1 Phylogenetic Analyses and Morphological Innovations in Land Plants
Abstract
An increasingly robust phylogenetic framework based on molecular and fossil data clarifies the sequence of evolutionary innovations in land plants. Oogamy and cellular novelties (phragmoplast, plasmodesmata, incipient meristems) evolved in aquatic streptophytes. Invasion of the land entailed interpolation of the sporophyte, jacketed gametangia and sporangia, and air-dispersed spores, followed by stomata. Origin of vascular plants involved branching of the sporophyte and stepwise evolution of vascular tissue. Leaves originated independently in lycophytes and euphyllophytes; in some euphyllophytes leaves were derived from single dichotomous branches, in others from whole branch systems. In seed plants, secondary growth evolved before the seed. Pinnately compound leaves were replaced by simple leaves in coniferophytes. The origin of the angiosperm flower remains unresolved, but bitegmic ovules may be derived from cupules, and the ancestral carpel can be reconstructed as ascidiate. Evolution of double fertilization was a stepwise process that continued within angiosperms; vessels also evolved within the group. Monocots show major reorganization tied to loss of secondary growth, while pentamerous flowers evolved from dimerous within eudicots.
1.1 Introduction
As in other groups of terrestrial organisms, the evolution of land plants involved a series of radiations linked with major evolutionary innovations, many of them clearly adaptations that allowed progressively more efficient and varied occupation of the land environment. The morphology of familiar plants such as the model system Arabidopsis thaliana therefore represents a hierarchical accumulation of structural features that arose at different points on the line from their distant aquatic ancestors, with older advances shared with a successively wider range of relatives. This chapter attempts to summarize the present picture of the sequence of evolutionary innovations in the latest phylogenetic framework, as well as outstanding unresolved issues.
Some of the main events in the evolutionary history of land plants have been recognized since the late nineteenth century. Key insights were recognition of the alternation of haploid and diploid generations, seen in its most basic and obvious form in “bryophytes” such as mosses and “lower vascular plants” such as ferns, and realization that this life cycle persists in modified form into seed plants (Hofmeister 1862; Bower 1890, 1908; Strasburger 1894). However, the details have become much clearer over the past century as a result of many factors, including fossil discoveries that show intermediate stages and character combinations no longer preserved in the living flora, technical advances that revealed new suites of characters at the microscopic and ultrastructural level, development of more explicit methods of analysis of phylogenetic relationships, and the application of these methods to molecular sequence data. Methods of phylogeny reconstruction, many derived from earlier partial insights (notably Zimmermann 1931; Donoghue & Kadereit 1992) but first clearly synthesized in English by Hennig (1966), were elaborated under the rubric of “cladistics” in the 1970s and 1980s and used in analyses of morphological characters. These methods used the principle of parsimony to search for the phylogenetic tree involving the fewest character state changes, on the assumption that this is the tree most consistent with the totality of characters recognized.
Whereas some precladistic discussions assumed that phylogeny could only be approached by consideration of fossils and identification of direct ancestors, which implied that the phylogeny of groups such as angiosperms with a supposedly poor fossil record could not be understood, cladistic methods could be applied to both living and fossil organisms. These methods also made it possible to draw conclusions on the origin of groups and their ancestral states by recognition of closest outgroups without identification of actual direct ancestors. At the level of land plants, many analyses included both fossil and living taxa. There was considerable discussion of the relative importance of the two sorts of data, some arguing that the main relationships among living organisms could be reconstructed without fossil data, which necessarily have far fewer characters due to lack of preservation of parts (Patterson 1981). Others argued that inclusion of fossils was necessary to obtain correct relationships, as in amniote vertebrates (Gauthier et al. 1988), and even when fossils were not required to infer the correct topology of the tree of living organisms, they could be needed to reconstruct the evolutionary steps leading to living clades, which are often separated from their closest relatives by large numbers of morphological changes (Donoghue et al. 1989). In molecular hindsight, morphological cladistic analyses correctly resolved many contentious problems that had plagued earlier intuitive approaches, such as the monophyly of land plants and angiosperms. On other questions, however, such as rooting of the angiosperm phylogenetic tree and relationships among vascular plant and seed plant lines, the results varied from one analysis to another, presumably due to homoplasy (evolutionary convergence and reversal), different interpretations of characters, and variable sampling of both extant and fossil taxa.
This picture has improved dramatically in the past two decades with the accumulation of vast quantities of molecular sequence data from more and more species, which has led to increasingly complete, consistent, and statistically well-supported trees of living organisms. The first analyses of sequences of single genes showed many of the same sorts of inconsistencies seen in morphological analyses. However, as more genes have been sequenced and combined into multigene and even whole-genome analyses, many tentative early results have stabilized and become statistically robust, and with some conspicuous exceptions, most early conflicts between genes have been firmly resolved. These studies started with parsimony analysis, but newer maximum likelihood and Bayesian methods take a more statistical approach to changes on branches. This has led to a role reversal—whereas formerly ideas on the evolution of morphological characters were used to reconstruct phylogenies, phylogenies based on molecular data are now used to reconstruct the evolution of morphological characters, by plotting (optimization) of character states on trees derived from molecular data, using parsimony or likelihood-based methods, thus avoiding dangers of circular reasoning.
For understanding of major evolutionary innovations, a major weakness of the increasingly exclusive reliance on molecular data for phylogeny reconstruction is that purely molecular analyses cannot include fossil taxa, since with the exception of very recent fossils (such as human relatives) no DNA remains. There is much discussion of whether morphological data have any role at all in reconstruction of phylogenies of living organisms (Scotland et al. 2003; Wiens 2004). Because of the vastly greater number of DNA characters, combined analyses of morphological and molecular data tend to be dominated by molecular data. Morphology may still have some role in resolving parts of the phylogeny where molecular analyses of different genes give conflicting or poorly supported results, such as among seed plants and major groups above the base of the angiosperms, but it may be argued that this represents only a brief intermediate phase before all relationships are cemented by genomic data.
Even if molecular analyses completely resolve all relationships among living taxa, other approaches will be needed to address those cases where fossils provide evidence on transitions that are not preserved in the living flora. Molecular analyses consider only relationships among crown groups—where a crown group includes the most recent common ancestor of the living members of a clade and all its derivatives—and not fossils on or attached to the stem lineages leading to these clades, known as stem relatives (Doyle & Donoghue 1993). Integration of these fossils will still require compilation and analysis of morphological data from both the fossils and all relevant living taxa. The ideal approach may be a “total evidence” analysis that combines both molecular and morphological data (Hermsen & Hendricks 2008), but this may not be so easy because of problems in choice of molecular data sets and differences in taxon sampling: single species in molecular data sets, but often higher taxa for which ancestral states have been reconstructed in morphological data sets. A few studies of land plants have used a total evidence approach to integrate morphological data from fossils with molecular data (e.g., Rothwell & Nixon 2006), but in the meantime others have used a “molecular scaffold” approach (Springer et al. 2001; Manos et al. 2007; Doyle & Endress 2010) that integrates fossils by analyzing a morphological data set for both living and fossil taxa with the relationships among living taxa fixed to a “constraint tree” based on molecular data. Such analyses essentially ask what additional insights fossils provide if the relationships inferred from molecular data are correct.
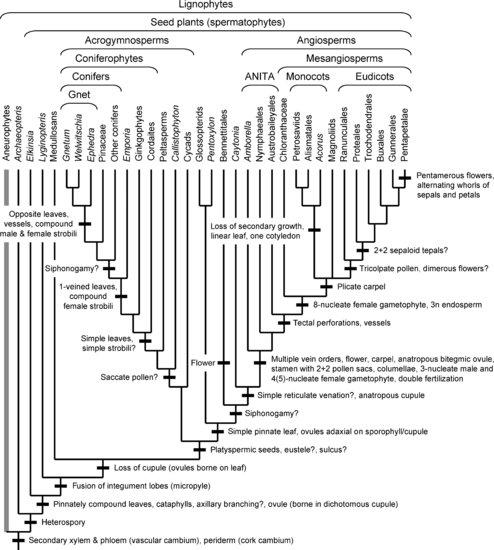
This chapter does not pretend to be a comprehensive review of the literature, but rather a selective though hopefully balanced survey of current ideas and evidence. Currently understood phylogenetic relationships and the placement of morphological innovations are summarized in Figures 1.1 and 1.2. A good general review of land plant phylogeny is provided by Judd et al. (2008). Except where noted, most information on morphological characters of the living and fossil taxa discussed here can be found in standard plant morphology and paleobotany texts (Smith 1955; Gifford & Foster 1989; Stewart & Rothwell 1993; Crum 2001; Taylor et al. 2009) and Graham (1993) for algal outgroups. Not all the innovations mentioned are equally “major” in an evolutionary sense, but some more obscure ones are of interest as providing morphological support for relationships. In most cases, I refer to clades above the ordinal level with anglicized versions of names in the phylogenetic nomenclature of Cantino et al. (2007), and I use quotes to mark traditional paraphyletic groups when these are first mentioned (e.g., “bryophytes”). Hopefully this summary will be useful as a framework for investigations on the developmental-genetic and functional bases of the evolutionary changes inferred.
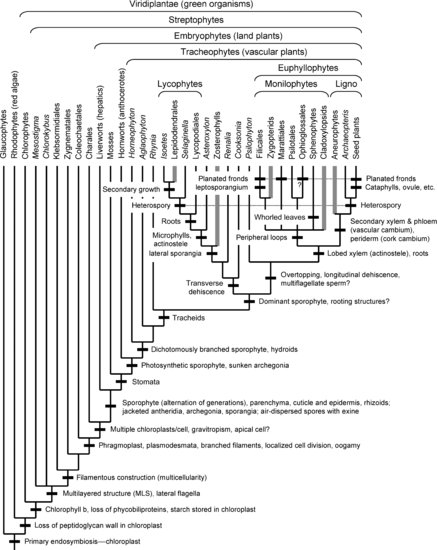
1.2 Basic Innovations in Cell Structure and Life Cycle: Aquatic Streptophytes
Both morphological and molecular phylogenetic analyses confirm the long-standing view that land plants are members of the clade of green organisms, or Viridiplantae, in which other members have been traditionally called green algae. The most conspicuous innovations that unite this clade are the origin of chlorophyll b (in addition to chlorophyll a) and storage of starch in the chloroplasts. Green organisms are in turn linked by molecular data to red algae (rhodophytes) and the unicellular glaucophytes. Molecular phylogenies indicate that these three groups were derived from the line in which the chloroplast first originated by primary endosymbiosis with a cyanobacterium, from which chloroplasts of all other photosynthetic eukaryotes were derived by secondary endosymbiosis (incorporation of a red or green alga) or tertiary endosymbiosis (Delwiche & Palmer 1997; Keeling 2004). The chloroplasts of glaucophytes retain a remnant of the peptidoglycan cell wall seen in free-living cyanobacteria and other eubacteria, whereas both glaucophytes and red algae retain phycobiliproteins, the characteristic photosynthetic accessory pigments of cyanobacteria, which were lost in green plants.
