in incipient leaf primordia, and not expressed in mature leaves (Vollbrecht et al. 1991; Lincoln et al. 1994; Nishimura et al. 1999).
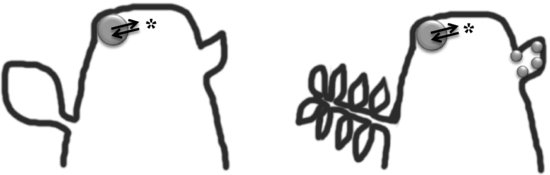
However, in angiosperms with compound leaves (e.g., tomato), KNOX proteins are expressed in the incipient leaf primordia but not in mature leaves (Janssen et al. 1998). In tomato, overexpression of KNOX produces young leaves that are coiled like a fern fiddlehead (circinate vernation) and mature leaves that are highly dissected (Hareven et al. 1996; Chen et al. 1997; Janssen et al. 1998). This coiled morphology in tomato KNOX overexpression plants is hypothesized to be due to faster cell growth on the abaxial compared to the adaxial side of the leaf. In addition, the natural variation in the level of leaf dissection found in various tomato cultivars is due to differences in KNOX expression and its interactions (Kimura et al. 2008).
Developmental genetic studies in Cardamine hirsuta have nicely illustrated how changes in the leaf developmental network result in changes in leaf morphology (Hay & Tsiantis 2006). C. hirsuta is closely related to Arabidopsis but has compound leaves. Similar to what is found in other compound leaved angiosperm species, C. hirsuta KNOX expression is reactivated in the leaflet primordia (Bharathan et al. 2002; Hay & Tsiantis 2006). In addition, an increase in KNOX expression in C. hirsuta results in more complex leaves while a reduction in KNOX expression gives simple leaves (Hay & Tsiantis 2006). Hay and Tsiantis (2006) also found that the difference in C. hirsuta KNOX expression in leaves compared to Arabidopsis KNOX expression are due to changes in the cis-regulatory region of C. hirsuta KNOX.
Studies on the regulation of these leaf developmental networks are important for understanding how changes in this regulatory network can result in different leaf morphologies. Analyses of angiosperm KNOX sequences have shown that a conserved noncoding sequence in the 5' regulatory region termed the K-box is found in angiosperms with simple and complex leaves (Uchida et al. 2007). Deletion of the K-box in simple leaved species results in an as1 (ARP) mutant phenotype. These results show that the K-box is necessary for the persistent repression of KNOX in leaves.
Although KNOX gene expression is reactivated in the leaflet primordia of many compound leaved angiosperms, the compound leaved Pisum sativum (pea) is an exception (Hofer et al. 1997; Bharathan et al. 2002). The pea leaf mutant unifoliata has a simple leaf and the mutation mapped to an ortholog of the meristem identity gene LEAFY (LFY) (Hofer et al. 1997). This likely represents a co-option of a protein necessary for flower development to leaf development in pea.
Not only are Class I KNOX proteins reactivated in the leaflet primordia of most compound leaved angiosperms, but research so far indicates that the entire leaf developmental network is redeployed for leaflet formation in compound leaved species (Figure 9.4). For example, auxin maxima are detected at the sites of leaflet primordia initiation of several compound-leaved angiosperm species (Barkoulas et al. 2008; DeMason & Polowick 2009; Koenig et al. 2009). In addition, in C. hirsuta the accumulation of auxin coincides with a reduction in Class I KNOX expression in developing leaflet primordia similar to what is found in developing leaf primordia on the flanks of the SAM (Barkoulas et al. 2008). The NAC transcription factors also play a role in defining the boundary between the leaflets and rachis of the compound leaf similar to their role in defining the boundary between the leaf primordia and SAM (Blein et al. 2008, 2009). Surprisingly, pea LFY interacts in the developmental network with auxin and NAC transcription factors to form compound leaves similar to the role played by KNOX with auxin and NAC transcription factors in forming compound leaves in other angiosperms (Blein et al. 2008, 2010). The developmental genetic research on compound leaves illustrates how the simple leaf developmental network has been redeployed to specify leaflets in compound leaved species.