Within green organisms, studies of cell ultrastructure in the 1960s and 1970s led to the view that certain groups of “green algae” are more closely related to land plants than others (Pickett-Heaps 1969, 1972, 1975, 1979; Stewart & Mattox 1975; Graham 1993), such as Charales (complex filaments with whorled branches), Coleochaetales (Chaetosphaeridium, with branched filaments, and Coleochaete, with disks made up of radiating branched filaments or a solid sheet of cells), and Zygnematales (including the unbranched filamentous alga Spirogyra and single-celled desmids). This conclusion has been confirmed and extended by molecular data (Karol et al. 2001; Lewis & McCourt 2004; Qiu 2008; Becker & Marin 2009). The clade of land plants and their aquatic algal relatives is known as the streptophytes, while the sister clade including most green algae (such as the familiar genera Chlamydomonas, Acetabularia, and Ulva, as well as basal unicellular lines known as “prasinophytes”) is called the chlorophytes.
Aquatic streptophytic algae form a paraphyletic grade, that is, a series of successive branches diverging from the line leading to land plants. Molecular analyses are inconsistent on which of these groups is most closely related to land plants (their sister group), but most recent studies (Karol et al. 2001; Lewis & McCourt 2004; Qiu et al. 2006b; Qiu 2008; Becker & Marin 2009) identify the sister group as Charales and the next outgroup as Coleochaetales. This view has been challenged by analyses that linked Zygnematales with land plants (Turmel et al. 2006; Chang & Graham 2011; Wodniok et al. 2011), although Qiu (2008) argued that this result may be a function of rearrangements in the chloroplast genome of Zygnematales and sparse taxon sampling. Zygnematales are morphologically simpler than Coleochaete and Charales; if they are sister to land plants, their simplicity may be due to reversal (Wodniok et al. 2011). The unicellular flagellate Mesostigma, Chlorokybus (with small clusters of cells), and Klebsormidiales (unbranched filaments) appear to be lower on the tree, with Mesostigma alone or Mesostigma plus Chlorokybus (Lemieux et al. 2007) most basal. All these organisms are freshwater or soil algae, indicating that plants invaded the land not from the sea but from freshwater, a less radical step from a physiological point of view. In fact, adaptation to freshwater may have been a preadaptation for invasion of the land (Becker & Marin 2009).
Aquatic streptophytes show varying numbers of innovations retained in land plants, which were the morphological evidence (presumed synapomorphies) that originally led to their recognition as land plant relatives (Pickett-Heaps 1969, 1979; Stewart & Mattox 1975; Graham 1993). Even Mesostigma, which was formerly placed in the prasinophytes with unicellular chlorophytes, has two laterally inserted flagella attached to a distinctive multilayered structure (MLS) of microtubules (Rogers et al. 1981; Melkonian 1989), features retained in the motile sperm cells well into land plants, until flagella were lost within seed plants.
More important for the origin of land plants, the most derived aquatic streptophyte lines, Coleochaetales and Charales, show the appearance of the characteristic land plant mode of cell division. Cell division in these groups involves a phragmoplast, where the spindle fibers persist in their original orientation between the two daughter nuclei and cell wall material is deposited centrifugally to form the cell plate, leading to a transverse wall rigidly connected to the sidewalls. In these algae, perforations left in the cell plate by the spindle fibers become the sites of plasmodesmata, or connections between the cytoplasms of adjacent cells, a major feature of multicellularity in land plants (Lucas et al. 1993; Qiu 2008). This contrasts with the predominant mode of cell division in chlorophytes, with a phycoplast (Pickett-Heaps 1972), where the spindle fibers collapse to the plane between the two nuclei, and cell separation occurs by centripetal furrowing of the cell membrane. More basal streptophytes have a persistent spindle but the cells divide by furrowing, with late formation of a rudimentary phragmoplast in some Zygnematales, indicating that the phragmoplast evolved within streptophytes (Pickett-Heaps 1969, 1979; Stewart & Mattox 1975; Graham 1993).
The land plant mode of cell division, which is so familiar that it may be taken for granted, ultimately formed the basis for the characteristic solid, three-dimensional tissue construction of land plants (Hagemann 1999). It originated, however, in algae with a simpler, basically filamentous construction. Although Zygnematales grow by intercalary divisions of cells along the filament, Coleochaete and Charales resemble land plants in having incipient meristems. Cell divisions are localized at the margin of the disk or the ends of the radiating filaments in Coleochaete and in a distinct apical cell in Charales (Graham 1993). An additional innovation that Charales share with land plants is gravitropism, a feature that took on greater importance in the land environment (Qiu 2008).
A significant consideration for the origin of land plants is the fact that aquatic streptophytes have a haploid life cycle, like most though not all members of the chlorophyte clade. This means that all mitotic divisions occur in the haploid phase and the only diploid cell is the zygote, which undergoes meiosis directly (usually after forming a resting zygospore). Within the aquatic streptophyte grade, the most important reproductive innovation was the origin of oogamy, seen in Coleochaetales and Charales, with a sessile egg and motile sperm rather than undifferentiated, usually motile isogametes. The exact point of origin of oogamy is uncertain, since sexual reproduction has not been described in the most basal streptophytes (Graham 1993). In Zygnematales filaments or cells conjugate and a protoplast migrates from one cell into another as a nonflagellated gamete. This is presumably autapomorphic, but without better evidence on relationships and data on life cycles in basal streptophytes it is uncertain whether it was derived from an oogamous or an isogamous condition. From a phylogenetic point of view, the oogamous haploid life cycle of “higher” aquatic streptophytes forms the starting point for the alternation of generations of land plants.
Coleochaete and Charales resemble land plants in additional reproductive innovations: retention of the zygote in the haploid thallus of Coleochaete, its investment by haploid cells in Charales, and presence of cells around the egg in Coleochaete that resemble placental transfer cells in the archegonium of land plants (Graham & Wilcox 1983). Which, if any, of these features are synapomorphies shared with land plants depends in part on which line is more closely related to land plants (Haig 2008).
1.3 Invasion of the Land: “Bryophytes”
The evolution of land plants (embryophytes) from their freshwater ancestors involved many major innovations, most of which are obvious adaptations to the radically different requirements for structural support, uptake of water, prevention of desiccation, and gas exchange in a terrestrial environment. The sequence of origin of these and subsequent land adaptations has been clarified by improved resolution of phylogenetic relationships, particularly where the land plant tree is rooted.
Since discovery of the basic life cycle of land plants, with alternating haploid (gametophyte) and diploid (sporophyte) generations (Hofmeister 1862; Strasburger 1894), it has been recognized that “bryophytes” and “lower” vascular plants, which have free-swimming sperm cells, represent a primitive “amphibious” stage in land plant evolution (Bower 1890, 1908). However, the relationship between bryophytes, with a dominant gametophyte, and vascular plants, with a dominant sporophyte, remained unsettled until the advent of morphological cladistic analyses, which made much use of data on cellular characters in aquatic streptophytes (Mishler & Churchill 1984, 1985; Kenrick & Crane 1997).
Although it had been widely thought that bryophytes and vascular plants were divergent sister groups, or even separate lines of evolution from aquatic ancestors, these analyses indicated that bryophytes are a paraphyletic series of three lines diverging below vascular plants, a result confirmed by molecular studies (Qiu et al. 1998, 2006b; Karol et al. 2001; Qiu 2008). This indicates that the bryophytic life cycle is ancestral, as is also implied by the fact that it is less removed from the life cycle in aquatic streptophytes, where the diploid phase consists of only one cell, the zygote (Mishler & Churchill 1984; Haig 2008). The morphological analysis of Mishler and Churchill (1984) indicated that liverworts (hepatics) were basal (sister to all other land plants), followed by hornworts (anthocerotes), with mosses sister to vascular plants. Although earlier molecular studies gave inconsistent rootings, multigene analyses appear to be stabilizing on the basal position for liverworts, but with hornworts rather than mosses as the sister group of vascular plants (Qiu et al. 2006b; Qiu 2008; Chang & Graham 2011).
A key innovation in land plants was origin of the alternation of generations, or specifically, given the haploid life cycle of the outgroups, origin of a multicellular diploid sporophyte, a topic of debate for more than a century (Haig 2008). The present view corresponds to the classic antithetic or interpolation theory (Bower 1890, 1908, 1935): that the sporophyte was a new phase interpolated into a haploid life cycle by a delay of meiosis, so that the zygote underwent mitotic divisions that produced a mass of diploid cells, some of which underwent meiosis. Compared to an alga with zygotic meiosis, this would result in production of vastly more numerous and genetically more varied haploid spores per fertilization event (Bower 1908; Becker & Marin 2009). This contrasts with the opposing homologous or transformation theory (Scott 1895; Zimmermann 1952; Remy 1982): that the ancestral life cycle was already an alternation of generations, but with isomorphic haploid and diploid phases that were subsequently modified in opposite ways.
In vegetative morphology, a major advance of land plants was evolution of more bulky three-dimensional parenchymatous construction, which would have retarded desiccation by decreasing the surface to volume ratio. The closest approach to parenchyma in aquatic streptophytes is in some species of Coleochaete, where areas around the zygote become several cells thick. Other antidesiccation devices were origin of a waxy cuticle and differentiation of epidermal cells at the surface of the plant body. Another innovation was origin of hair-like rhizoid cells on parts of the gametophyte in contact with the soil, which function for anchoring and water uptake (Kenrick 2002). Fragmentary Silurian fossils may represent bryophytic cuticles with attached rhizoids (Graham et al. 2004). The symbiotic association of land plants with mycorrhizal fungi, which has been considered an important key to their success (Pirozynski & Malloch 1975), presumably also originated near this point, since it occurs in liverworts and hornworts. Genes required for mycorrhiza formation also occur in mosses, although mycorrhizae themselves are known only in the basal genus Takakia (Wang et al. 2010).
Reproductive innovations of land plants include multicellular gametangia and sporangia with a jacket of sterile cells: antheridia producing numerous motile sperm cells, archegonia with neck canal cells and a basal egg, and sporangia with numerous sporogenous cells that undergo meiosis to produce the spores. The sterile jacket is functionally comparable to the epidermis, as a layer protecting the inner cells from desiccation. Although the initial stages differ, development of all these structures involves periclinal division of one or more surface cells, followed by derivation of the jacket from the outer cell(s) and the fertile cells from the inner cell(s) (Smith 1955; Crum 2001). This is an innovation relative to Coleochaete and Charales, where the egg is surrounded by sterile cells, but these are not derived by periclinal division of the same initial. There are closer approaches to land plant antheridia in the male structures of algal outgroups. In some species of Coleochaete, asymmetrical divisions produce both sterile and spermatogenous cells, while in some Charales periclinal divisions produce outer shield cells and inner cells that give rise to spermatogenous filaments (Pickett-Heaps 1975; Graham 1993).
One of the most important adaptations to the land environment was evolution of spores with a highly resistant outer wall or exine, which allowed dispersal of the meiotic products by air rather than water (Becker & Marin 2009). Being produced by meiosis, these spores are formed in tetrads, but in most living groups they separate before being shed from the sporangium. In the fossil spore record, however, there is an initial phase in which spores remained united in tetrads, extending back to the Middle Ordovician and probably the Cambrian (Taylor & Strother 2008). Discovery of masses of such tetrads in a fragmentary sporangium (Wellman et al. 2003) demonstrated that they were produced by a multicellular sporophyte. Tetrads are joined in the Late Ordovician (Steemans et al. 2009) by single spores with a prominent trilete (triradiate) tetrad scar, which represents the junction of the three contact faces with other spores in the tetrad. Steemans et al. (2009) suggested that these spores were produced by vascular plants, but this is unwarranted; although a trilete scar is absent or poorly developed in most liverworts and mosses (Gray 1985), it is well developed in hornworts (Shaw & Renzaglia 2004) and presumably originated in their common ancestor with vascular plants.
The bryophytic lines show the stepwise origin of additional new terrestrial adaptations. The most striking was the origin of stomata for gas exchange, seen in the sporophytes of mosses and hornworts. The sporophyte is largest, longest-lived, photosynthetic, and almost independent in hornworts, a possible synapomorphy supporting their sister group relationship to vascular plants (Campbell 1924; Qiu et al. 2006b). Tubular conducting cells occur in one or both generations of many mosses (Hébant 1977), but the water-conducting cells are hydroids that lack the internal secondary wall thickenings of the tracheids of living vascular plants. The fact that hydroids are absent in basal groups such as Takakia, Sphagnum, and Andraeales implies that they evolved within mosses. In the reproductive sphere, there was a noteworthy change in the archegonia: from long, narrow, and stalked in liverworts and mosses, to basally sunken with a short neck in hornworts and vascular plants.
1.4 Origin of Vascular Plants: The Importance of Fossils
The two most important innovations of vascular plants (tracheophytes) are the origin of (1) a branched sporophyte that produces numerous sporangia, and (2) vascular tissue consisting of xylem, made up of dead cells that conduct water and minerals from the soil to aerial parts, and phloem, with living cells that conduct sugars and other products of photosynthesis to the rest of the plant. Present data indicate that living vascular plants consist of three main clades: two clades of spore-bearing plants, lycophytes and monilophytes, which include ferns, Equisetum (the only living representative of sphenophytes), and Psilotales; and seed plants, including “gymnosperms” and angiosperms. All of these groups have a dominant sporophyte consisting of leaves, stems, and roots (except in Psilotales, apparently as a result of secondary loss). However, fossil data indicate that these were preceded by more primitive plants consisting entirely of dichotomously branched stems, with terminal sporangia. Cladistic analyses by Kenrick and Crane (1997) confirmed that these “rhyniophytes” were stem relatives of living (crown group) vascular plants. Such plants are known in best anatomical detail in the remarkably preserved Early Devonian Rhynie Chert (Kidston & Lang 1917, 1920) but extend back into the Middle Silurian.
Rhyniophytes provide unique evidence that the origin of vascular tissue, or xylem and phloem, was a stepwise process. Two Rhynie plants, Horneophyton and Aglaophyton, which represent the first two branches in the phylogeny of Kenrick and Crane (1997), had xylem consisting not of tracheids, with secondary wall thickenings laid down on the inside of the primary cell wall, but rather of hydroids, with no secondary thickenings, as in mosses (Hébant 1977; Edwards 1986). Mishler and Churchill (1984, 1985) considered vascular tissue a synapomorphy of mosses and vascular plants, which were sister groups in their analysis. However, with hornworts sister to vascular plants and the absence of hydroids in basal mosses this now appears to be a convergence. Rhynia, in the third branch of Kenrick and Crane (1997), had true tracheids but secondary thickenings of a different structure than those of living vascular plants (Edwards 1980; Kenrick & Crane 1991, 1997).
A major breakthrough in understanding early vascular plants was the discovery that some axes in the Rhynie Chert bore antheridia and archegonia. Moreover, it was recognized that similar plants had already been described from other Devonian floras under the name Sciadophyton, which had a rosette of radiating axes and flared tips bearing gametangia (Remy & Remy 1980; Remy 1982; Remy et al. 1993; Kenrick 1994). The Rhynie Chert axes had vascular tissue similar to that of the co-occurring sporophytes.
Remy (1982) and other fairly recent authors (e.g., Stewart & Rothwell 1993) have cited the similarity of the sporophytes and gametophytes in rhyniophytes as evidence for the homologous or transformation theory—that the common ancestor of land plants had an isomorphic alternation of generations, which was modified by reduction of the sporophyte in bryophytes but elaboration of the sporophyte and reduction of the gametophyte in vascular plants. This has been refuted by morphological and molecular phylogenetic evidence that bryophytes are a paraphyletic group consisting of three successive branches, all with a simple, unbranched sporophyte. Maintaining the homologous theory would require that the sporophyte was independently reduced in each of these lines, a much less parsimonious scenario. The existence of two comparable phases in rhyniophytes is entirely consistent with the interpolation theory, since at some point in its elaboration the sporophyte must have “passed” the gametophyte in complexity. Furthermore, it would not be surprising if genes involved in development of the gametophyte were co-opted by the sporophyte as it became independent (Haig 2008).
The rhyniophytic stem relatives of vascular plants also furnish insights on evolution of the vegetative architecture of the vascular plant sporophyte that could not be gained directly from living plants. Rhyniophytes are famous for their dichotomously branched creeping rhizomes and erect aerial stems, in which each successive dichotomy occurred at right angles to the last, giving a bushy, three-dimensional structure, with terminal sporangia at the tips of some axes. The rhizomes bore rhizoids that are presumably homologous with the rhizoids of bryophytes and the root hairs of more derived vascular plants. This architecture served as the basis for the telome theory of Zimmermann (1930, 1952), where the term “telome” refers to the free tips above the last dichotomy. According to the telome theory, leaves were derived by a series of elementary processes, each representing a simple change in developmental processes. These were (1) overtopping, a shift from equal to unequal branching of the apical meristem; (2) planation, restriction of branching from three dimensions to one plane; and (3) webbing, transformation of an open dichotomous system, where the apical meristem splits into two distinct meristems, into a sheet, by formation of a continuous marginal meristem. The telome theory has been much criticized for being overly simplistic and even untestable, but in explicitly relating evolutionary changes to changes in development it anticipated current evo-devo thinking (Stein & Boyer 2006).
1.5 Early Innovations within Vascular Plants: Leaves, Roots, and Heterospory
Among living vascular plants, most morphological and molecular phylogenetic analyses indicate that lycophytes (club mosses and relatives) are the sister group of the remaining vascular plants, or euphyllophytes, which in turn consist of monilophytes and seed plants (Raubeson & Jansen 1992; Kenrick & Crane 1997; Pryer et al. 2001; Qiu et al. 2007). Although the sporophyte and gametophyte were similar in complexity in the rhyniophytic stem relatives of vascular plants, the sporophyte is dominant in all members of the crown group and presumably had become so in their most recent common ancestor. Based on a tree of living taxa only, Schneider et al. (2002) inferred that leaves and roots originated once in the common ancestor of living vascular plants, but as argued by Friedman et al. (2004) inclusion of fossil outgroups of the living clades indicates that both organs arose more than once.
Typical roots, with positive geotropism and a root cap, appear to have originated at least twice, in lycophytes and euphyllophytes (Kenrick 2002; Friedman et al. 2004). However, stem relatives of both clades, such as Zosterophyllum, Bathurstia, and Asteroxylon on the line leading to lycophytes (Gensel et al. 2001) and Psilophyton on the line to euphyllophytes (Banks et al. 1975; Doran 1980), had downward-growing dichotomous “rooting structures” transitional between rhizomes and roots, suggesting that such structures may have arisen below the crown node of vascular plants. Their independent modification into typical roots may be reflected in differences between roots of living lycophytes and other vascular plants, notably apical dichotomous branching rather than endogenous lateral branching from the pericycle.
An early anatomical innovation in vascular plants was origin of periderm (cork, consisting of rows of suberized cells). The oldest known example of this tissue is in Psilophyton (Banks 1981; Banks & Colthart 1993), where it acted as a mechanism for repairing wounds in the epidermis, doubtless its original function. Since periderm of this sort also occurs in living lycophytes (Lu 1996), it presumably evolved before the crown node of vascular plants, but it is not known in rhyniophytes (Banks 1981).
In lycophytes, the most conspicuous innovations are simple one-veined leaves, known as microphylls, and the position of the sporangia in the axils or on the adaxial surface of fertile leaves, or sporophylls. Another is lobing of the xylem in the stele (actinostele). The origin of the leaves and the sporangial position pose special problems, which are closely linked.
The sporangia of lycophytes are more derived than those of rhyniophytes in being globose or reniform and having transverse dehiscence (with two valves opening horizontally relative to the stem), rather than elongate with no visible structural modification for dehiscence. In both characters they resemble sporangia of several Late Silurian and Early Devonian fossil taxa: Cooksonia, with dichotomously branched, leafless stems bearing terminal sporangia; Renalia, with small dichotomous lateral branches with terminal sporangia; Zosterophyllum and other “zosterophylls,” with sporangia borne laterally on leafless stems; and the Rhynie Chert plant Asteroxylon, which had leaves but lateral sporangia borne directly on the stem. According to the cladistic analysis of Kenrick and Crane (1997), all these fossils are stem relatives of lycophytes. Their arrangement is consistent with a scenario in which the sporangia became lateral by overtopping and then reduction of fertile branches.
Since Bower (1935), it has been widely assumed that the one-veined leaves of lycophytes were derived from nonvascularized outgrowths or enations rather than overtopped branches (e.g., Stewart & Rothwell 1993). This view seemed to be supported by the occurrence of enations in Early Devonian “spiny zosterophylls” such as Sawdonia and the fact that the leaves of Asteroxylon were intermediate in having a vascular strand that went only to the base. However, the analysis of Kenrick and Crane (1997) separated Asteroxylon and typical lycophytes from the spiny zosterophylls and nested them among zosterophylls that lack enations, implying that enations and microphylls were not homologous. As an alternative hypothesis, Kenrick and Crane (1997) proposed that microphylls originated by sterilization of lateral sporangia in a nonspiny zosterophyll, which would be consistent with the fact that sporangia and microphylls are intermixed in Asteroxylon. The association of sporangia with sporophylls would presumably be a later event.
Within lycophytes, an important innovation was the origin of heterospory, a synapomorphy of the living genera Selaginella and Isoetes and numerous Late Devonian and Carboniferous fossils, where instead of producing spores of one size that develop into bisexual gametophytes (homospory), sporophytes produce microspores and much larger megaspores that develop into male and female gametophytes, respectively. Because the gametophytes are retained inside the spore wall (endospory), this represents another step in reduction of the gametophyte generation. The shift from homospory to heterospory was repeated in several other fossil and living groups of vascular plants, most notably seed plants, making it one of the most conspicuous iterative trends in plant evolution (Bateman & DiMichele 1994). Proposed adaptive causes for this trend range from primarily genetic, such as assuring outcrossing and genetic diversity or allowing sporophytic control over sex expression, to nutritional and ecological, as a means of parental investment in the next sporophyte generation, by provisioning the megaspore and thus the female gametophyte with nutrients (Chaloner & Sheerin 1981; Bateman & DiMichele 1994).
Another major innovation in Late Paleozoic lycophytes (Lepidodendrales, or Isoetales: Bateman et al. 1992; Kenrick & Crane 1997) was the origin of secondary growth and the tree habit. This secondary growth differed from that of seed plants in involving a unifacial cambium that produced secondary xylem but no secondary phloem. There was also a cork cambium that produced periderm (so-called secondary cortex) near the outside of the trunk, which differed from the periderm of seed plants in functioning as the main support tissue. These innovations allowed Lepidodendrales to dominate Carboniferous coal swamp vegetation, but they have little significance for modern plants. They persist only in vestigial form in the radically reduced aquatic genus Isoetes, which has a single cambium that produces a mixture of xylem and phloem to the inside and parenchymatous cortical tissue to the outside. Some Lepidodendrales also showed a convergent origin of functionally seed-like structures (Lepidocarpon), derived by envelopment of the megasporangium by the sporophyll.