9.2.2.5 Leaf Genes and Pathways Across the Land Plants
The identification of the leaf regulatory networks throughout the land plants will be important for understanding when these networks evolved and to elucidate the evolution of land plant leaves. KNOX homologs have been isolated throughout the land plants (Bharathan et al. 2002; Harrison et al. 2005). Functional analyses of KNOX genes in Physcomitrella patens indicate that KNOX proteins are essential for proper development of the diploid sporophytes but not for the development of the leafy gametophyte (Sakakibara et al. 2008). These results support the idea that the leaves of bryophytes are not homologous to the leaves of vascular plants (Domin 1931). HD-ZIP and KAN transcription factors have been identified in bryophytes (P. patens), lycophytes (Selaginella spp.), and seed plants, and are likely to be found in ferns (Sakakibara et al. 2001; Floyd et al. 2006; Prigge & Clark 2006; Floyd & Bowman 2007). YABBY transcription factors have only been identified thus far in seed plants and it has been suggested that the evolution of the YABBY protein family was important for the evolution of the seed plant leaf (Floyd & Bowman 2007; Sarojam et al. 2010). KNOX expression patterns have been assessed in three fern species with compound leaves (Ceratopteris richardii, Osmunda regalis, and Anogramma chaeophylla) and found to be expressed in the apical meristem, leaf primordia, and leaf margins (Figure 9.3; Bharathan et al. 2002; Harrison et al. 2005; Sano et al. 2005). Unlike angiosperms, it appears that KNOX genes are not downregulated in incipient leaf primordia in ferns. ARP expression has only been studied in O. regalis and found in the apical meristem and leaf primordia (Harrison et al. 2005).
9.2.2.6 Leaf Genes and Leaf Homology
Studies of KNOX, ARP, and HD-ZIP expression patterns have been used to provide evidence for various hypotheses on microphyll and megaphyll origins and megaphyll homology (Harrison et al. 2005; Floyd & Bowman 2006). The leaves of vascular plants are all lateral organs that are vascularized, have determinate growth, and have adaxial–abaxial polarity (Gifford & Foster 1989). The KNOX-ARP and HD-ZIP-KAN interactions are important for the specification of these defining leaf characteristics. KNOX proteins are expressed in the apical region in the lycophyte Selaginella kraussiana but not in its leaf primordia (Figure 9.3; Harrison et al. 2005). ARP homologs were also isolated from S. kraussiana and are expressed in the apical region, leaf primordia, and developing leaves.
The expression of KNOX and ARP genes in the lycophyte S. kraussiana has been interpreted as support for a common genetic mechanism specifying leaf determinacy in microphylls and megaphylls (Harrison et al. 2005). Although, it has been argued that the KNOX-ARP interaction is important for the determinate growth of leaves, others have suggested that KNOX genes are not important for the determinacy of a leaf but whether a leaf is simple or compound (Harrison et al. 2005; Floyd & Bowman 2006). A thorough investigation of KNOX and ARP expression patterns in ferns and gymnosperms with simple and compound leaves will provide further data to compare to lycophyte and seed plant expression patterns.
The HD-ZIPs and KANADIs are important for leaf development, vascular patterning, and adaxial–abaxial polarity in leaves of seed plants (Floyd & Bowman 2007). HD-ZIP homologs have been isolated from across the streptophytes, and phylogenetic analyses indicate that one HD-ZIP protein was present in the common ancestor of all embryophytes (Floyd & Bowman 2004; Floyd et al. 2006; Prigge & Clark 2006). Two Class III HD-ZIP proteins each have been isolated from the lycophytes Selaginella moellendorffii, S. kraussiana, and C. richardii, and three from Psilotum nudum (Floyd & Bowman 2004, 2006, 2007; Floyd et al. 2006; Prigge & Clark 2006). In situ hybridization in S. kraussiana showed that one HD-ZIP gene is strongly expressed in the apical cells and the provascular tissue, whereas the other HD-ZIP gene is weakly expressed in the apical cells, adaxial region of the developing microphyll where the ligule will form, and the provascular tissue (Figure 9.3; Floyd & Bowman 2006). This suggests that HD-ZIP proteins have a role in apical meristem function and vascular development. A comparison of HD-ZIP expression patterns in seed plants and the lycophyte S. kraussiana has been interpreted as support for the independent origin of megaphylls and microphylls (Floyd & Bowman 2006). HD-ZIP and KAN expression patterns have not been assessed in any ferns but are integral to our understanding of megaphyll evolution and development. These comparative studies are crucial to address questions of homology and may illustrate how regulatory networks underlying development constrain the evolution of form.