The most important innovation of the euphyllophyte clade, seen in stem relatives such as the Early Devonian genus Psilophyton, was differentiation of one or more orders of main axes with dichotomous lateral branches, as a result of unequal dichotomy of the apical meristem (Banks et al. 1975)—a prime example of the telome process of overtopping. Another innovation was longitudinal dehiscence of the sporangia, that is, opening along a slit down one side, a feature retained in most later euphyllophytes, as illustrated by the pollen sacs of angiosperms. A synapomorphy seen in living euphyllophytes is a shift from biflagellate to multiflagellate sperm. Psilophyton had a round xylem cylinder, but this had become lobed (giving an actinostele) in Pertica (Gensel 1984) and basal crown euphyllophytes.
The dichotomous lateral branches of Psilophyton have often been interpreted as illustrating a stage in the origin of leaves, the next steps being planation and establishment of dorsiventral (adaxial-abaxial) polarity. However, later euphyllophytes suggest that leaves were derived in more than one way from structures in a Psilophyton-like ancestor, such that leaves in different lines have different homologies (Beck 1970; Doyle 1998; Galtier 2010). Either individual ultimate dichotomous branches could become simple, dichotomously organized leaves, or whole branch systems could be modified into pinnately compound leaves, with leaflets derived from the dichotomous ultimate branches. Leaves of the first sort might be homologous with leaflets of the second sort (setting aside later fusion of leaflets and other complications). Both types of leaves have been traditionally described as megaphylls, an unfortunate term that glosses over their presumably different origins (Tomescu 2009). Evolution of a continuous lamina with dichotomous venation, as a result of origin of a marginal meristem, occurred many times in leaves of both types (Boyce & Knoll 2002).
The finding that the living members of the monilophyte clade included not only ferns but also sphenophytes (with whorled simple leaves) and Psilotales (with one-veined or nonvascularized simple leaves and no roots) has been regarded as an unexpected result of molecular studies (Pryer et al. 2001). However, it had been anticipated by the morphological cladistic analysis of Kenrick and Crane (1997) and earlier suggestions that both ferns and sphenophytes were derived from Middle and Late Devonian “cladoxylopsids,” which had branch systems with dichotomous ultimate appendages and a stele with lobed or subdivided xylem (Scheckler 1974; Stein et al. 1984). Ironically, the clearest morphological synapomorphy of monilophytes is an obscure feature not seen in living ferns but characteristic of cladoxylopsids and some Paleozoic plants thought to be early ferns, including the Late Devonian genus Rhacophyton, Carboniferous “zygopterids,” and Ankyropteris, a probable stem relative of the main living fern order Filicales—an area of parenchymatous protoxylem near the tip of each lobe of the xylem, surrounded by a “peripheral loop” of metaxylem. If these fossils are related to living ferns, this character was lost or modified beyond recognition in the latter. However, it may be represented by the protoxylem canals of Equisetum and related Paleozoic sphenophytes (Equisetales), modified by stretching of the internodes due to intercalary meristematic growth.
A relationship of cladoxylopsids and zygopterids to living ferns was rejected by Rothwell and Nixon (2006), based on a morphological and molecular analysis, in which molecular data prevailed in uniting living members of the monilophyte clade, but cladoxylopsids and zygopterids formed a basal grade below all living vascular plants. However, this may be a result of incorrect rooting of vascular plants: lycophytes were linked with seed plants, whereas most other analyses place lycophytes at the base of vascular plants and unite monilophytes and seed plants. This may be a result of insufficient taxon sampling, especially the lack of fossils such as Cooksonia and zosterophylls as outgroups of lycophytes. If all groups in the tree of Rothwell and Nixon (2006) except Aglaophyton are rerooted between lycophytes and the remaining groups, cladoxylopsids and zygopterids are linked with crown monilophytes, roughly consistent with the view of Kenrick and Crane (1997).
The most important evolutionary innovation in monilophytes was evolution of the compound leaf (frond) of ferns. Another innovation was the whorled phyllotaxis of sphenophytes, which also occurred in some members of the cladoxylopsid grade (Berry & Stein 2000; Soria & Meyer-Berthaud 2003; Cordi & Stein 2005). In sphenophytes, available data support the view that leaves were derived from single dichotomous branches like those of Psilophyton, or the more leaf-like ultimate appendages of cladoxylopsids. Although Equisetum and its closest fossil relatives, including the Carboniferous tree genus Calamites, have one-veined leaves (typologically microphylls), other sphenophytes had leaves that were more like dichotomous branches. Examples include the wedge-shaped, dichotomously veined leaves of Sphenophyllum and the unwebbed dichotomous leaves of Archaeocalamites, a basal member of the Equisetales. However, Ophioglossales, Marattiales, and Filicales have basically compound leaves more plausibly derived from whole branch systems bearing dichotomous appendages.
A bizarre but potentially significant feature of some cladoxylopsids (e.g., Arachnoxylon), Rhacophyton, Carboniferous zygopterids, and the Carboniferous genus Stauropteris is quadriseriate branching, with secondary axes or pinnae produced in alternating pairs perpendicular to the rachis (Cornet et al. 1976; Stein 1981; Phillips & Galtier 2005; Galtier 2010). In Rhacophyton, Cornet et al. (1976) showed various degrees of reduction of one pinna per pair, and they suggested that reduction of this sort led to the biseriate (pinnate) pinna arrangement of living ferns. A connection of such fossils with Filicales in particular is supported by Ankyropteris, which had normal biseriate pinnae but resembled zygopterids in having an H-shaped vascular strand with peripheral loops in the petiole. These considerations suggest that planation proceeded from higher to lower orders (Galtier 2010). First, the ultimate appendages became biseriate, resulting in pinnae with two rows of pinnules, but pinnae were still borne in a quadriseriate arrangement. Second, the whole frond became planated by a shift from quadriseriate to biseriate pinnae. Such a scenario might never be suspected without fossil evidence. This hypothesis requires further phylogenetic testing, since the only analysis to include relevant taxa is that of Rothwell and Nixon (2006), which linked Ankyropteris and Filicales but separated them from groups with quadriseriate fronds. There is no evidence on the mode of origin of the leaves of Marattiales and Ophioglossales, since these groups had planated fronds as far back as they are known (Late Carboniferous for Marattiales, earliest Tertiary for Ophioglossales: Rothwell & Stockey 1989), and they have not been associated with more primitive fossil relatives.
Psilotales, traditionally associated with rhyniophytes because of their dichotomous shoot organization and lack of roots, are strongly linked with Ophioglossales by molecular data (Manhart 1995; Pryer et al. 2001; Rothwell & Nixon 2006). Possible morphological synapomorphies are axial mycotrophic gametophytes and fertile appendages with an adaxial sporangium-bearing portion (Doyle 1998). The fact that young appendages of Psilotum resemble primordia of fern fronds (Kaplan 1977, 2001) is consistent with the view that they were reduced from fronds. Although the lack of roots in Psilotales has been considered primitive (e.g., Bremer 1985), current phylogenies indicate that it is a result of loss.
In contrast to living monilophytes, some Paleozoic forms had secondary growth, or possible precursor conditions. Tree sphenophytes (Calamites) had extensive secondary wood, and a bifacial cambium is well documented in Sphenophyllum (Eggert & Gaunt 1973; Cichan 1985). Secondary xylem has also been reported in some cladoxylopsids, Rhacophyton, and zygopterid ferns, but it is unusual in being limited in amount. In cladoxylopsids this tissue has been interpreted as aligned metaxylem, as in extant Ophioglossales (Rothwell & Karrfalt 2008), because it lacks rays (Scheckler 1974). However, rays are known in Rhacophyton and Zygopteris (Dittrich et al. 1983; Phillips & Galtier 2005). Banks et al. (1975) reported aligned metaxylem in larger stems of Psilophyton. Expanding on a suggestion of Kenrick and Crane (1997), this might mean that a first step toward secondary growth, namely, periclinal divisions of tracheid initials, occurred on the stem lineage of euphyllophytes. This is of minor consequence for living monilophytes, since even if their ancestors had secondary growth it must have been subsequently lost.
In the main fern clade, Filicales, the most conspicuous innovation is the leptosporangium (hence their name “leptosporangiate ferns”). This differs from the eusporangium of other groups in its smaller size, fewer spores, narrow stalk, thin wall, and a row of thick-walled cells, the annulus, which corresponds to a patch of cells in Ankyropteris and other Carboniferous stem relatives. Current phylogenies (Pryer et al. 1995) confirm the traditional view that the sporangium underwent a shift from longitudinal to transverse dehiscence within Filicales. This trend culminated in the famous snapping dehiscence of Polypodiaceae sensu lato, which contrary to older views form a clade. Another innovation within Filicales was reduction of the number of jacket cells in the antheridium, which may have occurred once or twice (Pryer et al. 1995). Heterospory evolved in water ferns (Marsileaceae and Salviniaceae), which were previously interpreted as two unrelated lines but now appear to form a clade.
1.6 Innovations on the Line to Seed Plants: “Progymnosperms” and “Seed Ferns”
Two major paleobotanical advances in the last century shed new light on the origin of seed plants (spermatophytes) and their evolutionary innovations, which include not only the seed but also secondary growth. First was the recognition of “seed ferns” or “pteridosperms” (Oliver & Scott 1903), based on the association of fern-like pinnately compound leaves with woody stems and seeds in the Late Carboniferous genus Lyginopteris. This discovery showed that early seed plants had a leaf type not retained in any living members, while later studies of more primitive seeds from the Late Devonian and Early Carboniferous (Long 1966, 1975; Pettitt & Beck 1968; Serbet & Rothwell 1992) revealed apparent steps in the origin of the seed.
Second was recognition of the “progymnosperms,” based on association of woody trunks and leafy branch systems with sporangium-bearing appendages in the Late Devonian tree Archaeopteris and Middle and Late Devonian “aneurophytes” (Beck 1960, 1970, 1971; Scheckler & Banks 1971a). If these fossils are stem relatives of seed plants, as confirmed by morphological cladistic analyses (Doyle & Donoghue 1986; Rothwell & Serbet 1994; Hilton & Bateman 2006), they show that the first major seed plant innovation was not the seed. Rather it was secondary growth, with secondary xylem and phloem produced indefinitely by a bifacial vascular cambium and periderm produced by a cork cambium. The resulting origin of large trees with a woody trunk marked another step in the trend for elaboration of the sporophyte generation. The clade including both progymnosperms and seed plants has been called lignophytes, after the secondary wood. Origin of a cork cambium was an extension of the original role of periderm as a wound-repair device and was clearly an adaptation to distension and splitting of the epidermis and cortex due to growth of the vascular cylinder.
Progymnosperms also clarify the origin of the original fern-like leaf of seed plants. The more primitive aneurophytes had three-dimensional branch systems, with spiral or opposite-decussate secondary branches bearing dichotomous appendages. In Archaeopteris, however, the secondary axes were in one plane, resulting in a branch system that was originally misinterpreted as a fern-like frond. In all these groups the ultimate appendages, usually called leaves, were spiral or decussate. Such branch systems could be transformed into fronds of the seed fern type by planation, with the primary axis becoming the rachis, secondary axes becoming pinna rachises, and ultimate appendages becoming leaflets (Beck 1970; Doyle 1998; Galtier 2010).
In contrast to ferns, there is no sign that planation occurred first in higher order branches—in fact, the combination of spiral leaves and biseriate secondary axes in Archaeopteris might suggest the opposite sequence. However, the related genus Svalbardia had three-dimensional branch systems, suggesting that planation occurred independently in Archaeopteris and seed plants (the alternative, that seed plants are more closely related to Archaeopteris than to Svalbardia, is less likely because the two genera share anatomical advances not seen in basal seed plants, such as grouped pitting). This reasoning implies that seed plants are from an ancestor with branch systems like those of aneurophytes rather than Archaeopteris. Late Devonian and Carboniferous seed ferns differed from both ferns and Archaeopteris in showing more or less extensive dichotomy of the frond rachis (Walton 1931; Serbet & Rothwell 1992; Galtier 2010), but dichotomy of main axes is known in at least one aneurophyte, Proteokalon (Scheckler & Banks 1971b).
This scenario implies that origin of the seed plant leaf proceeded first by overtopping of simple dichotomous appendages, at the level of basal euphyllophytes, and then by planation of a whole branch system bearing such leaves into a compound leaf, between progymnosperms and seed ferns. It is suggestive that two gene families that specify adaxial-abaxial polarity in angiosperms, KANADI and Class III HD-Zip, occur throughout vascular plants, whereas the YABBY family is known across seed plants but has not been identified in ferns or more basal groups (Floyd & Bowman 2007). This raises the possibility that KANADI and Class III HD-Zip genes were involved in the initial overtopping seen in basal euphyllophytes, but YABBY genes were involved in the subsequent planation of branch systems into fronds in seed plants, which was independent of that in ferns (Sarojam et al. 2010).
Another major innovation of seed plants was axillary branching, a fundamental aspect of the architecture of living seed plants. Its exact point of origin is unclear, partly because early seed plants rarely branched, but it is known in Lyginopteris (Brenchley 1913) and the Early Carboniferous genus Calamopitys (Galtier & Holmes 1982; Galtier 1988). A less commonly noted synapomorphy of seed plants is the presence of pointed cataphylls (scale leaves) at the bases of shoots and around buds, in addition to fronds. These may represent leaf primordia whose development was arrested before production of pinnae.
Inside the stem, the most conspicuous innovation of living seed plants is the eustele, with a ring of primary vascular bundles around a pith. A series of intermediates in basal seed ferns show steps in origin of a eustele from a lobed protostele (actinostele), as in aneurophytes, by differentiation of the central tissue into parenchyma rather than xylem, leaving the lobes as separate vascular strands (Beck 1970). Archaeopteris also had a eustele, but the fact that basal seed ferns had actinosteles implies that this was a convergence, like planation of the branch systems. A eustele was firmly established in crown group seed plants, but its number of origins is unclear. Lyginopteris had a eustele, but medullosans, which all analyses place nearer the crown group, had either an actinostele (Quaestora) or a special type of eustele with tangentially elongate primary vascular strands and internal as well as external secondary wood (Basinger et al. 1974).
The first major reproductive innovation in lignophytes was heterospory, an essential step toward the seed. This step is seen in Archaeopteris, where it is the main evidence that this group was more closely related to seed plants than were aneurophytes (Doyle & Donoghue 1986; Hilton & Bateman 2006). In Archaeopteris, there was no external difference between either the microsporangia and megasporangia or the fertile appendages bearing them, which were dichotomous structures arranged like leaves on secondary axes of a branch system.
The key process in origin of the seed itself, or more precisely its immature stage, the ovule, was formation of an integument (future seed coat) around the megasporangium (nucellus), with a micropyle at the apex for pollen capture. Evidence on this process comes from Late Devonian (Elkinsia) and Early Carboniferous seed ferns (Long 1966, 1975; Rothwell & Scheckler 1988; Serbet & Rothwell 1992), which appear to attach to the seed plant stem lineage (Doyle & Donoghue 1986; Rothwell & Serbet 1994; Hilton & Bateman 2006). In these fossils the megasporangium was surrounded by a ring of integument lobes that show every degree of fusion from the base up, culminating in a complete integument with a typical micropyle. Reduction to one functional megaspore had occurred by this point. The lobes have been interpreted as outer telomes of a dichotomous branch (Andrews 1963) or sterilized outer sporangia of a synangium (Kenrick & Crane 1997), but these alternatives are not as different as they may seem, since synangia would presumably represent modified fertile telomic branches. The ovules were borne in so-called cupules, which were dichotomous structures resembling two facing hands with ovules on the palms. These cupules have been homologized with the similarly constructed fertile appendages of progymnosperms; in at least one aneurophyte, Tetraxylopteris (Bonamo & Banks 1967), the sporangia were in clusters that could be transformed into ovules (Kenrick & Crane 1997). In the Late Carboniferous genus Lyginopteris, the cupule had been simplified to several lobes around a single ovule.
Subsequent evolution of the ovule presents a striking story of transfer of function. In living gymnospermous seed plants, the pollen is caught by a pollination drop exuded from micropyle, but this was impossible before fusion of the integument lobes. Instead the pollination drop was exuded by an extension of the nucellus, called the lagenostome or salpinx, in which the epidermis separated from the inner tissue to form a central column surrounded by a pollen chamber (hydrasperman reproduction: Rothwell & Scheckler 1988). At first the pollen did not differ externally from the trilete spores of “lower” plants, with a trilete scar that presumably opened to release motile sperm that swam in liquid in the pollen chamber to archegonia at the apex of the female gametophyte (Chaloner 1970). The female gametophyte, inside the megaspore membrane, was relatively large, but the archegonia had been further simplified by reduction of the number of neck cells. The distinctive male gametophyte of living seed plants, with two sperm cells and a row of sterile cells, presumably evolved from a gametophyte with a larger number of cells. Its point of origin is unclear, but two sperm cells have been described in the pollen of medullosan seed ferns (Stewart 1951). In medullosans and crown group seed plants, the cupule was apparently lost and the ovule came to be borne directly on a leaf.
Another innovation of crown group seed plants that has been less discussed is the typical embryo, in which the root apex is located at the opposite pole from the shoot apex, rather than in a lateral position, as in other vascular plants. Understanding the timing and mode of its origin is prevented by the absence of mature embryos in Carboniferous seeds (Rothwell 1988). Embryos of a modern type are known in conifers near the Carboniferous–Permian boundary (Mapes et al. 1989), but these were already in the crown group.
1.7 Innovations within Seed Plants, Especially Conifers
Tracing evolutionary innovations within seed plants is hampered by uncertain relationships among clades, especially angiosperms and Gnetales, the two most derived groups. Prior to cladistic analyses (e.g., Chamberlain 1935), seed plants were widely assumed to be diphyletic, with the seed originating independently in “cycadophytes” (cycads, seed ferns, and fossils such as Bennettitales) and “coniferophytes” (conifers, ginkgophytes, and Paleozoic cordaites). This was thought to be reflected in the symmetry of the seeds: radial in cycadophytes (radiospermic), and bilateral or biradial in coniferophytes (platyspermic). After the recognition of progymnosperms, Beck (1966) argued that cycadophytes were related to aneurophytes, while coniferophytes were related to Archaeopteris. In this view, the fronds of seed ferns were derived from whole branch systems but the simple leaves of coniferophytes were derived from dichotomous ultimate appendages of the Archaeopteris type. However, these schemes were contradicted by morphological cladistic analyses, which nested coniferophytes within seed plants (Crane 1985; Doyle & Donoghue 1986; Nixon et al. 1994; Rothwell & Serbet 1994; Doyle 1996, 2006; Hilton & Bateman 2006; Rothwell et al. 2009). Furthermore, although molecular data do not directly address relationships between living taxa and progymnosperms, they refute the classic diphyletic hypothesis by failing to split seed plants into cycadophytes and coniferophytes (except Mathews et al. 2010).
Most morphological cladistic analyses have linked angiosperms and Gnetales, along with Mesozoic Bennettitales and Pentoxylon, in a clade called anthophytes because its members have more or less flower-like reproductive structures—an updated version of the anthostrobilus hypothesis of Arber & Parkin (1907). Some analyses placed anthophytes among “seed ferns” such as Permian glossopterids and Mesozoic corystosperms and Caytonia (Crane 1985; Doyle & Donoghue 1986; Hilton & Bateman 2006), which had often been proposed as angiosperm relatives (Gaussen 1946; Stebbins 1974; Doyle 1978; Retallack & Dilcher 1981). Others, however, related the anthophytes to coniferophytes (Nixon et al. 1994; Rothwell & Serbet 1994; Rothwell et al. 2009).
In contrast, only a few molecular analyses, of ribosomal genes, have linked angiosperms and Gnetales, with weak support (Hamby & Zimmer 1992; Stefanovic et al. 1998; Rydin et al. 2002). Most multigene analyses (reviewed in Mathews 2009) have either nested Gnetales within conifers, with Pinaceae (the “gnepine” hypothesis: Qiu et al. 1999, 2007; Bowe et al. 2000; Chaw et al. 2000; Hajibabaei et al. 2006), or placed them at the base of seed plants (e.g., Rai et al. 2008). The latter result is implausible from a geological point of view, since Gnetales and angiosperms were the last major groups to appear in the fossil record. There is evidence that trees with Gnetales basal are a result of long branch attraction, where homoplastic changes on lines with large amounts of molecular evolution lead to incorrect inferences on relationships. Those data sets that give Gnetales-basal trees when analyzed with parsimony place Gnetales within conifers when analyzed with likelihood methods, which are thought to correct for long branch effects (Sanderson et al. 2000; Magallón & Sanderson 2002; Soltis et al. 2002; Burleigh & Mathews 2004). The conflict with morphology may be less severe than is often thought. Views that Gnetales are related to conifers rather than angiosperms were common before cladistics (Bailey 1944, 1949; Eames 1952; Bierhorst 1971; Doyle 1978), and in the morphological analysis of Doyle (2008) trees with the two positions of Gnetales became equally parsimonious after inclusion of recently described conifer-like features in Gnetales (e.g., Carlquist 1996).
In most molecular analyses that place Gnetales in conifers, angiosperms are the sister group of living gymnosperms, with the result that gymnosperms, long assumed to be paraphyletic, form a monophyletic group. However, this conclusion applies only to living gymnosperms. All morphological analyses that include fossils, including those with living taxa constrained to a molecular arrangement (Doyle 2006, 2008), place a series of seed fern taxa, which are typologically gymnosperms, below the crown seed plant clade. With this in mind, the name acrogymnosperms has been proposed for the clade of living gymnosperms (Cantino et al. 2007). The greatest uncertainty concerns cycads, which different analyses have placed at the base of acrogymnosperms, with Ginkgo, or with angiosperms (Mathews et al. 2010).
Setting aside angiosperms, the most striking innovations within crown group seed plants occur in coniferophytes. Coniferophytes differ markedly from seed ferns in their simple leaves: fan-shaped or strap-shaped with dichotomous venation in ginkgophytes and cordaites, scale-like or needle-like with one vein in most conifers (conifers with several veins, such as some Araucariaceae and Podocarpaceae, appear to be derived, judging from their nested positions). The idea that coniferophytes were derived from a seed fern prototype was proposed by Rothwell (1982), based on recognition of conifer-like platyspermic seeds and saccate pollen (as in cordaites, Paleozoic conifers, and living Pinaceae and Podocarpaceae) in the Late Carboniferous seed fern Callistophyton. Given that seed ferns had compound fronds, this would require a radical change in leaf morphology, which Rothwell suggested could have been a result of heterochrony, a change in the timing of developmental events. Like other seed ferns, Callistophyton had not only fronds but also pointed cataphylls—bud scales at the base of the axillary branches. Rothwell suggested that the leaves of conifers might be derived not from fronds but from cataphylls, which were already essentially like conifer leaves, if the plant continued to produce cataphylls throughout its life, without ever shifting to fronds. This hypothesis explains the leaves of conifers better than the dichotomously veined leaves of cordaites and ginkgophytes, although it might be a smaller step to derive leaves of the latter sort from cataphylls than from large fronds.