9.3 Future Directions
9.3.1 Morphological Features
We have learned a lot about the developmental pathways necessary to build flowers and leaves. Many of the evo-devo studies in flowers and leaves have contributed important molecular data to the discussion on organ homology. The gene regulatory networks have also been well studied for flowering time, meristem maintenance, and root development in angiosperms. Yet much remains to be discovered in plant evolutionary developmental biology, not only in elucidating the gene regulatory networks important for the development of these plant structures but also how these networks evolve. Further studies are needed to understand the developmental genetics of the alternation of generations, gametophyte development, vasculature, spores, and seeds. To understand the gene regulatory networks underlying these features we will need to perform plant evolutionary developmental studies across the land plants. We will briefly discuss two examples that require more developmental genetic studies (alternation of generations and sporangia) and one (meristems) that warrants a comparative approach across the land plants. The comparative approach is a critical aspect of evo-devo studies and to understand the evolution of morphological features across the land plants we will need to develop model organisms across the land plants.
9.3.2 Alternation of Generations
One of the most dramatic changes in body plan is the alternation of generations exhibited by all land plants. All land plants alternate between two multicellular forms—the haploid gametophyte and the diploid sporophyte—and during land plant evolution there has also been a reduction in one phase and an elaboration in the other phase. In bryophytes, the gametophyte is the dominant phase while in vascular plants the sporophyte is the dominant phase of the life cycle.
Several theories, including the antithetic theory and homologous theory, have been proposed to explain the evolution of the alternation of generation in land plants (Gifford & Foster 1989). The antithetic or interpolation theory proposes that a delay in meiosis of the zygote and subsequent rounds of mitosis produced the multicellular sporophyte. Although the homologous or transformation theory proposes that the sporophyte and gametophyte generations evolved different morphologies from an ancestor with an isomorphic life cycle. Current data strongly support the antithetic theory (Bower 1972; Kenrick & Crane 1997). Phylogenetic analyses indicate that the closest relatives to the land plants are charophytes (Karol et al. 2001), which have a dominant multicellular haploid phase and a diploid phase represented by a unicellular zygote. The antithetic theory proposes that land plants evolved from an algal ancestor with a charophyte-type life cycle and that the multicellular diploid generation evolved by a delay in meiosis and an increase in mitotic divisions (Bower 1972; Kenrick & Crane 1997; Graham & Wilcox 2000).
One of the most intriguing aspects of the alternation of generations is that two morphologically distinct forms are generated from the same genome. Meiosis and syngamy are the turning points for the generation of the gametophyte and sporophyte phases, respectively. In fact, regardless of ploidy the gametophyte generation follows meiosis and the sporophyte generation follows syngamy. However, other switches besides meiosis and syngamy clearly exist. For example, apogamy and apospory occur in ferns and bryophytes, where the alternate generation is formed without syngamy or meiosis, respectively (Bower 1935; Niklas & Kutschera 2010). New resources and comparative analyses are beginning to shed some light on the developmental genetics of this dramatic change in body plan and the evolution of the two generations during land plant evolution.
A single plant genome is able to generate two morphologically distinct plant forms by differential gene expression. This has recently been demonstrated in Arabidopsis with large-scale expression techniques. Several studies found that the transcriptomes from Arabidopsis male and female gametophytes were unique from sporophyte transcriptomes (Pina et al. 2005; Yu et al. 2005). For example, in Arabidopsis, the pollen transcriptome is smaller than any sporophyte transcriptome but it is unique and has more selectively expressed and enriched genes compared to any other sporophyte transcriptome (Pina et al. 2005). However, we still do not know the developmental mechanism for how large-scale differential gene expression is generated in the two generations. It is clear that not only is activation of gene expression important but so is repression of gene expression. Gene silencing mechanisms are known to be important for the regulation of developmental processes in plants and animals, including regulation by small RNAs, DNA methylation, and histone modification (Baulcombe 2004; Loidl 2004; Chan et al. 2005). We are now beginning to uncover the molecular basis of gene silencing mechanisms that may be involved in the alternation of generations.
Histone modification likely plays a large role in differential gene expression between the gametophyte and sporophyte phases. Different heterochromatin banding patterns of chromosomes were noted between the gametophyte and sporophyte phases in the liverworts (Newton 1987). Heterochromatin banding is associated with methylation of histones. The Polycomb recruiting complex 2 (PRC2 or Polycomb group protein (PcG complex)) mediates the methylation of lysine 27 on histone 3 (K3K27me3) (Hennig et al. 2005; Hennig & Derkacheva 2009). PRC2 complexes are found in animals and plants. PcG complexes are modular in organization and several different PcG complexes have been found in A. thaliana. These A. thaliana PcG complexes are composed of two WD-40 proteins (FIE and MSI1), one SET domain protein (CLF, SWN, or MEA), and one zinc finger protein (EMF2, VRN2, or FIS2). We know a lot about the role of PcG complexes in A. thaliana development, however we have little data from across the land plants. However, recent research in P. patens has uncovered roles for PpFIE and PpCLF in moss development (Mosquna et al. 2009; Okano et al. 2009). Mutations in PpFIE or PpCLF result in gametophore meristems that overproliferate and do not reach the reproductive stage and produce gametes. However, PpFIE and PpCLF mutants produce sporophyte structures that look like sporophytes and express sporophyte specific genes. These results suggest that both PpCLF and PpFIE are necessary to repress apogamy in the gametophyte of P. patens.
The antithetic theory proposes a delay in meiosis and an increase in mitotic divisions to explain the evolution of the multicellular sporophytic phase. Therefore, analyses of cell cycle genes could provide some molecular data to further assess hypotheses on the alternation of generations. Not only is the PcG complex found in plants and animals but some genes involved in the cell cycle are also conserved across the eukaryotes. One conserved negative regulator of the cell cycle is Retinoblastoma (Ebel et al. 2004), and functional analyses of the A. thaliana RETINOBLASTOMA RELATED (RBR) protein has shown that it is required for differentiation of the male and female gametophytes (Johnston et al. 2008, 2010; Borghi et al. 2010). Functional analyses in A. thaliana have shown that RBR is important to derepress expression of genes involved in the PRC2 complex as well as genes involved in DNA methylation. Further research will provide great insights into the connection between gene silencing and the cell cycle and perhaps those developmental pathways involved in the alternation of generations.
9.3.3 Gametophytes
We know a lot more about the molecular genetics of sporophyte development compared to gametophyte development. This is mainly due to the fact that a majority of developmental genetic studies have been performed in angiosperms that have a dominant sporophyte phase and a much smaller gametophyte phase. However, we still have a surprising amount of information about the transcriptomes and development of the female gametophyte (embryo sac) and the male gametophyte (pollen) of Arabidopsis (Pina et al. 2005; Yu et al. 2005; Yang et al. 2010). Mutant screens for gametophyte development are helping to elucidate the function of gametophyte expressed genes as well as novel genes (Pagnussat et al. 2005; Boavida et al. 2009).
Overall, transcription factors are underrepresented in the pollen transcriptome. However, Type I MADS-box and Type II MIKC* MADS-box genes are transcription factors that are overrepresented in the pollen transcriptome (Pina et al. 2005). Mutations in Type II MIKC* MADS-box genes have reduced pollen germination that results in reduced fertility (Verelst et al. 2007; Adamczyk and Fernandez 2009). In addition, Type II MIKC* MADS-box genes have been shown to be part of a pollen developmental network that is composed of other MADS-box genes, particularly Type I MADS-box genes (Verelst et al. 2007; Adamczyk & Fernandez 2009). Type I MADS-have recently been shown to have important functions in female gametophyte development in Arabidopsis (Portereiko et al. 2006; Yoo et al. 2006; Bemer et al. 2008; Colombo et al. 2008; Kang et al. 2008; Steffen et al. 2008; Ambrose 2010; Masiero et al. 2011). Interestingly, of all the MADS-box genes present in the P. patens genome, more than half are Type II MIKC* MADS-box genes while there are only two Type I MADS-box genes (Rensing et al. 2008). Comparative functional analyses in Arabidopsis and P. patens will reveal the roles of these particular types of MADS-box genes in gametophyte development and evolution. Further developmental genetic studies in P. patens, which has a conspicuous gametophytic phase, will see leaps and bounds in our knowledge about the evolution and development of the gametophyte.
9.3.4 Sporangia and Spores
Sporophytes produce sporangia and meiosis occurs in the sporangia to produce haploid spores (Gifford & Foster 1989). Sporangia and spores have been well studied in the angiosperms, however, sporangia position, morphology, and development are diverse throughout the land plants (Kenrick & Crane 1997). In the lycophytes, the sporangia are affiliated with the microphyll and one hypothesis for the evolution of microphylls is through sporangia sterilization. Developmental studies of sporangia revealed the differences between leptosporangiate and eusporangiate species more than a century ago (Gifford & Foster 1989). The development of sporangia is well studied across the land plants and is an important character for taxonomic purposes. Therefore the framework for understanding the molecular genetic basis for sporangia development is well established and needs to be extended across the land plants.