Origin of coniferophyte reproductive structures also involved a change from fertile fronds, like those of Callistophyton, which had microsporangia or ovules on the abaxial surface, to simple sporophylls. In ginkgophytes these sporophylls were apparently grouped into simple male and female strobili. The female strobili were reduced to a stalk with two sessile ovules in Ginkgo but still bore several ovules in Mesozoic fossils (Zhou & Zhang 1992; Zhou 2009). In contrast, cordaites had compound strobili consisting of an axis bearing bracts and axillary short shoots (simple strobili) with scale leaves and simple sporophylls. In conifers the male cones are simple strobili with scale-like sporophylls, but the female cones have woody cone scales bearing one or more ovules. Because each cone scale is subtended by a bract, there had been suggestions since the 1800s that the cone scales are axillary branches and the whole cone is derived from a compound strobilus, as in cordaites (Worsdell 1900). This was confirmed by studies of Late Carboniferous and Permian conifer stem relatives by Florin (1951, 1954), who showed that the female cones had bracts and obvious axillary short shoots bearing scale leaves and sporophylls. Modern cone scales would be derived by transformation of the fertile short shoot into a woody scale; Florin described intermediate conditions in Permian and Triassic conifers, although some of the details have required modification (Mapes & Rothwell 1984; Clement-Westerhof 1988).
These comparisons have often been taken as evidence for derivation of conifers from cordaites. The simple organization of the conifer male cone, however, suggests that the two groups were derived from a common ancestor that had both simple male and simple female strobili. Both types of strobili would be grouped into compound strobili in cordaites, but only the female strobili in conifers. However, this picture is complicated by recognition that there were Late Paleozoic conifers with compound male strobili (Thucydia: Hernandez-Castillo et al. 2001).
Platyspermic seeds occur not only in Callistophyton and coniferophytes (best seen in cordaites) but also in Permian and Mesozoic “seed ferns,” including peltasperms, corystosperms, glossopterids, and Caytonia. Rothwell and Serbet (1994) questioned the distinction between platyspermy and radiospermy, but it can be made fairly consistently if defined in terms of anatomy (Doyle 1996). The shift from radiospermic to platyspermic seeds marks a clade called platysperms, which may be equivalent to crown group seed plants, depending on the position of Callistophyton and cycads. Many analyses that include fossils have nested cycads within platysperms, which would imply that their classic radiospermic seeds are a reversal, a conclusion consistent with the bilateral anatomy of seeds of Cycas (Stevenson 1990).
Other innovations of crown group seed plants involved modifications of the original spore-like pollen of more basal seed plants. One was origin of saccate pollen, with a single tire-like air sac or two sacs, formed by separation of the inner and outer layers of the exine. Today sacs are restricted to the conifer families Pinaceae and Podocarpaceae, but in the past they also occurred in Callistophyton, cordaites, Paleozoic conifers, some peltasperms (Autunia), corystosperms, glossopterids, and Caytonia. Whether sacs evolved once and were lost in the many nonsaccate seed plant taxa or arose several times is unclear because of uncertainty on the position of various fossils near the crown group node. However, the phylogenetic trees of Doyle (2008) imply that sacs are homologous in Callistophyton and other members of the acrogymnosperm line and were lost in Ginkgo, Araucariaceae, other conifers, and Gnetales (Doyle 2010). Experimental studies show that the sacs function to float the pollen upward in the liquid in the micropylar canal to the nucellus of the ovule, which is oriented downward, while their loss is correlated with a shift to upward-oriented ovules (Doyle 1945; Leslie 2010).
Another pollen innovation, also seen in Callistophyton, was the origin of a sulcus, an elongate thin area that serves as the site of germination of a pollen tube (actually known in Callistophyton: Rothwell 1972), correlated with loss of the proximal tetrad scar. Some cordaites and Paleozoic conifers had a tetrad scar and no sulcus, interpreted by Poort et al. (1996) as representing a stage before origin of a pollen tube. However, the nested position of these taxa in most analyses makes it more parsimonious to assume that the sulcus was lost. This may seem implausible, especially if one assumes that loss of the sulcus would imply loss of the pollen tube. This could mean that the sulcus was lost but the tube was retained, that the tube evolved once but a sulcus originated later in several lines (Doyle & Donoghue 1986; Friedman 1993), or that cordaites and conifers are more basal than current analyses indicate.
A final related innovation was a shift from swimming to nonmotile sperm, correlated with a change in function of the pollen tube: from anchoring the male gametophyte and absorption of nutrients (haustorial pollen tube, as in cycads and Ginkgo) to transfer of sperm to the archegonia (siphonogamy, as in living conifers, Gnetales, and angiosperms). The long, slender pollen tube of Callistophyton has been taken as evidence for siphonogamy (Rothwell 1981; Nishida et al. 2004), but Taylor (1988) and Friedman (1993) considered the function of the tube to be unknown. In acrogymnosperms, if ginkgophytes are sister to fossil and living conifers (Doyle 2008) and Paleozoic conifers were zooidogamous (Poort et al. 1996), siphonogamy originated on the line leading to living conifers and Gnetales.
Additional innovations evolved in Gnetales, many of them convergences with angiosperms if Gnetales are nested in conifers, such as presence of an outer tunica layer in the apical meristem and cellular embryogeny. Some would be interpreted as convergences with angiosperms even if the two groups were related, as noted in morphological cladistic studies (Doyle & Donoghue 1986; Doyle 1996), such as the angiosperm-like leaves of Gnetum, considering the scale-like and strap-shaped leaves of Ephedra and Welwitschia, which are consistent with a position in conifers, and vessels in the wood, which apparently arose within angiosperms. Although the reproductive units making up the strobili have been compared with flowers, they can also be interpreted as axillary shoots of a compound strobilus, like that of cordaites and Paleozoic conifers, with the “perianth” of the male “flower” and the outer integument around the ovule derived from sterile scale leaves on the axillary fertile shoot (cf. Eames 1952; Doyle 1994). The details need further examination in light of developmental evidence that the male structures are more complex than previously assumed (Mundry & Stützel 2004).
1.8 Origin of Angiosperms and Their Innovations
Phylogenetic analyses show a marked contrast between strong evidence on relationships within angiosperms and great uncertainty on their closest relatives. Early morphological analyses appeared to narrow the list of outgroups to Gnetales, Bennettitales, and Pentoxylon, but some linked the resulting anthophyte clade with corystosperms, glossopterids, and Caytonia (Crane 1985; Doyle & Donoghue 1986), while others nested the clade in coniferophytes (Nixon et al. 1994; Rothwell & Serbet 1994; Rothwell et al. 2009). Some later studies diluted the anthophyte concept by moving Caytonia up to a position as the sister group of angiosperms (Doyle 1996, 2006, 2008; Hilton & Bateman 2006). However, molecular analyses that place Gnetales in the conifers break up the anthophytes still more, and if angiosperms and acrogymnosperms are sister groups, no living gymnosperm taxon is closer to the angiosperms than any other, so any evidence on the origin of angiosperm innovations must come from fossils. Determining that cycads are sister to angiosperms (Mathews et al. 2010) would not help much, as cycads share few innovations with angiosperms. In either case, the angiosperm line would have diverged in the Carboniferous, since the oldest known acrogymnosperms are Late Carboniferous (cordaites, early conifers) and the oldest known cycads are Early Permian.
This picture offers interesting parallels and contrasts with the situation in vertebrates, where the lines leading to living mammals and reptiles (including birds) also diverged in Carboniferous. However, there is a long series of uncontested fossil stem relatives attached to the mammalian stem lineage, the so-called mammal-like reptiles (Gauthier et al. 1988). In contrast, there is no consensus among paleobotanists that any known fossils are angiosperm stem relatives. For example, glossopterids, Pentoxylon, Bennettitales, and Caytonia were identified as stem relatives in the morphological analysis of Doyle (2008) when living taxa were constrained to a molecular arrangement, with Gnetales in conifers, and in some trees obtained without constraints. However, glossopterids and Caytonia were far removed from angiosperms in morphological analyses of Rothwell et al. (2009), in which angiosperms were sister to Gnetales and nested in conifers (a result strongly contradicted by molecular data). Both studies associated Bennettitales and Pentoxylon with angiosperms, but reproductive structures of these taxa are highly modified, so even if they are related to angiosperms they shed little light on the origin of angiosperm reproductive features.
In contrast, although morphological cladistic analyses varied greatly on rooting of the angiosperms (Dahlgren & Bremer 1985; Donoghue & Doyle 1989; Loconte & Stevenson 1991; Nixon et al. 1994; Doyle 1996, 2006), molecular analyses of many separate and combined genes have given remarkably consistent results (Mathews & Donoghue 1999; Parkinson et al. 1999; Qiu et al. 1999, 2006a, 2010; Soltis et al. 1999, 2000; Barkman et al. 2000; Graham & Olmstead 2000; Zanis et al. 2002; Jansen et al. 2007; Moore et al. 2007). The so-called ANITA lines, namely, the New Caledonian endemic Amborella, Nymphaeales (water lilies), and Austrobaileyales, form a grade at the base. The remaining groups, called mesangiosperms, form five major clades: Chloranthaceae (notable for their highly simplified flowers), the reduced aquatic genus Ceratophyllum, magnoliids, monocots, and eudicots (united by tricolpate pollen). Rootings on Ceratophyllum (based on rbcL: Chase et al. 1993) and grasses (Goremykin et al. 2003) appear to be effects of long branch attraction and inadequate taxon sampling (e.g., Qiu et al. 2001; Degtjareva et al. 2004; Soltis et al. 2004). The main uncertainty concerns whether Amborella and Nymphaeales form two successive basal lines or a clade (Barkman et al. 2000; Qiu et al. 2006a, 2010; Goremykin et al. 2009), with the latter arrangement supported especially by mitochondrial genes. Relationships among the mesangiosperm clades remain poorly resolved, but relationships within them are largely stable. These results make it possible to reconstruct many features of the ancestral angiosperms by character optimization, independent of assumptions about outgroups (e.g., any state shared by all three ANITA lines can be reconstructed as ancestral).
Besides the flower and other reproductive advances, angiosperms share major vegetative innovations, the most obvious being in the leaves. These can be reconstructed as originally simple, with pinnate major venation and a hierarchy of reticulate higher vein orders (Doyle 2007), so their evolution must have involved several changes from the ancestral seed fern leaf, which had leaflets with a midrib and one order of dichotomous fine venation. If glossopterids, Pentoxylon, Bennettitales, and Caytonia are angiosperm stem relatives, the first step toward the angiosperm leaf would be a marked simplification to the “simple pinnate” type (Doyle & Donoghue 1986) seen in the first three of these taxa. This type includes both simple leaves with a midrib and one order of secondary veins, as in Pentoxylon and glossopterids, and once-compound leaves with a rachis bearing leaflets with only one order of dichotomous or parallel venation, as in modern cycads, both of which occurred in Bennettitales and fossil cycads (Caytonia had four leaflets that resemble glossopterid leaves; if it is nested within this clade, its leaves are presumably secondarily compound). This shift would occur earlier if cycads are located below these fossils (Mathews et al. 2010). In addition, glossopterids and Caytonia had simple reticulate venation, with a network consisting of one vein order, suggesting that this may have been a first step toward the complex reticulate venation of angiosperms. If so, it must have been followed by elaboration of several vein orders, a change ascribed to a shift from marginal to diffuse meristematic activity (Doyle & Hickey 1976; Boyce & Knoll 2002; Boyce 2005; Doyle 2006).