The spores, and sporopollenin wall surrounding the spores, are homologous across the land plants (Blackmore & Barnes 1987). The seed plants produce microspores or pollen grains, and developmental genetic pathways underlying Arabidopsis sporopollenin and microspore development are beginning to be understood (Ma 2005; Ariizumi & Toriyama 2011). For example, the gene SPOROCYTELESS in Arabidopsis is important for the initiation of sporogenesis and is regulated by the MADS-box gene AGAMOUS (Yang et al. 1999; Ito et al. 2004). However, a comparative approach is needed to understand the evolution of spore developmental genetics in the land plants (Brown & Lemmon 2011).
Homosporous land plants produce spores that are all the same size and these produce gametophytes with both antheridium (male) and archegonium (female) (Gifford & Foster 1989). Heterosporous land plants have two different sporangia that produce spores of two different sizes; the microspores will produce male gametophytes while megaspores will produce female gametophytes. Heterospory is found in distinct lineages and heterospory is thought to have been important for the evolution of the seed (Bateman & DiMichele 1994). It is necessary to study the evolutionary genetics of heterospory to understand the transition from heterospory to seed, and homospory to understand the transition from homospory to heterospory. To understand these transitions, developmental genetic studies will need to be performed across the land plants.
9.3.5 Meristems
The shoot apical meristem is found at the apex of land plant sporophyte shoots. The organization of the meristem has been described by one of seven different types (Wardlaw 1968), but even though the cellular organization may appear distinct, all meristems perform the same function. The apical meristem gives rise to all adult organs of the plant, and the plant must also maintain this totipotent group of cells. However the apical meristem of seed plants is composed of a pool of cells spanning several cell layers while the apical meristem of ferns is represented by a single apical cell with two or three cutting faces (Wardlaw 1968; Gifford & Foster 1989; Steeves & Sussex 1989). In lycophytes, members of the Selaginellaceae have one or two apical cells or a group of cells while members of the Lycopodiaceae and Isoetaceae have an apical meristem composed of a pool of cells similar to the apical meristem found in seed plants (Gifford & Foster 1989). Although, these meristems appear to differ, many are organized similarly with one quiescent cell or a pool of quiescent cells surrounded by small cytoplasmically dense rapidly dividing cells (McAlpin & White 1974; Stevenson 1976; Gifford & Foster 1989). Therefore, it is not surprising that homologs of genes necessary for meristem maintenance are found throughout the land plants (Floyd & Bowman 2007).
Meristems must balance the proliferation of cells to make lateral organs and maintain a set of pluripotent cells. The gene regulatory network needed to maintain meristems cells in an undifferentiated state has been well studied in angiosperms (Ha et al. 2010). Molecular genetic studies in A. thaliana have shown that a KNOX regulatory network and a CLAVATA-WUSCHEL regulatory network are both important for maintaining the shoot apical meristem. There has been little comparative work on the CLAVATA-WUSCHEL pathway outside of angiosperms. There has, however, been some comparative research on the role of KNOX genes in meristem development.
In P. patens, the gametophyte develops by apical tip growth and gives rise to the leafy plant and the sporophyte meristem is determinate and gives rise to the sporophyte (Lee et al. 2005; Vidali et al. 2007). Functional analyses of KNOX orthologs in P. patens indicate that KNOX proteins are important for the proper development of the sporophytes but have no role in the indeterminate haploid gametophyte (Sakakibara et al. 2008). Sakakibara et al. (2008) concluded that the KNOX pathway for specifying indeterminate meristems arose de novo in the angiosperms. KNOX genes function in both bryophyte and angiosperm sporophytes but the meristem in bryophytes is determinate while the angiosperm meristem is indeterminate. It is possible that the bryophyte intercalary meristem constrains the developmental potential of the sporophyte. An analysis of the KNOX developmental network and its regulation is necessary to better understand the role of KNOX proteins in the determinate sporophyte meristem of bryophytes. In C. richardii, KNOX orthologs are expressed in the sporophyte meristem and the margins of developing leaves but are not detected in the gametophyte meristem (Sano et al. 2005). KNOX orthologs are expressed similarly in the C. richardii meristem despite the meristem appearing structurally different from angiosperm meristems. These results also support the idea that KNOX orthologs are dispensable for gametophyte meristem development (Sano et al. 2005; Sakakibara et al. 2008).