Another innovation that had great physiological and ecological consequences was the origin of vessels from tracheids, with perforations formed by loss of the primary cell wall between vessel members, rather than pits. This apparently occurred within angiosperms, since vessels are lacking not only in putative outgroups (assuming Gnetales are in conifers) but also in Amborella and Nymphaeales (the latter have transitional conducting cells with porose pit membranes that have been called vessels but may be better considered tracheids: Carlquist & Schneider 2009). The famous cases of vessel-less wood in Winteraceae (magnoliids) and Trochodendraceae (eudicots), which are deeply nested within angiosperms, are most parsimoniously interpreted as due to reversal (Doyle & Endress 2000; Feild et al. 2002).
The angiosperm flower is often regarded as the signature innovation of the group, but it is not easy to define how it differs from the strobili of other seed plants (Doyle 2008; Rudall & Bateman 2010). It is unusual in having a much shorter axis relative to the length of the appendages, as also seen in so-called flowers of Bennettitales, presumably an adaptation for attraction of pollinators. The presence of a sterile perianth (reconstructed as present at the crown group node: Endress & Doyle 2009) is not a radical advance, since there are sterile appendages at the base of the strobili in many other seed plants (Doyle 2008), although their elaboration for attraction may have been new. More distinctive is bisexual organization, which is unique among living plants but did occur in some Bennettitales. However, it is not certain whether bisexuality was ancestral in angiosperms. Because Amborella and other members of the basal angiosperm grade (Hydatellaceae, Trimeniaceae, Schisandraceae) are unisexual and others are bisexual, the ancestral state is equivocal on parsimony grounds, although the presence of nonfunctional stamens in the female flowers of Amborella suggests it was bisexual (Endress & Doyle 2009). Possible developmental genetic mechanisms for the transition from separate male and female strobili to a bisexual strobilus have been the subject of much discussion (Frohlich & Chase 2007).
Both the anthostrobilus hypothesis of Arber and Parkin (1907) and the anthophyte hypothesis postulated that the flower originated well before the angiosperms, in their common ancestor with Bennettitales and Gnetales. However, if Caytonia is the sister group of angiosperms and Bennettitales are the second outgroup, flowers either arose separately in angiosperms and Bennettitales or were lost in Caytonia, since Caytonia had relatively large sporophylls that are unlikely to have been closely aggregated (Doyle 2008). Of these, the former scenario may be more plausible on functional grounds.
In the fertile parts of flower, the best-known innovation is enclosure of the ovules in the carpel, or angiospermy. However, the ovules themselves differ from those of other seed plants in being bitegmic (having two integuments), except in some derived taxa (e.g., most asterids), and usually anatropous (reflexed). Presumably the nucellus and the inner integument represent the original seed plant ovule, while the outer integument had a different origin. Hypotheses on the origin of the carpel and its homologies with structures in other groups should also explain the bitegmic ovule.
Molecular data on relationships within angiosperms have led to important new insights on the ancestral carpel. Formerly it was widely thought that the most primitive carpels were plicate (conduplicate), like a leaf folded down the middle, as in magnoliids such as Degeneria and Winteraceae (Bailey & Swamy 1951). Such carpels were often described as unsealed, but actually they are closed by postgenital fusion of the margins of the U-shaped carpel primordium (Igersheim & Endress 1997; Endress & Igersheim 2000). However, the basal ANITA groups have carpels of the ascidiate type, considered primitive by Leinfellner (1969) and van Heel (1981). Here there is a cross-zone of meristematic tissue between the margins of the primordium, and the whole structure grows up like a tube. The carpel is sealed by secretion in the narrow canal leading from the stigma to the ovary (Endress & Igersheim 2000)—what might be considered an incompletely angiospermous condition. The number and position of ovules vary among near-basal groups, but optimization of characters on molecular trees (Endress & Doyle 2009) indicates that the ancestral carpel had a single pendent ovule attached to the cross-zone, as in Amborella, Hydatellaceae (Nymphaeales), and Trimenia (Austrobaileyales).
During the development of anatropous bitegmic ovules in the ANITA grade and magnoliids, the inner integument grows up from a ring around the nucellus, but the outer integument grows over from one side like a hood (Umeda et al. 1994; Imaichi et al. 1995; Igersheim & Endress 1997; Yamada et al. 2001a, 2003). The ovule of Amborella is orthotropous (erect rather than reflexed) but dorsiventral early in development, suggesting derivation from an anatropous ancestor (Yamada et al. 2001b). There is evidence that the outer integument is a leaf-like structure with the nucellus and inner integument on its adaxial surface. First, when there are vascular bundles in the outer integument, the xylem is to the inside, implying that this side is adaxial (Frohlich & Chase 2007). Second, in Arabidopsis the gene INO, a member of the YABBY family, which specifies abaxial identity and was implicated above in origin of the seed plant leaf from a progymnosperm branch system, is expressed in the outer epidermis of the outer integument (Balasubramanian & Schneitz 2000; Meister et al. 2002; Skinner et al. 2004) but not in the inner integument (McAbee et al. 2006). This is consistent with the view that the two integuments have different origins and the outer integument is leaf-like (Skinner et al. 2004; McAbee et al. 2006; Doyle 2008).
More specific ideas on homologies of the outer integument have centered on the cupules of various “seed fern” groups, which appear to have different homologies in different taxa. As discussed above, the cupules of the oldest seed ferns were dichotomous structures that were probably derived from the fertile appendages of progymnosperms and lost in more derived seed ferns, such as medullosans and Callistophyton. In contrast, younger peltasperms, corystosperms, glossopterids, and Caytonia had dorsiventral cupules that are more likely modified leaves (sporophylls) or leaflets, with ovules on one surface. Among these, anatomical and positional evidence (reviewed in Doyle 2008) indicates that the ovules were on the abaxial surface of the cupule in peltasperms and corystosperms but on the adaxial surface in glossopterids. Glossopterids had one or more cupules attached to the midrib of a leaf, most simply interpreted as sporophylls on an adnate axillary branch (Retallack & Dilcher 1981). Caytonia had reflexed cupules that have been interpreted as leaflets borne along the rachis of a sporophyll, with adaxial ovules (Harris 1940; Gaussen 1946; Harris 1951; Doyle 1978). However, as emphasized by Rothwell et al. (2009), this has not been confirmed by anatomical data, and there are putatively related fossils with cupules arranged in a spiral, suggesting that they were whole sporophylls borne on a branch (Schweitzer & Kirchner 1998; Wang 2010).
These observations are consistent with precladistic hypotheses that the angiosperm outer integument is homologous with the cupule of glossopterids and/or Caytonia (Gaussen 1946; Stebbins 1974; Doyle 1978; Retallack & Dilcher 1981). They are also consistent with analyses in which glossopterids and Caytonia are angiosperm stem relatives, although the fact that the same analyses also associate angiosperms with Pentoxylon and Bennettitales poses problems, since these taxa had ovule-bearing structures that are difficult to interpret in these terms (or any others; Doyle 2008). Caytonia is more like angiosperms in having anatropous cupules, which could be transformed into anatropous bitegmic ovules by reduction to one ovule per cupule.
Gaussen (1946) and later authors (Doyle 1978; Crane 1985) proposed that the carpel was derived by broadening and folding of a cupule-bearing rachis of the Caytonia type. However, others have homologized the carpel with a glossopterid leaf-cupule complex, with the carpel wall derived from the subtending leaf and the bitegmic ovule from a cupule on an axillary branch (Stebbins 1974; Retallack & Dilcher 1981; Doyle 1996; Doyle 2008). This would be consistent with the inference that the ancestral carpel had one bitegmic ovule on the cross-zone, corresponding to an axillary position, and with hints that the carpel wall and placenta in Arabidopsis are under separate genetic control (Skinner et al. 2004). However, ovule position appears to have been highly labile in early angiosperms (Frohlich & Chase 2007; Endress & Doyle 2009), suggesting that positional arguments for homology should be used with caution. A problem in relating angiosperms to both glossopterids and Caytonia is the fact that it is difficult to homologize the cupule-bearing structures of these fossils with each other; this might be easier if the supposed Caytonia sporophyll turns out to be a sporophyll-bearing branch (Doyle 2008).
Although discussions on the origin of the flower tend to emphasize the carpel, the stamens too show major innovations and present similar problems. They are unique in having four microsporangia fused into two thecae, one on either side of the medial plane. Thomas (1925) compared angiosperm stamens with microsynangia of Caytonia, which consisted of four sporangia, but as noted by Harris (1937) the four sporangia were not separated into two pairs and were borne on a branched structure interpreted as a sporophyll. If angiosperms are related to Caytonia, it might be better to homologize each theca with a synangium and the stamen with the whole sporophyll, drastically reduced to an unbranched structure with two synangia (Gaussen 1946; Doyle 2008). Character optimization on molecular trees (Endress & Doyle 2009) indicates that the microsporangia were originally lateral or adaxial (introrse). This could be compared to the situation in glossopterids, where microsporangia were borne on a branch from the adaxial side of a leaf, or in Bennettitales, where microsynangia were borne on the adaxial side of simple or pinnate structures interpreted as sporophylls. However, in both fossil groups the microsporangia were perpendicular to the leaf-like structure, not parallel to its surface as in angiosperms, and like ovule position, microsporangial position was highly labile in early angiosperms (Endress & Doyle 2009).
Molecular phylogenetic results confirm the standard view that the first angiosperms had monosulcate pollen. If angiosperms had ancestors with saccate pollen, as in glossopterids and Caytonia, the sacs must have been lost below the crown group. Rothwell et al. (2009) argued that the saccate pollen of Caytonia was evidence against a relationship with angiosperms. However, even if Caytonia is sister to angiosperms, it is not clear that their common ancestor had sacs, and as already noted loss of sacs was a common phenomenon in seed plants, tied to shifts away from capture of pollen by a pollination drop secreted by a downward-oriented ovule (Doyle 2010; Leslie 2010).
An important innovation in angiosperm pollen was columellar exine structure, with radial rods connecting the inner nexine and outer tectum layers. In potential outgroups, the infratectal structure was alveolar (glossopterids, Caytonia) or granular (Bennettitales, Pentoxylon, Gnetales), whereas in angiosperms it is granular or columellar. Earlier workers (Van Campo & Lugardon 1973; Doyle et al. 1975; Walker & Skvarla 1975; Walker 1976) argued that granular (including “atectate”) exine structure was ancestral, since it occurs both in gymnosperms and in Magnoliales, which were assumed to be primitive. However, the ANITA lines have columellar exines, or in Amborella an apparently related type with an undulating tectum (Sampson 1993; Hesse 2001), indicating that columellae evolved on the angiosperm stem lineage (Doyle 2005, 2009). Columellar exines often have perforations in the tectum, resulting in reticulate sculpture, but the tectum is continuous in Amborella and Nymphaeales, implying that perforations arose within angiosperms, below the Austrobaileyales node.
Another advance on the line leading to angiosperms was siphonogamy, which appears to have originated independently from siphonogamy in conifers and Gnetales, based on the placement of zooidogamous cycads and Ginkgo lower in the acrogymnosperms. Multiflagellated sperm cells have been described in glossopterids (Nishida et al. 2004), which would imply that siphonogamy originated after this group diverged from angiosperms, if the two are related. Based on similarities in the pattern of pollen tube growth in ovules of Bennettitales and araucariaceous conifers, Stockey and Rothwell (2003) argued that Bennettitales were siphonogamous.