Axillary meristems are formed from the flanks of the shoot apical meristem during the generation of lateral organs in gymnosperms and angiosperms (Gifford & Foster 1989). Axillary meristems contribute to the plasticity of form and the generation of novel forms, contributing to diverse architecture of seed plants. Although branching may sometimes occur in ferns, axillary meristems are not formed consistently and regularly in the ferns as they are in the seed plants. The genes that are responsible for the generation of meristems in the axils of lateral organs are not well known. However, it is clear that this pathway did not become well established until late in land plant evolution. Meristems are found not only in the shoot apex but also in the root tip and in vascular cambium, and although distinct regulatory networks function in the shoot, root, and vascular meristems, recent work has shown that some of the genes in these networks are homologous (Fiers et al. 2007; Hirakawa et al. 2010). In fact, recent work on unraveling the gene regulatory network present in vascular cambium has nicely illustrated the power of the candidate gene approach (Hirakawa et al. 2010).
However, there are some interesting aspects of meristems outside the flowering plants that warrant further study. One interesting structure is the rhizophore, which is found in lycophytes and arises from the angle meristem where the shoot branches (Webster 1992). The rhizophore is particularly intriguing because it can develop into a shoot or a root depending on the environmental and developmental conditions (Worsdell 1910; Imaichi & Kato 1989). In fact the identity of the rhizophore has been variously interpreted. It has been considered as either a root or shoot because it shares morphological features with each. The rhizophore has also been interpreted as a novel organ. The developmental plasticity of this meristem is unique among plant meristems and understanding the molecular genetic network underlying rhizophore development will illuminate mechanisms of stem cell maintenance and cell fate specification in plant meristems.
9.3.6 Development of Model Organisms
Much of what we know about the developmental genetics of these plant morphological features comes from studies in angiosperm species. A lot of focus on flower development and evolution reflects the availability of genetic and genomic tools in the angiosperms. The candidate gene approach to understand plant morphological diversity within the angiosperms and to some extent across the land plants has been very powerful and will remain very useful for lineages with large genomes and long life cycles. The candidate gene approach has also been useful for providing more data in the assessment of homology. Transcriptomes are being generated across the land plants, which will facilitate the candidate gene approach for evo-devo studies across the land plants (http://www.onekp.com/project.html; http://www.jgi.doe.gov/genome-projects). This may be particularly useful in gymnosperms and ferns, whose genomes are prohibitively large for current whole genome sequencing efforts. In addition, transcriptome and whole genome sequences have been generated for the lycophytes S. moellendorffii and S. apoda (Banks et al. 2011; D. Stevenson, personal communication). A comparison of plant genomes will allow a comparison of major plant transcription factor families and signaling proteins (expansion and contraction of plant families) that allow hypotheses to be proposed on the evolution of major features during land plant evolution (Floyd & Bowman 2007).
However, to understand the development of morphological features that are found outside the angiosperms and to understand the evolution of developmental pathways, we need to develop additional plant model systems. Model organisms are essential for unraveling the developmental genetics of particular morphological features and are also important for understanding how developmental pathways have evolved. For a species to be effectively used as a model, it needs to have certain characteristics and community resources: it must be easily and quickly grown, and it must possess natural variation, a genetic map, reliable expression protocols, reverse and forward genetic resources, transcriptome sequences, and genome sequences (Abzhanov et al. 2008). Major model systems have been developed across the animal kingdom, however, except for the bryophyte, P. patens, major plant model systems are restricted to angiosperms (Abzhanov et al. 2008). To fully understand land plant evolution and development, major model systems must be developed across the backbone of the land plant phylogenetic tree. To develop a major model system it is necessary to generate forward and reverse genetic resources. Homologous recombination in P. patens has been an important aspect of developing this bryophyte into a major model system (Schaefer & Zryd 1997). Genomes and functional tools are being developed for additional bryophytes, which are important resources for the development of a model organism. Sequencing of the liverwort genome, Marchantia polymorpha is currently underway and transformation of this species has also been documented (http://www.jgi.doe.gov/genome-projects; Ishizaki et al. 2008).
9.4 Conclusions
Evolutionary developmental biology studies in animals and plants have both demonstrated that they share some general concepts that show the logic of developmental pathways and how development influences evolution. Plant evolutionary developmental biology has provided many insights into the evolution and development of leaves and flowers. However, there is still much more work to be done on these plant morphological features as well as additional morphological features such as the alternation of generations, spores, and meristems. To better understand the evolution and development of plant form we need to expand our studies across the land plants and to do this we need to develop additional model organisms.