Some of the most remarkable innovations in angiosperms are related to radical reduction of the gametophyte generation. In the male gametophyte, loss of sterile cells left only two sperm cells and the tube nucleus. The ancestral female gametophyte (embryo sac) was long assumed to be of the eight-nucleate Polygonum type, with no recognizable archegonium but an egg and two synergid cells, two free polar nuclei, and three antipodal cells. This serves as the setup for double fertilization, where one of the two sperm cells fuses with the egg to produce the embryo, the other with the polar nuclei to produce the triploid endosperm, the nourishing tissue for the embryo in most angiosperms. However, recent studies confirmed earlier reports (e.g., Yoshida 1960; Batygina et al. 1982) that two of the ANITA lines, Nymphaeales and Austrobaileyales, have a four-nucleate female gametophyte with only an egg, two synergids, and one polar nucleus, which fuses with the second sperm to produce diploid endosperm (Williams & Friedman 2002; Friedman et al. 2003; Williams & Friedman 2004). Friedman et al. (2003) proposed that this type was ancestral and the Polygonum type was derived by duplication of the four-nucleate module, with the antipodals representing a sterilized egg and synergids.
A problem is that Amborella has a female gametophyte resembling the Polygonum type (with an exception discussed below), so it seems equally parsimonious to assume that the four-nucleate type was either ancestral or derived twice from eight-nucleate. However, Williams and Friedman (2004) showed that dissection of this character into developmental changes favors a scenario in which the first step, on the angiosperm stem lineage, was reduction from many nuclei to a four-nucleate module, which was duplicated once in Amborella and once after the divergence of Austrobaileyales, that is, at the base of the mesangiosperms. If so, endosperm originated as a diploid tissue and later become triploid. Its triploid state would be a byproduct of duplication of the polar nucleus, but advantages of triploidy in endosperm tissue may have been the selective factor that favored this change (Friedman & Williams 2004) and a key innovation that contributed to the vast diversity of the mesangiosperm clade (Williams & Friedman 2004), which includes around 99.9% of angiosperm species.
This scenario is complicated by the discovery that the female gametophyte of Amborella has nine nuclei rather than eight (Friedman 2006). The extra nucleus is derived from division of one of the micropylar cells to produce the egg and a outer cell. This could be the sole remnant in angiosperms of the periclinal division of the archegonial initial cell, established in the first land plants, or it could be an autapomorphy (Friedman & Ryerson 2009). In the former case, it could mean that the ancestral female gametophyte had five nuclei rather than four.
There is little evidence on the timing of these changes in the life cycle, because relevant details are rarely preserved in fossils. In the heyday of the anthophyte hypothesis, when a form of double fertilization was confirmed in Gnetales (where fusion of the second sperm with another nucleus of the female gametophyte produces an extra embryo), this was proposed as a step toward the angiosperm condition (Friedman 1990, 1994; Friedman & Carmichael 1996). However, in the light of molecular phylogenies this now appears to be a convergence (Friedman & Floyd 2001). Glossopterids and Bennettitales had relatively large female gametophytes, so if they are related to angiosperms the main reduction must have occurred between their divergence and the base of the angiosperms. Unfortunately none of these characters are preserved in Caytonia.
Another issue concerns embryogeny, which involves only cellular divisions in angiosperms but an initial free-nuclear phase in other seed plants, except Gnetales (Ephedra is a complex case discussed in Doyle 2006). An exception in angiosperms is Paeonia, which was considered primitive by Stebbins (1974) but is clearly derived based on its position in the eudicot order Saxifragales. However, if acrogymnosperms and angiosperms are sister groups, it is equivocal whether cellular or nuclear embryogenesis is ancestral for crown group seed plants. This is a case where a position of cycads on the line leading to angiosperms (Mathews et al. 2010) would have an effect on scenarios, since it would imply that the angiosperm condition was derived, but the point at which cellular embryogeny evolved on the angiosperm stem lineage would remain unknown.
1.9 Innovations within Angiosperms: Monocots and Eudicots
Evolutionary innovations within angiosperms are too numerous to treat thoroughly, but a few deserve special attention. One already mentioned was derivation of the plicate carpel, with margins sealed by postgenital fusion of the young epidermises, from the ancestral ascidiate carpel (Endress & Igersheim 2000). In trees based on combined morphological and molecular data, this occurred above the base of mesangiosperms, after divergence of the ascidiate Chloranthaceae and Ceratophyllum lines, but in trees with other arrangements of mesangiosperms it occurred either several times or once at the base of the clade, followed by reversals (Doyle & Endress 2000; Endress & Doyle 2009). Syncarpy, which allows fertilization of ovules in all carpels by pollen landing on any stigma, arose many times within angiosperms, usually by congenital fusion of the carpels (Endress 1994).
Monocots underwent a major reorganization of vegetative morphology tied with loss of secondary growth, including scattered primary vascular bundles (an atactostele), early abortion of the primary root, and production of adventitious roots from rhizomes (Arber 1925; Takhtajan 1969; Dahlgren et al. 1985). Similar changes also occurred in Nymphaeales, where it is clear that they are functionally related to an aquatic habit. This and the assumption that the most primitive monocots were aquatic members of the Alismatales (Helobiae) led to the hypothesis that monocots and Nymphaeales were derived from an aquatic common ancestor (e.g., Cronquist 1968; Takhtajan 1969), which seemed to be supported by morphological cladistic analyses that linked the two groups (Dahlgren & Bremer 1985; Donoghue & Doyle 1989; Loconte & Stevenson 1991). However, the molecular separation of monocots from Nymphaeales indicates that their similar derived features are purely convergent. Molecular data have further clarified the situation by showing that Acorus and Alismatales are basal to the remaining monocot groups (core monocots, or petrosaviids). Acorus is a marsh plant but less aquatic than classic Helobiae, which are nested in Alismatales among Araceae and Tofieldiaceae, many of which are also marsh or bog plants. This supports a more nuanced view that loss of secondary growth and related features evolved in wet but not fully aquatic habitats.
Because Acorus, Tofieldiaceae, and many basal Araceae are like other monocots in having linear leaves with “parallel” venation (actually joining at the apex: Kaplan 1973; Doyle et al. 2008), such leaves presumably evolved on the stem lineage of monocots. In most monocots the leaf blade develops from the lower zone of the leaf primordium, not the upper zone as in other groups, which implies that it corresponds to the leaf base of other angiosperms (Kaplan 1973). However, in Acorus, Alismatales, and many Liliales, the blade develops from the upper leaf zone (Kaplan 1973; Bharathan 1996; Rudall & Buzgo 2002), indicating that the change in blade morphology occurred first and the new mode of development later.
The signature innovation of monocots is the single cotyledon of the embryo, in contrast to the two cotyledons of other angiosperms and most gymnosperms. Whether the monocot condition evolved by loss of one cotyledon or fusion of two is a long-standing topic of discussion (Arber 1925; Takhtajan 1969). There are no intermediate conditions in taxa that might be phylogenetically relevant. In Hydatellaceae, minute aquatics once considered monocots but recently found to be related to Nymphaeales (Saarela et al. 2007), Sokoloff et al. (2008) described fusion of the cotyledons into a bilobed structure and suggested that this might be a step toward a single cotyledon, but in the current phylogenetic context this cannot be more than an analogy.
In precladistic classifications angiosperms were divided into monocots and dicots, but “dicots” have been abandoned as a grossly paraphyletic group. However, some 95% of dicot species form a clade, the eudicots, which include such familiar plants as the model system Arabidopsis. Their most definite synapomorphy is tricolpate pollen, with three germination furrows (colpi) running along lines of longitude relative to the polar axis (defined by the center of the meiotic tetrad and the center of the grain), rather than one furrow (sulcus) at the distal pole. Tricolpate pollen was ancestral to many other types, such as tricolporate and triporate. This innovation may have allowed more rapid penetration of the stigma by the pollen tube, regardless of the orientation of the pollen. Precladistic workers assumed that tricolpate pollen evolved independently in several lines (e.g., Cronquist 1968), but molecular studies confirmed preliminary indications from morphological analyses (Dahlgren & Bremer 1985; Donoghue & Doyle 1989) that eudicots are monophyletic and tricolpate pollen originated only once (except for a superficial convergence in Illicium and Schisandra, in the Austrobaileyales, where the three furrows are oriented differently: Huynh 1976; Doyle 2005). Phylogenetic analyses provide little evidence on the mode of origin of tricolpate pollen, since the potential outgroups are basically monosulcate (Doyle 2005), but some of the oldest Early Cretaceous tricolpate pollen has obliquely oriented and interconnected colpi, suggesting an origin by elongation, spiralization, and fragmentation of a sulcus (Doyle & Hotton 1991; Blackmore & Crane 1998).
Another change that may have occurred on the line to eudicots but was not recognized until recently is a shift from trimerous to dimerous flowers, with parts in whorls of two (Drinnan et al. 1994; Endress & Doyle 2009). It is equivocal whether dimery arose once at the base of eudicots or more than once within them. In the basal order Ranunculales the Papaveraceae are dimerous but most of the other families are trimerous, and in the other line the basal order Proteales includes both the dimerous family Proteaceae and taxa with other conditions (Nelumbo, Platanus). However, dimery was established in Trochodendraceae, Buxaceae, and Gunnerales, the lines closest to the remaining eudicots. In this part of the phylogeny the flower can be reconstructed as having two pairs of sepaloid tepals and two pairs of stamens (Ronse De Craene 2008; Endress & Doyle 2009). Given that most of these taxa (except Nelumbo and Proteaceae) are wind pollinated, whereas more basal groups are largely insect pollinated, this floral architecture appears to reflect a shift from insect to wind pollination.
Within eudicots, a major innovation was origin of the typical pentamerous (less commonly tetramerous) “dicot” flower of textbooks, with alternating whorls of five protective sepals, five attractive petals, and five or ten stamens. This appears to be a synapomorphy of a major clade, called core eudicots or Pentapetalae, which is the sister group of Gunnerales (Gunnera and Myrothamnus). The fact that Pentapetalae are nested among the dimerous groups suggests that their typically insect pollinated flowers were derived from simpler wind pollinated flowers, a reversal of the earlier trend. Wanntorp and Ronse De Craene (2005) argued that the flowers of Gunnera are not relevant for the origin of Pentapetalae because they are reduced for wind pollination, but this does not follow, since parsimony optimization indicates that the whole line went through a reduced phase (Ronse De Craene 2004; Endress & Doyle 2009). Based on the spiral perianth of Berberidopsis, near the base of the Pentapetalae, Ronse De Craene (2004) suggested that the pentamerous flower was derived from a flower with spiral phyllotaxis, but Berberidopsis is unlikely to represent a transitional state, since it is linked with Aextoxicon, which is pentamerous (Ronse De Craene & Stuppy 2010). Many authors have suggested that the petals of Pentapetalae were derived from stamens, but this was questioned by Ronse De Craene (2007) on developmental grounds, and it seems unlikely from a phylogenetic perspective, since the more basal groups have so few stamens. However the transition occurred, it involved an increase in the total number of floral parts from that in basal eudicots, whether by addition of cycles, increase in the number of parts per cycle, or both.
Acknowledgments
I thank Judy Jernstedt, Stefan Little, and Yin-Long Qiu for useful discussions and help in obtaining literature.



